Crosstalk of Mast Cells and Natural Killer Cells with Neurons in Chemotherapy-Induced Peripheral Neuropathy
Abstract
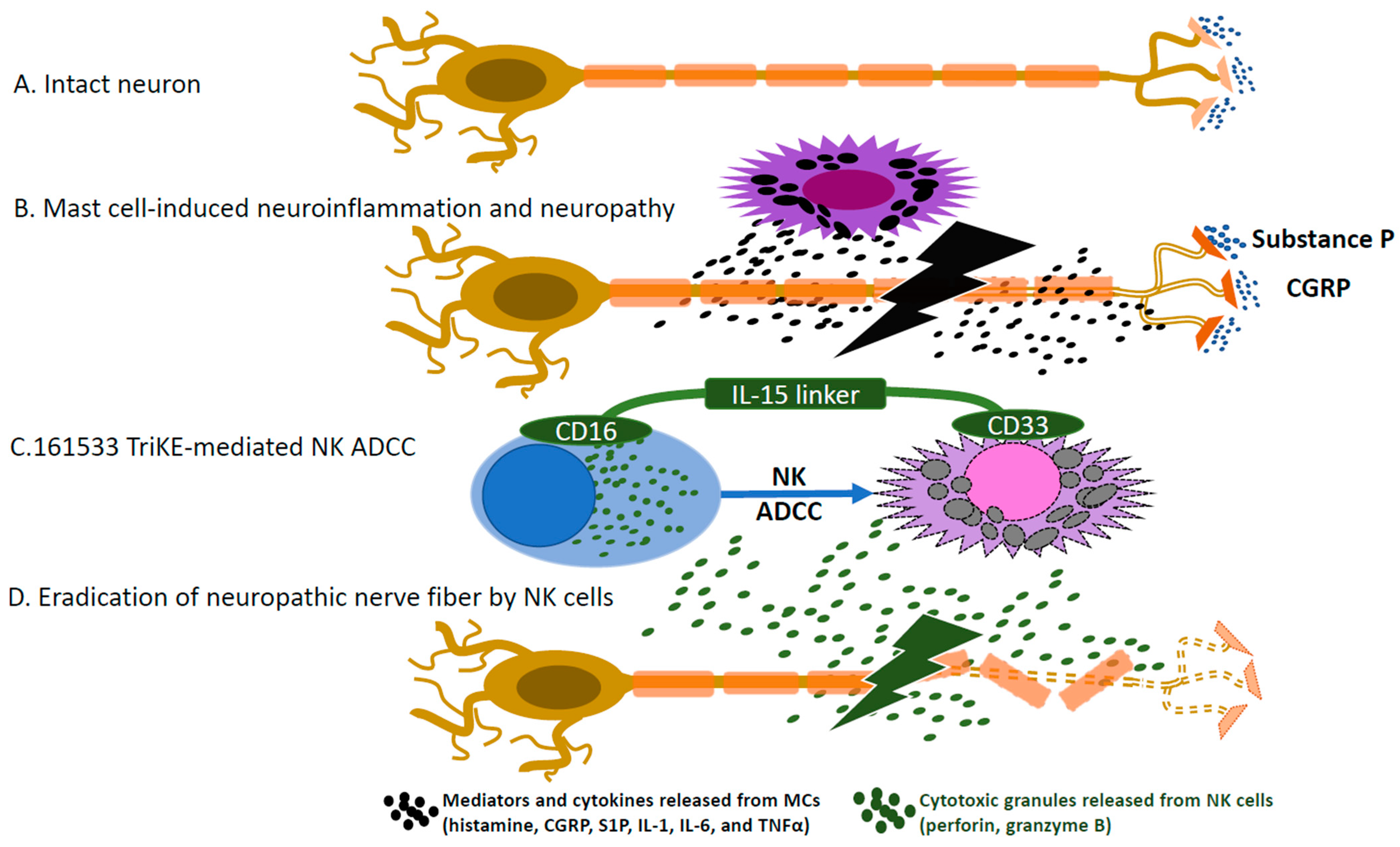
:1. Introduction
2. Mast Cells
3. Natural Killer (NK) Cells
4. Communication between Mast Cells and NK Cells
5. Proinflammatory Cytokines
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Staff, N.P.; Grisold, A.; Grisold, W.; Windebank, A.J. Chemotherapy-Induced Peripheral Neuropathy: A Current Review. Ann. Neurol. 2017, 81, 772–781. [Google Scholar] [CrossRef]
- Richardson, P.G.; Briemberg, H.; Jagannath, S.; Wen, P.Y.; Barlogie, B.; Berenson, J.; Singhal, S.; Siegel, D.S.; Irwin, D.; Schuster, M.; et al. Frequency, Characteristics, and Reversibility of Peripheral Neuropathy during Treatment of Advanced Multiple Myeloma with Bortezomib. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 3113–3120. [Google Scholar] [CrossRef] [PubMed]
- Postma, T.J.; Vermorken, J.B.; Liefting, A.J.; Pinedo, H.M.; Heimans, J.J. Paclitaxel-Induced Neuropathy. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 1995, 6, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Hammack, J.E.; Michalak, J.C.; Loprinzi, C.L.; Sloan, J.A.; Novotny, P.J.; Soori, G.S.; Tirona, M.T.; Rowland, K.M.; Stella, P.J.; Johnson, J.A. Phase III Evaluation of Nortriptyline for Alleviation of Symptoms of Cis-Platinum-Induced Peripheral Neuropathy. Pain 2002, 98, 195–203. [Google Scholar] [CrossRef]
- Kautio, A.-L.; Haanpää, M.; Kautiainen, H.; Kalso, E.; Saarto, T. Burden of Chemotherapy-Induced Neuropathy—A Cross-Sectional Study. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2011, 19, 1991–1996. [Google Scholar] [CrossRef] [PubMed]
- Mariotto, S.; Tecchio, C.; Sorio, M.; Bertolasi, L.; Turatti, M.; Tozzi, M.C.; Benedetti, F.; Cavaletti, G.; Monaco, S.; Ferrari, S. Clinical and Neurophysiological Serial Assessments of Brentuximab Vedotin-Associated Peripheral Neuropathy. Leuk. Lymphoma 2019, 60, 2806–2809. [Google Scholar] [CrossRef] [PubMed]
- Oaklander, A.L. Immunotherapy Prospects for Painful Small-Fiber Sensory Neuropathies and Ganglionopathies. Neurother. J. Am. Soc. Exp. Neurother. 2016, 13, 108–117. [Google Scholar] [CrossRef] [Green Version]
- Dubey, D.; David, W.S.; Reynolds, K.L.; Chute, D.F.; Clement, N.F.; Cohen, J.V.; Lawrence, D.P.; Mooradian, M.J.; Sullivan, R.J.; Guidon, A.C. Severe Neurological Toxicity of Immune Checkpoint Inhibitors: Growing Spectrum. Ann. Neurol. 2020, 87, 659–669. [Google Scholar] [CrossRef]
- Farooq, M.Z.; Aqeel, S.B.; Lingamaneni, P.; Pichardo, R.C.; Jawed, A.; Khalid, S.; Banskota, S.U.; Fu, P.; Mangla, A. Association of Immune Checkpoint Inhibitors With Neurologic Adverse Events: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2022, 5, e227722. [Google Scholar] [CrossRef]
- Speck, R.M.; Sammel, M.D.; Farrar, J.T.; Hennessy, S.; Mao, J.J.; Stineman, M.G.; DeMichele, A. Impact of Chemotherapy-Induced Peripheral Neuropathy on Treatment Delivery in Nonmetastatic Breast Cancer. J. Oncol. Pract. 2013, 9, e234–e240. [Google Scholar] [CrossRef]
- Gewandter, J.S.; Freeman, R.; Kitt, R.A.; Cavaletti, G.; Gauthier, L.R.; McDermott, M.P.; Mohile, N.A.; Mohlie, S.G.; Smith, A.G.; Tejani, M.A.; et al. Chemotherapy-Induced Peripheral Neuropathy Clinical Trials: Review and Recommendations. Neurology 2017, 89, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Kerckhove, N.; Collin, A.; Condé, S.; Chaleteix, C.; Pezet, D.; Balayssac, D. Long-Term Effects, Pathophysiological Mechanisms, and Risk Factors of Chemotherapy-Induced Peripheral Neuropathies: A Comprehensive Literature Review. Front. Pharmacol. 2017, 8, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgess, J.; Ferdousi, M.; Gosal, D.; Boon, C.; Matsumoto, K.; Marshall, A.; Mak, T.; Marshall, A.; Frank, B.; Malik, R.A.; et al. Chemotherapy-Induced Peripheral Neuropathy: Epidemiology, Pathomechanisms and Treatment. Oncol. Ther. 2021, 9, 385–450. [Google Scholar] [CrossRef]
- Loprinzi, C.L.; Lacchetti, C.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Hertz, D.L.; Kelley, M.R.; Lavino, A.; Lustberg, M.B.; Paice, J.A.; et al. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: ASCO Guideline Update. J. Clin. Oncol. 2020, 38, 3325–3348. [Google Scholar] [CrossRef]
- Rao, R.D.; Michalak, J.C.; Sloan, J.A.; Loprinzi, C.L.; Soori, G.S.; Nikcevich, D.A.; Warner, D.O.; Novotny, P.; Kutteh, L.A.; Wong, G.Y.; et al. Efficacy of Gabapentin in the Management of Chemotherapy-Induced Peripheral Neuropathy. Cancer 2007, 110, 2110–2118. [Google Scholar] [CrossRef] [PubMed]
- Rentsch, C.T.; Morford, K.L.; Fiellin, D.A.; Bryant, K.J.; Justice, A.C.; Tate, J.P. Safety of Gabapentin Prescribed for Any Indication in a Large Clinical Cohort of 571,718 US Veterans with and without Alcohol Use Disorder. Alcohol Clin. Exp. Res. 2020, 44, 1807–1815. [Google Scholar] [CrossRef]
- Ishida, J.H.; McCulloch, C.E.; Steinman, M.A.; Grimes, B.A.; Johansen, K.L. Gabapentin and Pregabalin Use and Association with Adverse Outcomes among Hemodialysis Patients. J. Am. Soc. Nephrol. 2018, 29, 1970. [Google Scholar] [CrossRef] [Green Version]
- Moehring, F.; Halder, P.; Seal, R.P.; Stucky, C.L. Uncovering the Cells and Circuits of Touch in Normal and Pathological Settings. Neuron 2018, 100, 349–360. [Google Scholar] [CrossRef] [Green Version]
- Moehring, F.; Cowie, A.M.; Menzel, A.D.; Weyer, A.D.; Grzybowski, M.; Arzua, T.; Geurts, A.M.; Palygin, O.; Stucky, C.L. Keratinocytes Mediate Innocuous and Noxious Touch via ATP-P2X4 Signaling. eLife 2018, 7, e31684. [Google Scholar] [CrossRef]
- Klusch, A.; Ponce, L.; Gorzelanny, C.; Schäfer, I.; Schneider, S.W.; Ringkamp, M.; Holloschi, A.; Schmelz, M.; Hafner, M.; Petersen, M. Coculture Model of Sensory Neurites and Keratinocytes to Investigate Functional Interaction: Chemical Stimulation and Atomic Force Microscope-Transmitted Mechanical Stimulation Combined with Live-Cell Imaging. J. Investig. Dermatol. 2013, 133, 1387–1390. [Google Scholar] [CrossRef] [Green Version]
- Ellis, S.R.; Vierra, A.T.; Millsop, J.W.; Lacouture, M.E.; Kiuru, M. Dermatologic Toxicities to Immune Checkpoint Inhibitor Therapy: A Review of Histopathologic Features. J. Am. Acad. Dermatol. 2020, 83, 1130–1143. [Google Scholar] [CrossRef]
- Quach, H.T.; Johnson, D.B.; LeBoeuf, N.R.; Zwerner, J.P.; Dewan, A.K. Cutaneous Adverse Events Caused by Immune Checkpoint Inhibitors. J. Am. Acad. Dermatol. 2021, 85, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Sibaud, V. Dermatologic Reactions to Immune Checkpoint Inhibitors: Skin Toxicities and Immunotherapy. Am. J. Clin. Dermatol. 2018, 19, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Goldinger, S.M.; Stieger, P.; Meier, B.; Micaletto, S.; Contassot, E.; French, L.E.; Dummer, R. Cytotoxic Cutaneous Adverse Drug Reactions during Anti-PD-1 Therapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 4023–4029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, A.J.; Rinaldi, S.; Costigan, M.; Oh, S.B. Cytotoxic Immunity in Peripheral Nerve Injury and Pain. Front. Neurosci. 2020, 14, 142. [Google Scholar] [CrossRef] [Green Version]
- Tsimberidou, A.M.; Fountzilas, E.; Nikanjam, M.; Kurzrock, R. Review of Precision Cancer Medicine: Evolution of the Treatment Paradigm. Cancer Treat. Rev. 2020, 86, 102019. [Google Scholar] [CrossRef]
- Pennock, G.K.; Chow, L.Q.M. The Evolving Role of Immune Checkpoint Inhibitors in Cancer Treatment. Oncologist 2015, 20, 812–822. [Google Scholar] [CrossRef] [Green Version]
- Ribatti, D.; Crivellato, E. Mast Cells, Angiogenesis, and Tumour Growth. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2012, 1822, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Somasundaram, R.; Connelly, T.; Choi, R.; Choi, H.; Samarkina, A.; Li, L.; Gregorio, E.; Chen, Y.; Thakur, R.; Abdel-Mohsen, M.; et al. Tumor-Infiltrating Mast Cells Are Associated with Resistance to Anti-PD-1 Therapy. Nat. Commun. 2021, 12, 346. [Google Scholar] [CrossRef]
- Myers, J.A.; Miller, J.S. Exploring the NK Cell Platform for Cancer Immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 85–100. [Google Scholar] [CrossRef]
- Foley, B.; Felices, M.; Cichocki, F.; Cooley, S.; Verneris, M.R.; Miller, J.S. The Biology of NK Cells and Their Receptors Affects Clinical Outcomes after Hematopoietic Cell Transplantation (HCT). Immunol. Rev. 2014, 258, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-M.; Lehky, T.J.; Brell, J.M.; Dorsey, S.G. Discovering Cytokines as Targets for Chemotherapy-Induced Painful Peripheral Neuropathy. Cytokine 2012, 59, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, K.; Harvima, I.T. Mast Cell-Neural Interactions Contribute to Pain and Itch. Immunol. Rev. 2018, 282, 168–187. [Google Scholar] [CrossRef] [PubMed]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2015, 6, 620. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Lei, Z.; Zhang, G.-M.; Li, D.; Song, C.; Li, B.; Liu, Y.; Yuan, Y.; Unkeless, J.; Xiong, H.; et al. SCF-Mediated Mast Cell Infiltration and Activation Exacerbate the Inflammation and Immunosuppression in Tumor Microenvironment. Blood 2008, 112, 1269–1279. [Google Scholar] [CrossRef]
- Komi, D.E.A.; Redegeld, F.A. Role of Mast Cells in Shaping the Tumor Microenvironment. Clin. Rev. Allergy Immunol. 2020, 58, 313–325. [Google Scholar] [CrossRef]
- Nguyen, J.; Luk, K.; Vang, D.; Soto, W.; Vincent, L.; Robiner, S.; Saavedra, R.; Li, Y.; Gupta, P.; Gupta, K.; et al. Morphine Stimulates Cancer Progression and Mast Cell Activation and Impairs Survival in Transgenic Mice with Breast Cancer. BJA Br. J. Anaesth. 2014, 113, i4–i13. [Google Scholar] [CrossRef] [Green Version]
- Novy, D.M.; Nelson, D.V.; Koyyalagunta, D.; Cata, J.P.; Gupta, P.; Gupta, K. Pain, Opioid Therapy, and Survival: A Needed Discussion. Pain 2020, 161, 496–501. [Google Scholar] [CrossRef]
- Aich, A.; Afrin, L.B.; Gupta, K. Mast Cell-Mediated Mechanisms of Nociception. Int. J. Mol. Sci. 2015, 16, 29069–29092. [Google Scholar] [CrossRef] [Green Version]
- Mittal, A.; Sagi, V.; Gupta, M.; Gupta, K. Mast Cell Neural Interactions in Health and Disease. Front. Cell. Neurosci. 2019, 13, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendriksen, E.; van Bergeijk, D.; Oosting, R.S.; Redegeld, F.A. Mast Cells in Neuroinflammation and Brain Disorders. Neurosci. Biobehav. Rev. 2017, 79, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.; Gupta, M.; Gupta, K. Targeting Novel Mechanisms of Pain in Sickle Cell Disease. Blood 2017, 130, 2377–2385. [Google Scholar] [CrossRef] [PubMed]
- Vincent, L.; Vang, D.; Nguyen, J.; Gupta, M.; Luk, K.; Ericson, M.E.; Simone, D.A.; Gupta, K. Mast Cell Activation Contributes to Sickle Cell Pathobiology and Pain in Mice. Blood 2013, 122, 1853–1862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, L.; Vang, D.; Nguyen, J.; Benson, B.; Lei, J.; Gupta, K. Cannabinoid Receptor-Specific Mechanisms to Alleviate Pain in Sickle Cell Anemia via Inhibition of Mast Cell Activation and Neurogenic Inflammation. Haematologica 2016, 101, 566–577. [Google Scholar] [CrossRef] [Green Version]
- Groetzner, P.; Weidner, C. The Human Vasodilator Axon Reflex—An Exclusively Peripheral Phenomenon? PAIN® 2010, 149, 71–75. [Google Scholar] [CrossRef]
- Tani, E.; Ishikawa, T. Histamine Acts Directly on Calcitonin Gene-Related Peptide- and Substance P-Containing Trigeminal Ganglion Neurons as Assessed by Calcium Influx and Immunocytochemistry. Auris. Nasus. Larynx 1990, 17, 267–274. [Google Scholar] [CrossRef]
- Schwenger, N.; Dux, M.; de Col, R.; Carr, R.; Messlinger, K. Interaction of Calcitonin Gene-Related Peptide, Nitric Oxide and Histamine Release in Neurogenic Blood Flow and Afferent Activation in the Rat Cranial Dura Mater. Cephalalgia Int. J. Headache 2007, 27, 481–491. [Google Scholar] [CrossRef]
- Wolf, S.; Barton, D.; Kottschade, L.; Grothey, A.; Loprinzi, C. Chemotherapy-Induced Peripheral Neuropathy: Prevention and Treatment Strategies. Eur. J. Cancer 2008, 44, 1507–1515. [Google Scholar] [CrossRef]
- Kidera, Y.; Satoh, T.; Ueda, S.; Okamoto, W.; Okamoto, I.; Fumita, S.; Yonesaka, K.; Hayashi, H.; Makimura, C.; Okamoto, K.; et al. High-Dose Dexamethasone plus Antihistamine Prevents Colorectal Cancer Patients Treated with Modified FOLFOX6 from Hypersensitivity Reactions Induced by Oxaliplatin. Int. J. Clin. Oncol. 2011, 16, 244–249. [Google Scholar] [CrossRef]
- Déry, O.; Corvera, C.U.; Steinhoff, M.; Bunnett, N.W. Proteinase-Activated Receptors: Novel Mechanisms of Signaling by Serine Proteases. Am. J. Physiol.-Cell Physiol. 1998, 274, C1429–C1452. [Google Scholar] [CrossRef]
- Vergnolle, N.; Bunnett, N.W.; Sharkey, K.A.; Brussee, V.; Compton, S.J.; Grady, E.F.; Cirino, G.; Gerard, N.; Basbaum, A.I.; Andrade-Gordon, P.; et al. Proteinase-Activated Receptor-2 and Hyperalgesia: A Novel Pain Pathway. Nat. Med. 2001, 7, 821–826. [Google Scholar] [CrossRef]
- Steinhoff, M.; Vergnolle, N.; Young, S.H.; Tognetto, M.; Amadesi, S.; Ennes, H.S.; Trevisani, M.; Hollenberg, M.D.; Wallace, J.L.; Caughey, G.H.; et al. Agonists of Proteinase-Activated Receptor 2 Induce Inflammation by a Neurogenic Mechanism. Nat. Med. 2000, 6, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, C.; Wang, Z. Activation of Mast Cell Tryptase and Protease-Activated Receptor 2 Mediate Neuropathic Pain Induced by Paclitaxel. J. Pain 2010, 11, S25. [Google Scholar] [CrossRef]
- Prieschl, E.E.; Csonga, R.; Novotny, V.; Kikuchi, G.E.; Baumruker, T. The Balance between Sphingosine and Sphingosine-1-Phosphate Is Decisive for Mast Cell Activation after Fc Epsilon Receptor I Triggering. J. Exp. Med. 1999, 190, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jolly, P.S.; Bektas, M.; Olivera, A.; Gonzalez-Espinosa, C.; Proia, R.L.; Rivera, J.; Milstien, S.; Spiegel, S. Transactivation of Sphingosine-1–Phosphate Receptors by FcεRI Triggering Is Required for Normal Mast Cell Degranulation and Chemotaxis. J. Exp. Med. 2004, 199, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Grenald, S.A.; Doyle, T.M.; Zhang, H.; Slosky, L.M.; Chen, Z.; Largent-Milnes, T.M.; Spiegel, S.; Vanderah, T.W.; Salvemini, D. Targeting the S1P/S1PR1 Axis Mitigates Cancer-Induced Bone Pain and Neuroinflammation. Pain 2017, 158, 1733–1742. [Google Scholar] [CrossRef] [Green Version]
- Janes, K.; Little, J.W.; Li, C.; Bryant, L.; Chen, C.; Chen, Z.; Kamocki, K.; Doyle, T.; Snider, A.; Esposito, E.; et al. The Development and Maintenance of Paclitaxel-Induced Neuropathic Pain Require Activation of the Sphingosine 1-Phosphate Receptor Subtype 1. J. Biol. Chem. 2014, 289, 21082–21097. [Google Scholar] [CrossRef] [Green Version]
- Kärre, K.; Ljunggren, H.G.; Piontek, G.; Kiessling, R. Selective Rejection of H-2-Deficient Lymphoma Variants Suggests Alternative Immune Defence Strategy. Nature 1986, 319, 675–678. [Google Scholar] [CrossRef]
- Binstadt, B.A.; Brumbaugh, K.M.; Dick, C.J.; Scharenberg, A.M.; Williams, B.L.; Colonna, M.; Lanier, L.L.; Kinet, J.P.; Abraham, R.T.; Leibson, P.J. Sequential Involvement of Lck and SHP-1 with MHC-Recognizing Receptors on NK Cells Inhibits FcR-Initiated Tyrosine Kinase Activation. Immunity 1996, 5, 629–638. [Google Scholar] [CrossRef] [Green Version]
- Guia, S.; Jaeger, B.N.; Piatek, S.; Mailfert, S.; Trombik, T.; Fenis, A.; Chevrier, N.; Walzer, T.; Kerdiles, Y.M.; Marguet, D.; et al. Confinement of Activating Receptors at the Plasma Membrane Controls Natural Killer Cell Tolerance. Sci. Signal. 2011, 4, ra21. [Google Scholar] [CrossRef]
- Cooley, S.; Weisdorf, D.J.; Guethlein, L.A.; Klein, J.P.; Wang, T.; Le, C.T.; Marsh, S.G.E.; Geraghty, D.; Spellman, S.; Haagenson, M.D.; et al. Donor Selection for Natural Killer Cell Receptor Genes Leads to Superior Survival after Unrelated Transplantation for Acute Myelogenous Leukemia. Blood 2010, 116, 2411–2419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooley, S.; Trachtenberg, E.; Bergemann, T.L.; Saeteurn, K.; Klein, J.; Le, C.T.; Marsh, S.G.E.; Guethlein, L.A.; Parham, P.; Miller, J.S.; et al. Donors with Group B KIR Haplotypes Improve Relapse-Free Survival after Unrelated Hematopoietic Cell Transplantation for Acute Myelogenous Leukemia. Blood 2009, 113, 726–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weisdorf, D.; Cooley, S.; Wang, T.; Trachtenberg, E.; Vierra-Green, C.; Spellman, S.; Sees, J.A.; Spahn, A.; Vogel, J.; Fehniger, T.A.; et al. KIR B Donors Improve the Outcome for AML Patients given Reduced Intensity Conditioning and Unrelated Donor Transplantation. Blood Adv. 2020, 4, 740–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachanova, V.; Weisdorf, D.J.; Wang, T.; Marsh, S.G.E.; Trachtenberg, E.; Haagenson, M.D.; Spellman, S.R.; Ladner, M.; Guethlein, L.A.; Parham, P.; et al. Donor KIR B Genotype Improves Progression-Free Survival of Non-Hodgkin Lymphoma Patients Receiving Unrelated Donor Transplantation. Biol. Blood Marrow Transplant. 2016, 22, 1602–1607. [Google Scholar] [CrossRef] [Green Version]
- Cooley, S.; Weisdorf, D.J.; Guethlein, L.A.; Klein, J.P.; Wang, T.; Marsh, S.G.E.; Spellman, S.; Haagenson, M.D.; Saeturn, K.; Ladner, M.; et al. Donor Killer Cell Ig-like Receptor B Haplotypes, Recipient HLA-C1, and HLA-C Mismatch Enhance the Clinical Benefit of Unrelated Transplantation for Acute Myelogenous Leukemia. J. Immunol. 2014, 192, 4592–4600. [Google Scholar] [CrossRef] [Green Version]
- Vivier, E.; Morin, P.; O’Brien, C.; Druker, B.; Schlossman, S.F.; Anderson, P. Tyrosine Phosphorylation of the Fc Gamma RIII(CD16): Zeta Complex in Human Natural Killer Cells. Induction by Antibody-Dependent Cytotoxicity but Not by Natural Killing. J. Immunol. 1991, 146, 206–210. [Google Scholar] [CrossRef]
- Nimmerjahn, F.; Ravetch, J.V. Fcγ Receptors as Regulators of Immune Responses. Nat. Rev. Immunol. 2008, 8, 34–47. [Google Scholar] [CrossRef]
- Adams, G.P.; Weiner, L.M. Monoclonal Antibody Therapy of Cancer. Nat. Biotechnol. 2005, 23, 1147–1157. [Google Scholar] [CrossRef]
- Yun, H.D.; Felices, M.; Vallera, D.A.; Hinderlie, P.; Cooley, S.; Arock, M.; Gotlib, J.; Ustun, C.; Miller, J.S. Trispecific Killer Engager CD16xIL15xCD33 Potently Induces NK Cell Activation and Cytotoxicity against Neoplastic Mast Cells. Blood Adv. 2018, 2, 1580–1584. [Google Scholar] [CrossRef]
- Felices, M.; Lenvik, T.R.; Davis, Z.B.; Miller, J.S.; Vallera, D.A. Generation of BiKEs and TriKEs to Improve NK Cell-Mediated Targeting of Tumor Cells. Methods Mol. Biol. Clifton NJ 2016, 1441, 333–346. [Google Scholar] [CrossRef] [Green Version]
- Gleason, M.K.; Ross, J.A.; Warlick, E.D.; Lund, T.C.; Verneris, M.R.; Wiernik, A.; Spellman, S.; Haagenson, M.D.; Lenvik, A.J.; Litzow, M.R.; et al. CD16xCD33 Bispecific Killer Cell Engager (BiKE) Activates NK Cells against Primary MDS and MDSC CD33+ Targets. Blood 2014, 123, 3016–3026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romee, R.; Foley, B.; Lenvik, T.; Wang, Y.; Zhang, B.; Ankarlo, D.; Luo, X.; Cooley, S.; Verneris, M.; Walcheck, B.; et al. NK Cell CD16 Surface Expression and Function Is Regulated by a Disintegrin and Metalloprotease-17 (ADAM17). Blood 2013, 121, 3599–3608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raulet, D.H.; Gasser, S.; Gowen, B.G.; Deng, W.; Jung, H. Regulation of Ligands for the NKG2D Activating Receptor. Annu. Rev. Immunol. 2013, 31, 413–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanier, L.L. NKG2D Receptor and Its Ligands in Host Defense. Cancer Immunol. Res. 2015, 3, 575–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eagle, R.A.; Trowsdale, J. Promiscuity and the Single Receptor: NKG2D. Nat. Rev. Immunol. 2007, 7, 737–744. [Google Scholar] [CrossRef]
- Groh, V.; Wu, J.; Yee, C.; Spies, T. Tumour-Derived Soluble MIC Ligands Impair Expression of NKG2D and T-Cell Activation. Nature 2002, 419, 734–738. [Google Scholar] [CrossRef]
- Cichocki, F.; Miller, J.S. Promoting T and NK Cell Attack: Preserving Tumor MICA/B by Vaccines. Cell Res. 2022, 32, 961–962. [Google Scholar] [CrossRef]
- Myers, J.A.; Schirm, D.; Bendzick, L.; Hopps, R.; Selleck, C.; Hinderlie, P.; Felices, M.; Miller, J.S. Balanced Engagement of Activating and Inhibitory Receptors Mitigates Human NK Cell Exhaustion. JCI Insight 2022, 7, e150079. [Google Scholar] [CrossRef]
- Kennedy, P.R.; Felices, M.; Miller, J.S. Challenges to the Broad Application of Allogeneic Natural Killer Cell Immunotherapy of Cancer. Stem Cell Res. Ther. 2022, 13, 165. [Google Scholar] [CrossRef]
- Yun, H.D.; Schirm, D.K.; Felices, M.; Miller, J.S.; Eckfeldt, C.E. Dinaciclib Enhances Natural Killer Cell Cytotoxicity against Acute Myelogenous Leukemia. Blood Adv. 2019, 3, 2448–2452. [Google Scholar] [CrossRef]
- Manning, P.T.; Russell, J.H.; Johnson, E.M. Immunosuppressive Agents Prevent Guanethidine-Induced Destruction of Rat Sympathetic Neurons. Brain Res. 1982, 241, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, P.; Hougen, H.P.; Christensen, H.B.; Rygaard, J.; Svendsen, O.; Juul, P. Identification of the Mononuclear Cell Infiltrate in the Superior Cervical Ganglion of Athymic Nude and Euthymic Rats after Guanethidine-Induced Sympathectomy. Int. J. Immunopharmacol. 1990, 12, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Backström, E.; Chambers, B.J.; Kristensson, K.; Ljunggren, H.-G. Direct NK Cell-Mediated Lysis of Syngenic Dorsal Root Ganglia Neurons In Vitro1. J. Immunol. 2000, 165, 4895–4900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, A.J.; Kim, H.W.; Gonzalez-Cano, R.; Choi, J.; Back, S.K.; Roh, S.E.; Johnson, E.; Gabriac, M.; Kim, M.-S.; Lee, J.; et al. Natural Killer Cells Degenerate Intact Sensory Afferents Following Nerve Injury. Cell 2019, 176, 716–728. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Zou, W.; Lee, K.; Wang, E.; Zhu, X.; Guo, Q. Different Symptoms of Neuropathic Pain Can Be Induced by Different Degrees of Compressive Force on the C7 Dorsal Root of Rats. Spine J. Off. J. North Am. Spine Soc. 2012, 12, 1154–1160. [Google Scholar] [CrossRef]
- Gao, Y.-H.; Wang, J.-Y.; Qiao, L.-N.; Chen, S.-P.; Tan, L.-H.; Xu, Q.-L.; Liu, J.-L. NK Cells Mediate the Cumulative Analgesic Effect of Electroacupuncture in a Rat Model of Neuropathic Pain. BMC Complement. Altern. Med. 2014, 14, 316. [Google Scholar] [CrossRef] [Green Version]
- Lassen, J.; Stürner, K.H.; Gierthmühlen, J.; Dargvainiene, J.; Kixmüller, D.; Leypoldt, F.; Baron, R.; Hüllemann, P. Protective Role of Natural Killer Cells in Neuropathic Pain Conditions. PAIN 2021, 162, 2366. [Google Scholar] [CrossRef]
- Mausberg, A.K.; Heininger, M.K.; Meyer Zu Horste, G.; Cordes, S.; Fleischer, M.; Szepanowski, F.; Kleinschnitz, C.; Hartung, H.-P.; Kieseier, B.C.; Stettner, M. NK Cell Markers Predict the Efficacy of IV Immunoglobulins in CIDP. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e884. [Google Scholar] [CrossRef]
- St. John, A.L.; Rathore, A.P.S.; Yap, H.; Ng, M.-L.; Metcalfe, D.D.; Vasudevan, S.G.; Abraham, S.N. Immune Surveillance by Mast Cells during Dengue Infection Promotes Natural Killer (NK) and NKT-Cell Recruitment and Viral Clearance. Proc. Natl. Acad. Sci. USA 2011, 108, 9190–9195. [Google Scholar] [CrossRef]
- Burke, S.M.; Issekutz, T.B.; Mohan, K.; Lee, P.W.K.; Shmulevitz, M.; Marshall, J.S. Human Mast Cell Activation with Virus-Associated Stimuli Leads to the Selective Chemotaxis of Natural Killer Cells by a CXCL8-Dependent Mechanism. Blood 2008, 111, 5467–5476. [Google Scholar] [CrossRef] [Green Version]
- Portales-Cervantes, L.; Haidl, I.D.; Lee, P.W.; Marshall, J.S. Virus-Infected Human Mast Cells Enhance Natural Killer Cell Functions. J. Innate Immun. 2017, 9, 94–108. [Google Scholar] [CrossRef]
- Ustun, C.; Williams, S.; Skendzel, S.; Kodal, B.; Arock, M.; Gotlib, J.; Vallera, D.A.; Cooley, S.; Felices, M.; Weisdorf, D.; et al. Allogeneic NK Cells Eradicate Myeloblasts but Not Neoplastic Mast Cells in Systemic Mastocytosis Associated with Acute Myeloid Leukemia. Am. J. Hematol. 2017, 92, E66–E68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krauth, M.-T.; Böhm, A.; Agis, H.; Sonneck, K.; Samorapoompichit, P.; Florian, S.; Sotlar, K.; Valent, P. Effects of the CD33-Targeted Drug Gemtuzumab Ozogamicin (Mylotarg) on Growth and Mediator Secretion in Human Mast Cells and Blood Basophils. Exp. Hematol. 2007, 35, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Starobova, H.; Vetter, I. Pathophysiology of Chemotherapy-Induced Peripheral Neuropathy. Front. Mol. Neurosci. 2017, 10, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javeed, A.; Ashraf, M.; Riaz, A.; Ghafoor, A.; Afzal, S.; Mukhtar, M.M. Paclitaxel and Immune System. Eur. J. Pharm. Sci. 2009, 38, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.R.; Coelho, M.S.; Neves, F.D.A.R.; Duarte, D.B. Cisplatin-Induced Changes in Calcitonin Gene-Related Peptide or TNF-α Release in Rat Dorsal Root Ganglia in Vitro Model of Neurotoxicity Are Not Reverted by Rosiglitazone. Neurotoxicology 2022, 93, 211–221. [Google Scholar] [CrossRef]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic Inflammation and Cytokines in the Tumor Microenvironment. J. Immunol. Res. 2014, 2014, e149185. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Elner, S.G.; Bian, Z.-M.; Till, G.O.; Petty, H.R.; Elner, V.M. Pro-Inflammatory Cytokines Increase Reactive Oxygen Species through Mitochondria and NADPH Oxidase in Cultured RPE Cells. Exp. Eye Res. 2007, 85, 462–472. [Google Scholar] [CrossRef] [Green Version]
- Mateen, S.; Moin, S.; Shahzad, S.; Khan, A.Q. Level of Inflammatory Cytokines in Rheumatoid Arthritis Patients: Correlation with 25-Hydroxy Vitamin D and Reactive Oxygen Species. PLoS ONE 2017, 12, e0178879. [Google Scholar] [CrossRef]
- Woo, C.H.; Eom, Y.W.; Yoo, M.H.; You, H.J.; Han, H.J.; Song, W.K.; Yoo, Y.J.; Chun, J.S.; Kim, J.H. Tumor Necrosis Factor-Alpha Generates Reactive Oxygen Species via a Cytosolic Phospholipase A2-Linked Cascade. J. Biol. Chem. 2000, 275, 32357–32362. [Google Scholar] [CrossRef] [Green Version]
- Salehi, F.; Behboudi, H.; Kavoosi, G.; Ardestani, S.K. Oxidative DNA Damage Induced by ROS-Modulating Agents with the Ability to Target DNA: A Comparison of the Biological Characteristics of Citrus Pectin and Apple Pectin. Sci. Rep. 2018, 8, 13902. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, C.; Pimpão, C.; Mósca, A.F.; Coxixo, A.S.; Lopes, D.; da Silva, I.V.; Pedersen, P.A.; Antunes, F.; Soveral, G. Human Aquaporin-5 Facilitates Hydrogen Peroxide Permeation Affecting Adaption to Oxidative Stress and Cancer Cell Migration. Cancers 2019, 11, 932. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.-L.; Maeda, S.; Hsu, L.-C.; Yagita, H.; Karin, M. Inhibition of NF-KappaB in Cancer Cells Converts Inflammation- Induced Tumor Growth Mediated by TNFalpha to TRAIL-Mediated Tumor Regression. Cancer Cell 2004, 6, 297–305. [Google Scholar] [CrossRef] [Green Version]
- Zheng, T.; Hong, X.; Wang, J.; Pei, T.; Liang, Y.; Yin, D.; Song, R.; Song, X.; Lu, Z.; Qi, S.; et al. Gankyrin Promotes Tumor Growth and Metastasis through Activation of IL-6/STAT3 Signaling in Human Cholangiocarcinoma. Hepatology 2014, 59, 935–946. [Google Scholar] [CrossRef]
- He, D.; Li, H.; Yusuf, N.; Elmets, C.A.; Athar, M.; Katiyar, S.K.; Xu, H. IL-17 Mediated Inflammation Promotes Tumor Growth and Progression in the Skin. PLoS ONE 2012, 7, e32126. [Google Scholar] [CrossRef] [Green Version]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. Mech. Dis. 2018, 13, 395–412. [Google Scholar] [CrossRef]
- Bates, R.C.; Mercurio, A.M. Tumor Necrosis Factor-Alpha Stimulates the Epithelial-to-Mesenchymal Transition of Human Colonic Organoids. Mol. Biol. Cell 2003, 14, 1790–1800. [Google Scholar] [CrossRef] [Green Version]
- Yadav, A.; Kumar, B.; Datta, J.; Teknos, T.N.; Kumar, P. IL-6 Promotes Head and Neck Tumor Metastasis by Inducing Epithelial-Mesenchymal Transition via the JAK-STAT3-SNAIL Signaling Pathway. Mol. Cancer Res. MCR 2011, 9, 1658–1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visciano, C.; Liotti, F.; Prevete, N.; Cali’, G.; Franco, R.; Collina, F.; de Paulis, A.; Marone, G.; Santoro, M.; Melillo, R.M. Mast Cells Induce Epithelial-to-Mesenchymal Transition and Stem Cell Features in Human Thyroid Cancer Cells through an IL-8-Akt-Slug Pathway. Oncogene 2015, 34, 5175–5186. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Chang, J.S.-M.; Syu, S.-H.; Wong, T.-S.; Chan, J.Y.-W.; Tang, Y.-C.; Yang, Z.-P.; Yang, W.-C.; Chen, C.-T.; Lu, S.-C.; et al. IL-1β Promotes Malignant Transformation and Tumor Aggressiveness in Oral Cancer. J. Cell. Physiol. 2015, 230, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Ju, M.K.; Jeon, H.M.; Lee, Y.J.; Kim, C.H.; Park, H.G.; Han, S.I.; Kang, H.S. Reactive Oxygen Species Induce Epithelial-mesenchymal Transition, Glycolytic Switch, and Mitochondrial Repression through the Dlx-2/Snail Signaling Pathways in MCF-7 Cells. Mol. Med. Rep. 2019, 20, 2339–2346. [Google Scholar] [CrossRef] [PubMed]
- McMahon, G. VEGF Receptor Signaling in Tumor Angiogenesis. Oncologist 2000, 5, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Feurino, L.W.; Zhang, Y.; Bharadwaj, U.; Zhang, R.; Li, F.; Fisher, W.E.; Brunicardi, F.C.; Chen, C.; Yao, Q.; Min, L. IL-6 Stimulates Th2 Type Cytokine Secretion and Upregulates VEGF and NRP-1 Expression in Pancreatic Cancer Cells. Cancer Biol. Ther. 2007, 6, 1096–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boreddy, S.R.; Sahu, R.P.; Srivastava, S.K. Benzyl Isothiocyanate Suppresses Pancreatic Tumor Angiogenesis and Invasion by Inhibiting HIF-α/VEGF/Rho-GTPases: Pivotal Role of STAT-3. PLoS ONE 2011, 6, e25799. [Google Scholar] [CrossRef]
- Leibovich, S.J.; Polverini, P.J.; Shepard, H.M.; Wiseman, D.M.; Shively, V.; Nuseir, N. Macrophage-Induced Angiogenesis Is Mediated by Tumour Necrosis Factor-α. Nature 1987, 329, 630–632. [Google Scholar] [CrossRef]
- Gagari, E.; Tsai, M.; Lantz, C.S.; Fox, L.G.; Galli, S.J. Differential Release of Mast Cell Interleukin-6 Via c-Kit. Blood 1997, 89, 2654–2663. [Google Scholar] [CrossRef]
- Huang, M.; Pang, X.; Karalis, K.; Theoharides, T.C. Stress-Induced Interleukin-6 Release in Mice Is Mast Cell-Dependent and More Pronounced in Apolipoprotein E Knockout Mice. Cardiovasc. Res. 2003, 59, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Gaje, P.N.; Amalia Ceausu, R.; Jitariu, A.; Stratul, S.I.; Rusu, L.-C.; Popovici, R.A.; Raica, M. Mast Cells: Key Players in the Shadow in Oral Inflammation and in Squamous Cell Carcinoma of the Oral Cavity. BioMed Res. Int. 2016, 2016, 9235080. [Google Scholar] [CrossRef] [Green Version]
- Nigrovic, P.A.; Binstadt, B.A.; Monach, P.A.; Johnsen, A.; Gurish, M.; Iwakura, Y.; Benoist, C.; Mathis, D.; Lee, D.M. Mast Cells Contribute to Initiation of Autoantibody-Mediated Arthritis via IL-1. Proc. Natl. Acad. Sci. USA 2007, 104, 2325–2330. [Google Scholar] [CrossRef]
- Hu, Z.Q.; Kobayashi, K.; Zenda, N.; Shimamura, T. Tumor Necrosis Factor-Alpha- and Interleukin-6-Triggered Mast Cell Development from Mouse Spleen Cells. Blood 1997, 89, 526–533. [Google Scholar] [CrossRef]
- Yanagida, M.; Fukamachi, H.; Ohgami, K.; Kuwaki, T.; Ishii, H.; Uzumaki, H.; Amano, K.; Tokiwa, T.; Mitsui, H.; Saito, H.; et al. Effects of T-Helper 2-Type Cytokines, Interleukin-3 (IL-3), IL-4, IL-5, and IL-6 on the Survival of Cultured Human Mast Cells. Blood 1995, 86, 3705–3714. [Google Scholar] [CrossRef] [PubMed]
- Cruse, G.; Cockerill, S.; Bradding, P. IgE Alone Promotes Human Lung Mast Cell Survival through the Autocrine Production of IL-6. BMC Immunol. 2008, 9, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulliksson, M.; Carvalho, R.F.S.; Ullerås, E.; Nilsson, G. Mast Cell Survival and Mediator Secretion in Response to Hypoxia. PLoS ONE 2010, 5, e12360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hültner, L.; Kölsch, S.; Stassen, M.; Kaspers, U.; Kremer, J.-P.; Mailhammer, R.; Moeller, J.; Broszeit, H.; Schmitt, E. In Activated Mast Cells, IL-1 Up-Regulates the Production of Several Th2-Related Cytokines Including IL-9. J. Immunol. 2000, 164, 5556–5563. [Google Scholar] [CrossRef]
- Marcenaro, S.; Gallo, F.; Martini, S.; Santoro, A.; Griffiths, G.M.; Aricó, M.; Moretta, L.; Pende, D. Analysis of Natural Killer–Cell Function in Familial Hemophagocytic Lymphohistiocytosis (FHL): Defective CD107a Surface Expression Heralds Munc13-4 Defect and Discriminates between Genetic Subtypes of the Disease. Blood 2006, 108, 2316–2323. [Google Scholar] [CrossRef] [Green Version]
- Villanueva, J.; Lee, S.; Giannini, E.H.; Graham, T.B.; Passo, M.H.; Filipovich, A.; Grom, A.A. Natural Killer Cell Dysfunction Is a Distinguishing Feature of Systemic Onset Juvenile Rheumatoid Arthritis and Macrophage Activation Syndrome. Arthritis Res. Ther. 2004, 7, R30. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.W.; Gardner, R.; Porter, D.L.; Louis, C.U.; Ahmed, N.; Jensen, M.; Grupp, S.A.; Mackall, C.L. Current Concepts in the Diagnosis and Management of Cytokine Release Syndrome. Blood 2014, 124, 188–195. [Google Scholar] [CrossRef] [Green Version]
- Cifaldi, L.; Prencipe, G.; Caiello, I.; Bracaglia, C.; Locatelli, F.; De Benedetti, F.; Strippoli, R. Inhibition of Natural Killer Cell Cytotoxicity by Interleukin-6: Implications for the Pathogenesis of Macrophage Activation Syndrome. Arthritis Rheumatol. 2015, 67, 3037–3046. [Google Scholar] [CrossRef]
- Wiley, S.R.; Schooley, K.; Smolak, P.J.; Din, W.S.; Huang, C.P.; Nicholl, J.K.; Sutherland, G.R.; Smith, T.D.; Rauch, C.; Smith, C.A. Identification and Characterization of a New Member of the TNF Family That Induces Apoptosis. Immunity 1995, 3, 673–682. [Google Scholar] [CrossRef] [Green Version]
- Prager, I.; Watzl, C. Mechanisms of Natural Killer Cell-Mediated Cellular Cytotoxicity. J. Leukoc. Biol. 2019, 105, 1319–1329. [Google Scholar] [CrossRef]
- Zamai, L.; Ahmad, M.; Bennett, I.M.; Azzoni, L.; Alnemri, E.S.; Perussia, B. Natural Killer (NK) Cell–Mediated Cytotoxicity: Differential Use of TRAIL and Fas Ligand by Immature and Mature Primary Human NK Cells. J. Exp. Med. 1998, 188, 2375–2380. [Google Scholar] [CrossRef] [PubMed]
- Jewett, A.; Cavalcanti, M.; Bonavida, B. Pivotal Role of Endogenous TNF-Alpha in the Induction of Functional Inactivation and Apoptosis in NK Cells. J. Immunol. 1997, 159, 4815–4822. [Google Scholar] [CrossRef]
- Jin, F.; Wu, Z.; Hu, X.; Zhang, J.; Gao, Z.; Han, X.; Qin, J.; Li, C.; Wang, Y. The PI3K/Akt/GSK-3β/ROS/EIF2B Pathway Promotes Breast Cancer Growth and Metastasis via Suppression of NK Cell Cytotoxicity and Tumor Cell Susceptibility. Cancer Biol. Med. 2019, 16, 38–54. [Google Scholar] [CrossRef] [Green Version]
- Romero, A.I.; Thorén, F.B.; Brune, M.; Hellstrand, K. NKp46 and NKG2D Receptor Expression in NK Cells with CD56dim and CD56bright Phenotype: Regulation by Histamine and Reactive Oxygen Species. Br. J. Haematol. 2006, 132, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Myers, T.J.; Brennaman, L.H.; Stevenson, M.; Higashiyama, S.; Russell, W.E.; Lee, D.C.; Sunnarborg, S.W. Mitochondrial Reactive Oxygen Species Mediate GPCR–Induced TACE/ADAM17-Dependent Transforming Growth Factor-α Shedding. Mol. Biol. Cell 2009, 20, 5236–5249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brill, A.; Chauhan, A.K.; Canault, M.; Walsh, M.T.; Bergmeier, W.; Wagner, D.D. Oxidative Stress Activates ADAM17/TACE and Induces Its Target Receptor Shedding in Platelets in a P38-Dependent Fashion. Cardiovasc. Res. 2009, 84, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Wiernik, A.; Foley, B.; Zhang, B.; Verneris, M.R.; Warlick, E.; Gleason, M.K.; Ross, J.A.; Luo, X.; Weisdorf, D.J.; Walcheck, B.; et al. Targeting Natural Killer Cells to Acute Myeloid Leukemia In Vitro with a CD16 × 33 Bispecific Killer Cell Engager and ADAM17 Inhibition. Clin. Cancer Res. 2013, 19, 3844–3855. [Google Scholar] [CrossRef] [Green Version]
- Tofaris, G.K.; Patterson, P.H.; Jessen, K.R.; Mirsky, R. Denervated Schwann Cells Attract Macrophages by Secretion of Leukemia Inhibitory Factor (LIF) and Monocyte Chemoattractant Protein-1 in a Process Regulated by Interleukin-6 and LIF. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 6696–6703. [Google Scholar] [CrossRef]
- Austin, P.J.; Moalem-Taylor, G. The Neuro-Immune Balance in Neuropathic Pain: Involvement of Inflammatory Immune Cells, Immune-like Glial Cells and Cytokines. J. Neuroimmunol. 2010, 229, 26–50. [Google Scholar] [CrossRef]
- Whitehead, K.J.; Smith, C.G.S.; Delaney, S.-A.; Curnow, S.J.; Salmon, M.; Hughes, J.P.; Chessell, I.P. Dynamic Regulation of Spinal Pro-Inflammatory Cytokine Release in the Rat in Vivo Following Peripheral Nerve Injury. Brain. Behav. Immun. 2010, 24, 569–576. [Google Scholar] [CrossRef]
- Schäfers, M.; Sorkin, L. Effect of Cytokines on Neuronal Excitability. Neurosci. Lett. 2008, 437, 188–193. [Google Scholar] [CrossRef]
- Pollock, J.; McFarlane, S.M.; Connell, M.C.; Zehavi, U.; Vandenabeele, P.; MacEwan, D.J.; Scott, R.H. TNF-α Receptors Simultaneously Activate Ca2+ Mobilisation and Stress Kinases in Cultured Sensory Neurones. Neuropharmacology 2002, 42, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Junger, H.; Sorkin, L.S. Nociceptive and Inflammatory Effects of Subcutaneous TNFα. Pain 2000, 85, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, H.; Kawatani, M.; Hisamitsu, T.; Takeshige, C. Cutaneous Hyperalgesia Induced by Peripheral Injection of Interleukin-1β in the Rat. Brain Res. 1994, 657, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.; Gabay, E.; Tal, M.; Yirmiya, R.; Shavit, Y. Genetic Impairment of Interleukin-1 Signaling Attenuates Neuropathic Pain, Autotomy, and Spontaneous Ectopic Neuronal Activity, Following Nerve Injury in Mice. Pain 2006, 120, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Schäfers, M.; Brinkhoff, J.; Neukirchen, S.; Marziniak, M.; Sommer, C. Combined Epineurial Therapy with Neutralizing Antibodies to Tumor Necrosis Factor-Alpha and Interleukin-1 Receptor Has an Additive Effect in Reducing Neuropathic Pain in Mice. Neurosci. Lett. 2001, 310, 113–116. [Google Scholar] [CrossRef]
- Tobinick, E.; Davoodifar, S. Efficacy of Etanercept Delivered by Perispinal Administration for Chronic Back and/or Neck Disc-Related Pain: A Study of Clinical Observations in 143 Patients. Curr. Med. Res. Opin. 2004, 20, 1075–1085. [Google Scholar] [CrossRef]
- Cohen, S.P.; Bogduk, N.; Dragovich, A.; Buckenmaier, C.C.; Griffith, S.; Kurihara, C.; Raymond, J.; Richter, P.J.; Williams, N.; Yaksh, T.L. Randomized, Double-Blind, Placebo-Controlled, Dose-Response, and Preclinical Safety Study of Transforaminal Epidural Etanercept for the Treatment of Sciatica. Anesthesiology 2009, 110, 1116–1126. [Google Scholar] [CrossRef] [Green Version]
- Ohtori, S.; Miyagi, M.; Eguchi, Y.; Inoue, G.; Orita, S.; Ochiai, N.; Kishida, S.; Kuniyoshi, K.; Nakamura, J.; Aoki, Y.; et al. Efficacy of Epidural Administration of Anti-Interleukin-6 Receptor Antibody onto Spinal Nerve for Treatment of Sciatica. Eur. Spine J. 2012, 21, 2079–2084. [Google Scholar] [CrossRef] [Green Version]
- Szatrowski, T.P.; Nathan, C.F. Production of Large Amounts of Hydrogen Peroxide by Human Tumor Cells. Cancer Res. 1991, 51, 794–798. [Google Scholar]
- Weinberg, F.; Ramnath, N.; Nagrath, D. Reactive Oxygen Species in the Tumor Microenvironment: An Overview. Cancers 2019, 11, 1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conklin, K.A. Chemotherapy-Associated Oxidative Stress: Impact on Chemotherapeutic Effectiveness. Integr. Cancer Ther. 2004, 3, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Areti, A.; Yerra, V.G.; Naidu, V.; Kumar, A. Oxidative Stress and Nerve Damage: Role in Chemotherapy Induced Peripheral Neuropathy. Redox Biol. 2014, 2, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Park, E.-S.; Gao, X.; Chung, J.M.; Chung, K. Levels of Mitochondrial Reactive Oxygen Species Increase in Rat Neuropathic Spinal Dorsal Horn Neurons. Neurosci. Lett. 2006, 391, 108–111. [Google Scholar] [CrossRef]
- Schwartz, E.S.; Lee, I.; Chung, K.; Mo Chung, J. Oxidative Stress in the Spinal Cord Is an Important Contributor in Capsaicin-Induced Mechanical Secondary Hyperalgesia in Mice. Pain 2008, 138, 514–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, E.S.; Kim, H.Y.; Wang, J.; Lee, I.; Klann, E.; Chung, J.M.; Chung, K. Persistent Pain Is Dependent on Spinal Mitochondrial Antioxidant Levels. J. Neurosci. 2009, 29, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Fidanboylu, M.; Griffiths, L.A.; Flatters, S.J.L. Global Inhibition of Reactive Oxygen Species (ROS) Inhibits Paclitaxel-Induced Painful Peripheral Neuropathy. PLoS ONE 2011, 6, e25212. [Google Scholar] [CrossRef]
- Soriani, A.; Iannitto, M.L.; Ricci, B.; Fionda, C.; Malgarini, G.; Morrone, S.; Peruzzi, G.; Ricciardi, M.R.; Petrucci, M.T.; Cippitelli, M.; et al. Reactive Oxygen Species– and DNA Damage Response–Dependent NK Cell Activating Ligand Upregulation Occurs at Transcriptional Levels and Requires the Transcriptional Factor E2F1. J. Immunol. 2014, 193, 950–960. [Google Scholar] [CrossRef] [Green Version]
- Ledeboer, A.; Jekich, B.M.; Sloane, E.M.; Mahoney, J.H.; Langer, S.J.; Milligan, E.D.; Martin, D.; Maier, S.F.; Johnson, K.W.; Leinwand, L.A.; et al. Intrathecal Interleukin-10 Gene Therapy Attenuates Paclitaxel-Induced Mechanical Allodynia and Proinflammatory Cytokine Expression in Dorsal Root Ganglia in Rats. Brain. Behav. Immun. 2007, 21, 686–698. [Google Scholar] [CrossRef] [Green Version]
- Echeverry, S.; Shi, X.Q.; Haw, A.; Liu, G.; Zhang, Z.; Zhang, J. Transforming Growth Factor-Β1 Impairs Neuropathic Pain through Pleiotropic Effects. Mol. Pain 2009, 5, 1744-8069-5-16. [Google Scholar] [CrossRef] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, H.D.; Goel, Y.; Gupta, K. Crosstalk of Mast Cells and Natural Killer Cells with Neurons in Chemotherapy-Induced Peripheral Neuropathy. Int. J. Mol. Sci. 2023, 24, 12543. https://doi.org/10.3390/ijms241612543
Yun HD, Goel Y, Gupta K. Crosstalk of Mast Cells and Natural Killer Cells with Neurons in Chemotherapy-Induced Peripheral Neuropathy. International Journal of Molecular Sciences. 2023; 24(16):12543. https://doi.org/10.3390/ijms241612543
Chicago/Turabian StyleYun, Hyun Don, Yugal Goel, and Kalpna Gupta. 2023. "Crosstalk of Mast Cells and Natural Killer Cells with Neurons in Chemotherapy-Induced Peripheral Neuropathy" International Journal of Molecular Sciences 24, no. 16: 12543. https://doi.org/10.3390/ijms241612543
APA StyleYun, H. D., Goel, Y., & Gupta, K. (2023). Crosstalk of Mast Cells and Natural Killer Cells with Neurons in Chemotherapy-Induced Peripheral Neuropathy. International Journal of Molecular Sciences, 24(16), 12543. https://doi.org/10.3390/ijms241612543




