Regenerating Myofibers after an Acute Muscle Injury: What Do We Really Know about Them?
Abstract
:1. Introduction
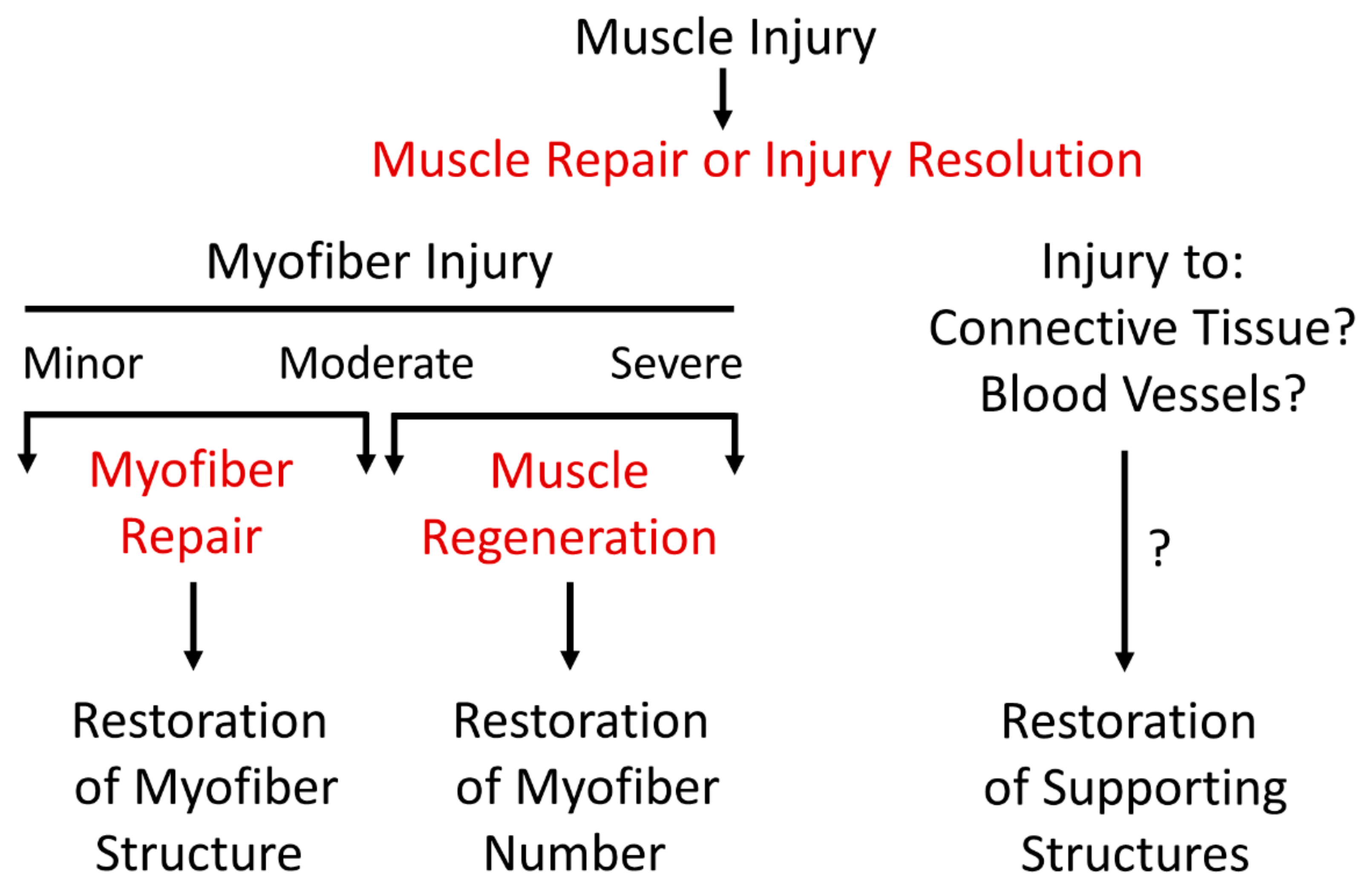
2. Semantics of Nomenclature
3. General Overview of Muscle Regeneration
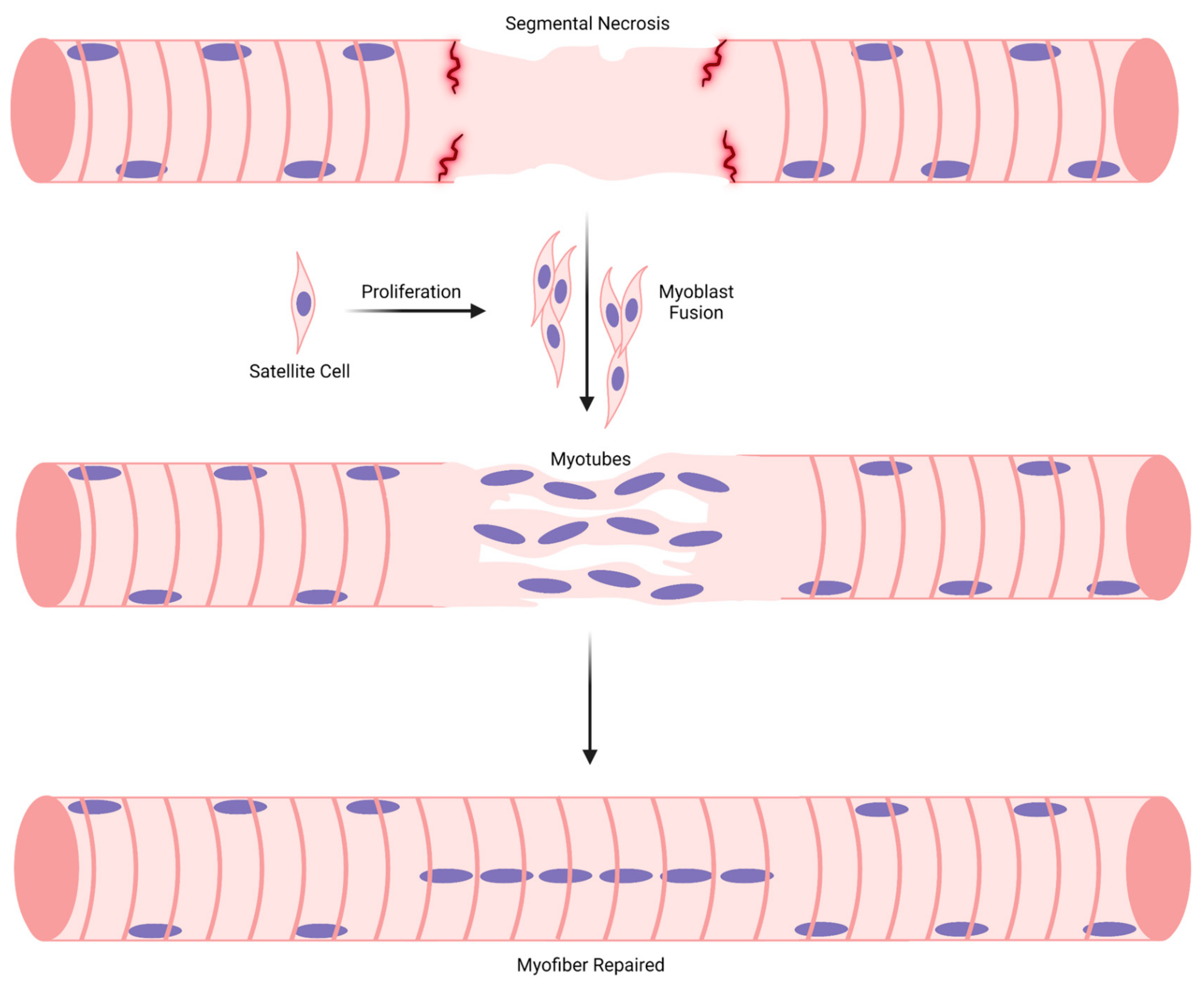
3.1. Stage 1: Myogenic Cell Proliferation
3.2. Stage 2: Myoblast Differentiation, Adhesion, and Fusion
3.3. Stage 3: Maturation of Myotubes into Regenerating Myofibers
3.4. Stage 4: Regenerating Myofiber Maturation
4. Embryonic and Postnatal Myogenesis vs. Muscle Regeneration
4.1. Myonuclear Positioning during Embryonic and Postnatal Myogenesis
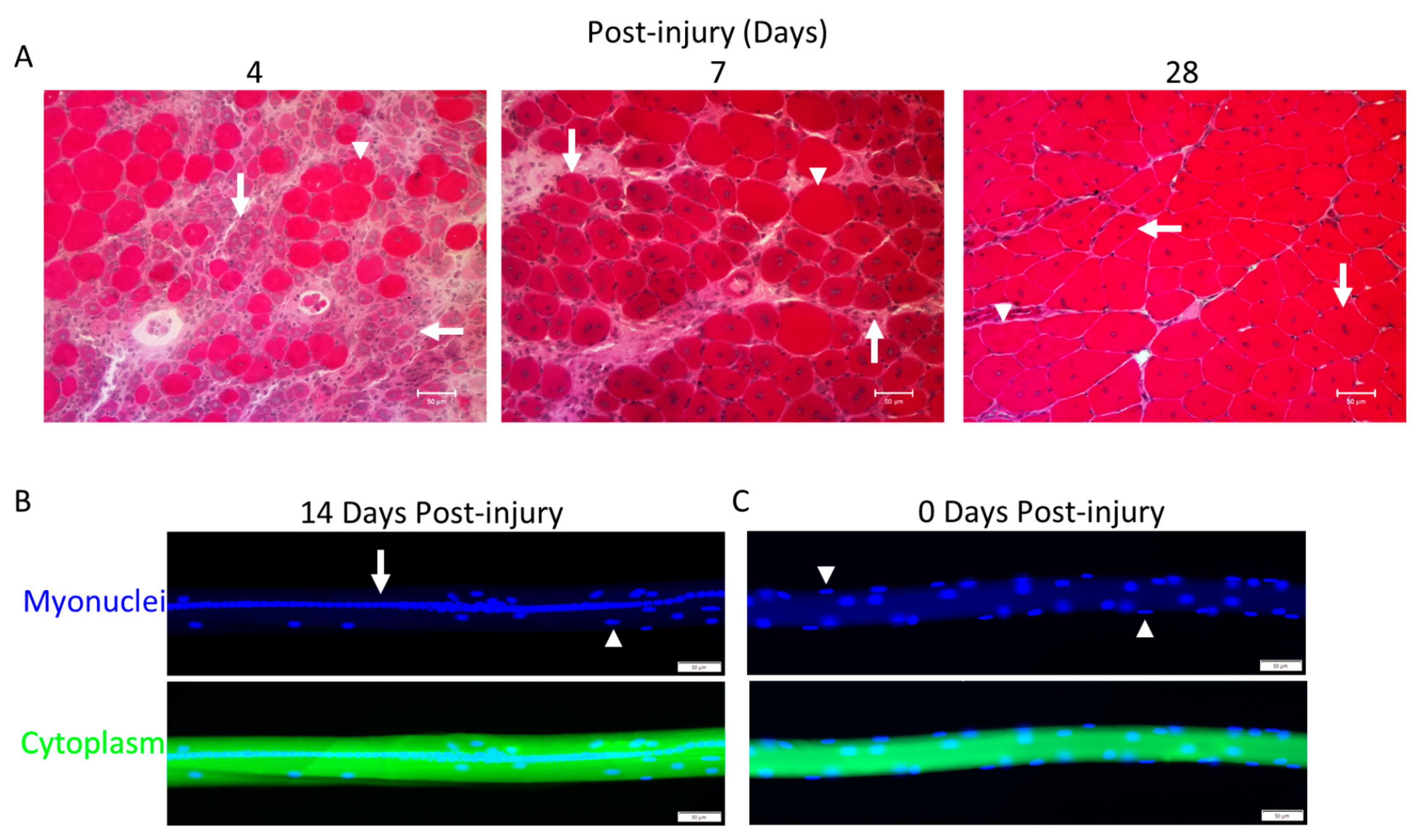
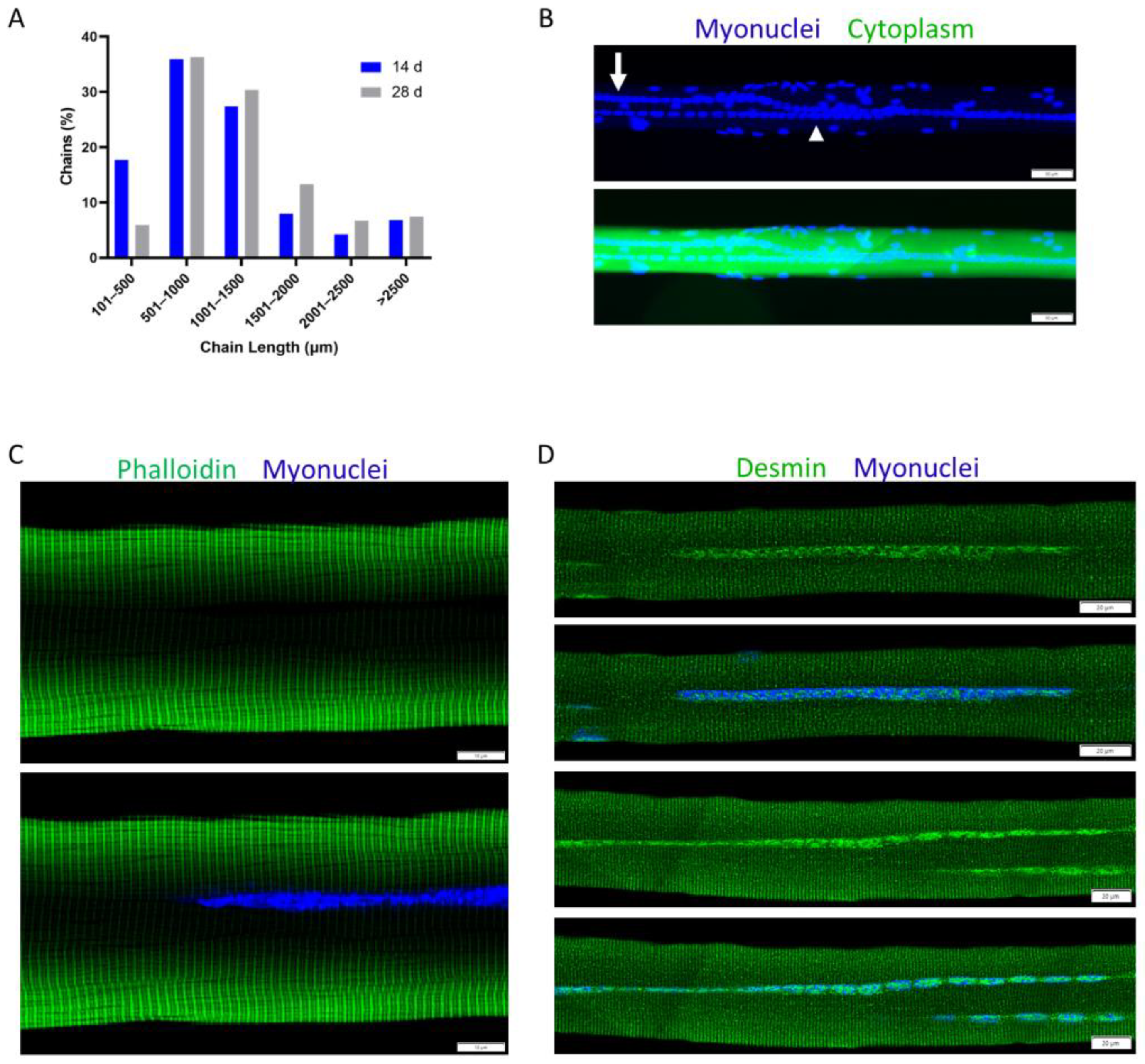
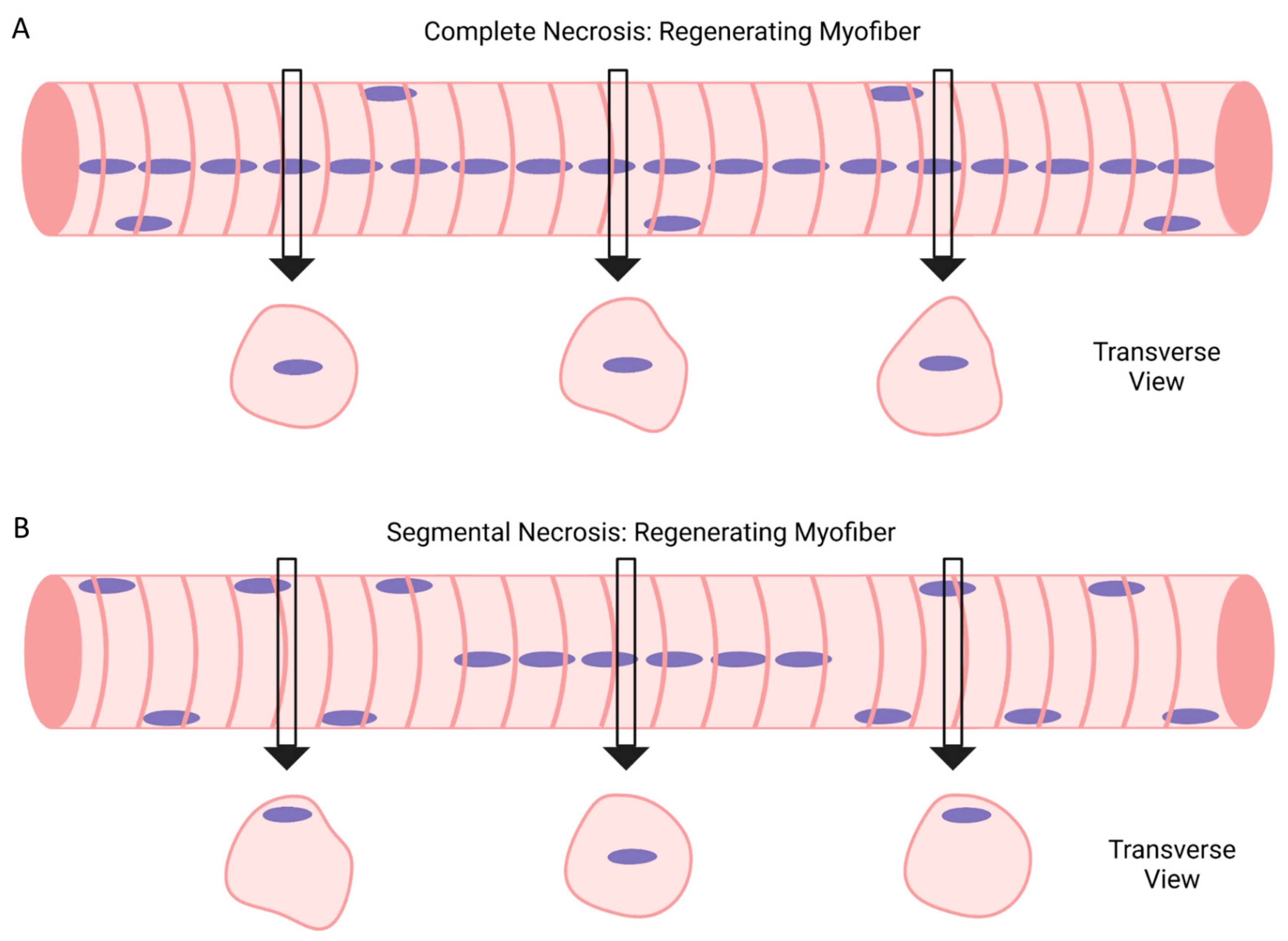
4.2. Myonuclear Positioning during Trauma-Induced Muscle Regeneration
4.3. Myonuclear Accretion during Embryonic and Postnatal Myogenesis
4.4. Myonuclear Accretion during Trauma-Induced Muscle Regeneration
4.5. Myofiber Hypertrophy during Postnatal Myogenesis
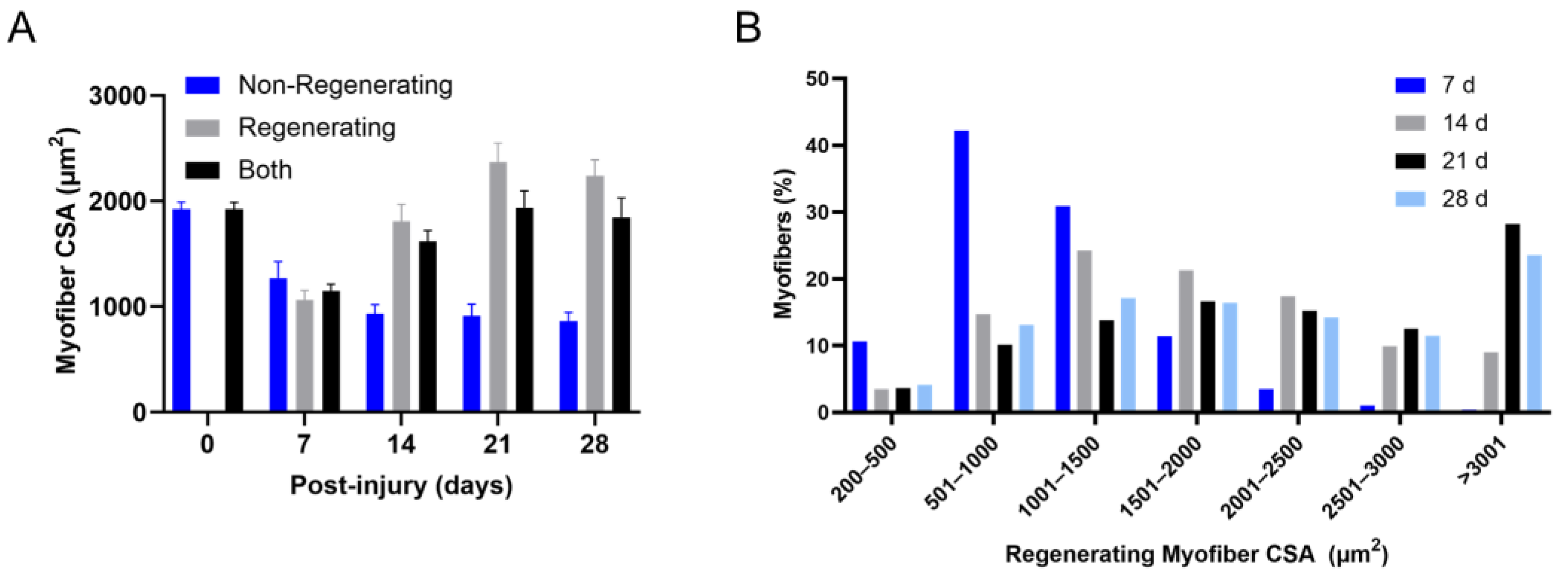
4.6. Regenerating Myofiber Hypertrophy after Trauma-Induced Muscle Regeneration
4.7. Myofiber Formation and Morphology during Embryonic Myogenesis
4.8. Myofiber Formation and Morphology during Trauma-Induced Muscle Regeneration
5. Complete vs. Segmental Necrosis of Myofibers and Ensuing Muscle Regeneration
6. Regenerating Myofibers in Models of Contraction- and Exercise-Induced Muscle Injury
6.1. Animal Models
6.2. Human Models
6.3. Summary
7. Concluding Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warren, G.L.; Lowe, D.A.; Armstrong, R.B. Measurement tools used in the study of eccentric contraction-induced injury. Sports Med. 1999, 27, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, P.M.; Hubal, M.J. Exercise-induced muscle damage in humans. Am. J. Phys. Med. Rehabil. 2002, 81 (Suppl. S11), S52–S69. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, J.A.; Brooks, S.V.; Opiteck, J.A. Injury to skeletal muscle fibers during contractions: Conditions of occurrence and prevention. Phys. Ther. 1993, 73, 911–921. [Google Scholar] [CrossRef]
- Proske, U.; Morgan, D.L. Muscle damage from eccentric exercise: Mechanism, mechanical signs, adaptation and clinical applications. J. Physiol. 2001, 537, 333–345. [Google Scholar] [CrossRef]
- Corona, B.T.; Wenke, J.C.; Ward, C.L. Pathophysiology of Volumetric Muscle Loss Injury. Cells Tissues Organs 2016, 202, 180–188. [Google Scholar] [CrossRef]
- Ogilvie, R.W.; Armstrong, R.B.; Baird, K.E.; Bottoms, C.L. Lesions in the rat soleus muscle following eccentrically biased exercise. Am. J. Anat. 1988, 182, 335–346. [Google Scholar] [CrossRef]
- Newham, D.J.; McPhail, G.; Mills, K.R.; Edwards, R.H. Ultrastructural changes after concentric and eccentric contractions of human muscle. J. Neurol. Sci. 1983, 61, 109–122. [Google Scholar] [CrossRef]
- Koh, T.J.; Escobedo, J. Cytoskeletal disruption and small heat shock protein translocation immediately after lengthening contractions. Am. J. Physiol. Cell Physiol. 2004, 286, C713–C722. [Google Scholar] [CrossRef]
- Lieber, R.L.; Shah, S.; Friden, J. Cytoskeletal disruption after eccentric contraction-induced muscle injury. Clin. Orthop. 2002, 403, S90–S99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamer, P.W.; McGeachie, J.M.; Davies, M.J.; Grounds, M.D. Evans Blue Dye as an in vivo marker of myofibre damage: Optimising parameters for detecting initial myofibre membrane permeability. J. Anat. 2002, 200, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Gibala, M.J.; MacDougall, J.D.; Tarnopolsky, M.A.; Stauber, W.T.; Elorriaga, A. Changes in human skeletal muscle ultrastructure and force production after acute resistance exercise. J. Appl. Physiol. 1995, 78, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Friden, J.; Sjostrom, M.; Ekblom, B. A morphological study of delayed muscle soreness. Experientia 1981, 37, 506–507. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, F.; Paulsen, G.; Raastad, T.; Bergersen, L.H.; Owe, S.G. Gross ultrastructural changes and necrotic fiber segments in elbow flexor muscles after maximal voluntary eccentric action in humans. J. Appl. Physiol. 2009, 107, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, J.D.; Woods, R.I. Hypertrophy of rat extensor digitorum longus muscle injected with bupivacaine. A sequential histochemical, immunohistochemical, histological and morphometric study. J. Anat. 1992, 181, 11–27. [Google Scholar]
- Rosenblatt, J.D. A time course study of the isometric contractile properties of rat extensor digitorum longus muscle injected with bupivacaine. Comp. Biochem. Physiol. Comp. Physiol. 1992, 101, 361–367. [Google Scholar] [CrossRef]
- Carlson, B.M. The regeneration of skeletal muscle. A review. Am. J. Anat. 1973, 137, 119–149. [Google Scholar] [CrossRef]
- Grounds, M.D. The need to more precisely define aspects of skeletal muscle regeneration. Int. J. Biochem. Cell Biol. 2014, 56, 56–65. [Google Scholar] [CrossRef]
- Hawke, T.J.; Garry, D.J. Myogenic satellite cells: Physiology to molecular biology. J. Appl. Physiol. 2001, 91, 534–551. [Google Scholar] [CrossRef]
- Horn, A.; Jaiswal, J.K. Cellular mechanisms and signals that coordinate plasma membrane repair. Cell. Mol. Life Sci. 2018, 75, 3751–3770. [Google Scholar] [CrossRef]
- Demonbreun, A.R.; McNally, E.M. Plasma Membrane Repair in Health and Disease. Curr. Top. Membr. 2016, 77, 67–96. [Google Scholar]
- Massenet, J.; Gardner, E.; Chazaud, B.; Dilworth, F.J. Epigenetic regulation of satellite cell fate during skeletal muscle regeneration. Skelet. Muscle 2021, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Thomson, D.M. The Role of AMPK in the Regulation of Skeletal Muscle Size, Hypertrophy, and Regeneration. Int. J. Mol. Sci. 2018, 19, 3125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wosczyna, M.N.; Rando, T.A. A Muscle Stem Cell Support Group: Coordinated Cellular Responses in Muscle Regeneration. Dev. Cell 2018, 46, 135–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millay, D.P. Regulation of the myoblast fusion reaction for muscle development, regeneration, and adaptations. Exp. Cell Res. 2022, 415, 113134. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.; Wankell, M.; Saxena, P.; McFarlane, C.; Hebbard, L. Cell adhesion an important determinant of myogenesis and satellite cell activity. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119170. [Google Scholar] [CrossRef]
- Panci, G.; Chazaud, B. Inflammation during post-injury skeletal muscle regeneration. Semin. Cell Dev. Biol. 2021, 119, 32–38. [Google Scholar] [CrossRef]
- Sousa-Victor, P.; Garcia-Prat, L.; Munoz-Canoves, P. Control of satellite cell function in muscle regeneration and its disruption in ageing. Nat. Rev. Mol. Cell Biol. 2022, 23, 204–226. [Google Scholar] [CrossRef]
- Enesco, M.; Puddy, D. Increase in the Number of Nuclei and Weight in Skeletal Muscle of Rats of Various Ages. Am. J. Anat. 1964, 114, 235–244. [Google Scholar] [CrossRef]
- Montgomery, R.D. Growth of human striated muscle. Nature 1962, 195, 194–195. [Google Scholar] [CrossRef]
- Rowe, R.W.; Goldspink, G. Muscle fibre growth in five different muscles in both sexes of mice. J. Anat. 1969, 104, 519–530. [Google Scholar]
- Li, M.; Zhou, X.; Chen, Y.; Nie, Y.; Huang, H.; Chen, H.; Mo, D. Not all the number of skeletal muscle fibers is determined prenatally. BMC Dev. Biol. 2015, 15, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumont, N.A.; Bentzinger, C.F.; Sincennes, M.C.; Rudnicki, M.A. Satellite Cells and Skeletal Muscle Regeneration. Compr. Physiol. 2015, 5, 1027–1059. [Google Scholar] [PubMed]
- Chazaud, B. Inflammation and Skeletal Muscle Regeneration: Leave It to the Macrophages! Trends Immunol. 2020, 41, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Helmbacher, F.; Stricker, S. Tissue cross talks governing limb muscle development and regeneration. Semin. Cell Dev. Biol. 2020, 104, 14–30. [Google Scholar] [CrossRef]
- Roman, W.; Pinheiro, H.; Pimentel, M.R.; Segales, J.; Oliveira, L.M.; Garcia-Dominguez, E.; Gomez-Cabrera, M.C.; Serrano, A.L.; Gomes, E.R.; Munoz-Canoves, P. Muscle repair after physiological damage relies on nuclear migration for cellular reconstruction. Science 2021, 374, 355–359. [Google Scholar] [CrossRef]
- Hardy, D.; Besnard, A.; Latil, M.; Jouvion, G.; Briand, D.; Thepenier, C.; Pascal, Q.; Guguin, A.; Gayraud-Morel, B.; Cavaillon, J.M.; et al. Comparative Study of Injury Models for Studying Muscle Regeneration in Mice. PLoS ONE 2016, 11, e0147198. [Google Scholar] [CrossRef] [Green Version]
- Webster, M.T.; Manor, U.; Lippincott-Schwartz, J.; Fan, C.M. Intravital Imaging Reveals Ghost Fibers as Architectural Units Guiding Myogenic Progenitors during Regeneration. Cell Stem Cell 2016, 18, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.M.; Lawson, J.A.; Mathew, S.J.; Hutcheson, D.A.; Kardon, G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 2011, 138, 3625–3637. [Google Scholar] [CrossRef] [Green Version]
- Vracko, R.; Benditt, E.P. Basal lamina: The scaffold for orderly cell replacement. Observations on regeneration of injured skeletal muscle fibers and capillaries. J. Cell Biol. 1972, 55, 406–419. [Google Scholar] [CrossRef]
- Schmalbruch, H. The morphology of regeneration of skeletal muscles in the rat. Tissue Cell 1976, 8, 673–692. [Google Scholar] [CrossRef]
- Caldwell, C.J.; Mattey, D.L.; Weller, R.O. Role of the basement membrane in the regeneration of skeletal muscle. Neuropathol. Appl. Neurobiol. 1990, 16, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Joanisse, S.; Snijders, T.; Nederveen, J.P.; Parise, G. The Impact of Aerobic Exercise on the Muscle Stem Cell Response. Exerc. Sport. Sci. Rev. 2018, 46, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Moss, F.P.; Leblond, C.P. Satellite cells as the source of nuclei in muscles of growing rats. Anat. Rec. 1971, 170, 421–435. [Google Scholar] [CrossRef]
- Lepper, C.; Partridge, T.A.; Fan, C.M. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 2011, 138, 3639–3646. [Google Scholar] [CrossRef] [Green Version]
- Lepper, C.; Conway, S.J.; Fan, C.M. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature 2009, 460, 627–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fry, C.S.; Lee, J.D.; Mula, J.; Kirby, T.J.; Jackson, J.R.; Liu, F.; Yang, L.; Mendias, C.L.; Dupont-Versteegden, E.E.; McCarthy, J.J.; et al. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat. Med. 2015, 21, 76–80. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, J.J.; Mula, J.; Miyazaki, M.; Erfani, R.; Garrison, K.; Farooqui, A.B.; Srikuea, R.; Lawson, B.A.; Grimes, B.; Keller, C.; et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 2011, 138, 3657–3666. [Google Scholar] [CrossRef] [Green Version]
- Sambasivan, R.; Yao, R.; Kissenpfennig, A.; Van Wittenberghe, L.; Paldi, A.; Gayraud-Morel, B.; Guenou, H.; Malissen, B.; Tajbakhsh, S.; Galy, A. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 2011, 138, 3647–3656. [Google Scholar] [CrossRef] [Green Version]
- Charge, S.B.; Rudnicki, M.A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004, 84, 209–238. [Google Scholar] [CrossRef]
- Braun, T.; Gautel, M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat. Rev. Mol. Cell Biol. 2011, 12, 349–361. [Google Scholar] [CrossRef]
- Krauss, R.S. Regulation of promyogenic signal transduction by cell-cell contact and adhesion. Exp. Cell Res. 2010, 316, 3042–3049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krauss, R.S.; Joseph, G.A.; Goel, A.J. Keep Your Friends Close: Cell-Cell Contact and Skeletal Myogenesis. Cold Spring Harb. Perspect. Biol. 2017, 9, a029298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrany, M.J.; Millay, D.P. Cell Fusion: Merging Membranes and Making Muscle. Trends Cell Biol. 2019, 29, 964–973. [Google Scholar] [CrossRef]
- Lehka, L.; Redowicz, M.J. Mechanisms regulating myoblast fusion: A multilevel interplay. Semin. Cell Dev. Biol. 2020, 104, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Abmayr, S.M.; Pavlath, G.K. Myoblast fusion: Lessons from flies and mice. Development 2012, 139, 641–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, P.; McAnally, J.R.; Shelton, J.M.; Sanchez-Ortiz, E.; Bassel-Duby, R.; Olson, E.N. Fusogenic micropeptide Myomixer is essential for satellite cell fusion and muscle regeneration. Proc. Natl. Acad. Sci. USA 2018, 115, 3864–3869. [Google Scholar] [CrossRef] [Green Version]
- Millay, D.P.; O’Rourke, J.R.; Sutherland, L.B.; Bezprozvannaya, S.; Shelton, J.M.; Bassel-Duby, R.; Olson, E.N. Myomaker is a membrane activator of myoblast fusion and muscle formation. Nature 2013, 499, 301–305. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wen, J.; Bigot, A.; Chen, J.; Shang, R.; Mouly, V.; Bi, P. Human myotube formation is determined by MyoD-Myomixer/Myomaker axis. Sci. Adv. 2020, 6, eabc4062. [Google Scholar] [CrossRef]
- Quinn, M.E.; Goh, Q.; Kurosaka, M.; Gamage, D.G.; Petrany, M.J.; Prasad, V.; Millay, D.P. Myomerger induces fusion of non-fusogenic cells and is required for skeletal muscle development. Nat. Commun. 2017, 8, 15665. [Google Scholar] [CrossRef] [Green Version]
- Millay, D.P.; Sutherland, L.B.; Bassel-Duby, R.; Olson, E.N. Myomaker is essential for muscle regeneration. Genes Dev. 2014, 28, 1641–1646. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; McLennan, I.S. During secondary myotube formation, primary myotubes preferentially absorb new nuclei at their ends. Dev. Dyn. 1995, 204, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.J.; Duxson, M.J.; Fitzsimons, R.B.; Rieger, F. Myonuclear birthdates distinguish the origins of primary and secondary myotubes in embryonic mammalian skeletal muscles. Development 1989, 107, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.; Baillie, H.; Caswell, A.; Wigmore, P. During fetal muscle development, clones of cells contribute to both primary and secondary fibers. Dev. Biol. 1994, 162, 348–353. [Google Scholar] [CrossRef]
- Pizza, F.X.; Martin, R.A.; Springer, E.M.; Leffler, M.S.; Woelmer, B.R.; Recker, I.J.; Leaman, D.W. Intercellular adhesion molecule-1 augments myoblast adhesion and fusion through homophilic trans-interactions. Sci. Rep. 2017, 7, 5094. [Google Scholar] [CrossRef] [Green Version]
- Jacquemin, V.; Furling, D.; Bigot, A.; Butler-Browne, G.S.; Mouly, V. IGF-1 induces human myotube hypertrophy by increasing cell recruitment. Exp. Cell Res. 2004, 299, 148–158. [Google Scholar] [CrossRef]
- Goh, Q.; Dearth, C.L.; Corbett, J.T.; Pierre, P.; Chadee, D.N.; Pizza, F.X. Intercellular adhesion molecule-1 expression by skeletal muscle cells augments myogenesis. Exp. Cell Res. 2015, 331, 292–308. [Google Scholar] [CrossRef]
- Musa, H.; Orton, C.; Morrison, E.E.; Peckham, M. Microtubule assembly in cultured myoblasts and myotubes following nocodazole induced microtubule depolymerisation. J. Muscle Res. Cell Motil. 2003, 24, 301–308. [Google Scholar] [CrossRef]
- Nowak, S.J.; Nahirney, P.C.; Hadjantonakis, A.K.; Baylies, M.K. Nap1-mediated actin remodeling is essential for mammalian myoblast fusion. J. Cell Sci. 2009, 122, 3282–3293. [Google Scholar] [CrossRef] [Green Version]
- Fidzianska, A. Human ontogenesis. I. Ultrastructural characteristics of developing human muscle. J. Neuropathol. Exp. Neurol. 1980, 39, 476–486. [Google Scholar] [CrossRef]
- Kelly, A.M.; Zacks, S.I. The histogenesis of rat intercostal muscle. J. Cell Biol. 1969, 42, 135–153. [Google Scholar] [CrossRef]
- Ishikawa, H. Formation of elaborate networks of T-system tubules in cultured skeletal muscle with special reference to the T-system formation. J. Cell Biol. 1968, 38, 51–66. [Google Scholar] [CrossRef]
- Larson, P.F.; Jenkison, M.; Hudgson, P. The morphological development of chick embryo skeletal muscle grown in tissue culture as studied by electron microscopy. J. Neurol. Sci. 1970, 10, 385–405. [Google Scholar] [CrossRef]
- Fischman, D.A. An electron microscope study of myofibril formation in embryonic chick skeletal muscle. J. Cell Biol. 1967, 32, 557–575. [Google Scholar] [CrossRef]
- Franzini-Armstrong, C. Simultaneous maturation of transverse tubules and sarcoplasmic reticulum during muscle differentiation in the mouse. Dev. Biol. 1991, 146, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Flucher, B.E.; Takekura, H.; Franzini-Armstrong, C. Development of the excitation-contraction coupling apparatus in skeletal muscle: Association of sarcoplasmic reticulum and transverse tubules with myofibrils. Dev. Biol. 1993, 160, 135–147. [Google Scholar] [CrossRef]
- Fischman, D.A. The synthesis and assembly of myofibrils in embryonic muscle. Curr. Top. Dev. Biol. 1970, 5, 235–280. [Google Scholar]
- Ontell, M.; Kozeka, K. The organogenesis of murine striated muscle: A cytoarchitectural study. Am. J. Anat. 1984, 171, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Ontell, M.; Kozeka, K. Organogenesis of the mouse extensor digitorum logus muscle: A quantitative study. Am. J. Anat. 1984, 171, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Roman, W.; Gomes, E.R. Nuclear positioning in skeletal muscle. Semin. Cell Dev. Biol. 2018, 82, 51–56. [Google Scholar] [CrossRef]
- Azevedo, M.; Baylies, M.K. Getting into Position: Nuclear Movement in Muscle Cells. Trends Cell Biol. 2020, 30, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Folker, E.S.; Baylies, M.K. Nuclear positioning in muscle development and disease. Front. Physiol. 2013, 4, 363. [Google Scholar] [CrossRef] [Green Version]
- Romero, N.B.; Mezmezian, M.; Fidzianska, A. Main steps of skeletal muscle development in the human: Morphological analysis and ultrastructural characteristics of developing human muscle. Handb. Clin. Neurol. 2013, 113, 1299–1310. [Google Scholar]
- Allbrook, D. An electron microscopic study of regenerating skeletal muscle. J. Anat. 1962, 96, 137–152. [Google Scholar] [PubMed]
- Jirmanova, I.; Thesleff, S. Ultrastructural study of experimental muscle degeneration and regeneration in the adult rat. Z. Zellforsch. Mikrosk. Anat. 1972, 131, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Robertson, T.A.; Papadimitriou, J.M.; Grounds, M.D. Fusion of myogenic cells to the newly sealed region of damaged myofibres in skeletal muscle regeneration. Neuropathol. Appl. Neurobiol. 1993, 19, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.A.; Buckley, K.H.; Mankowski, D.C.; Riley, B.M.; Sidwell, A.N.; Douglas, S.L.; Worth, R.G.; Pizza, F.X. Myogenic Cell Expression of Intercellular Adhesion Molecule-1 Contributes to Muscle Regeneration after Injury. Am. J. Pathol. 2020, 190, 2039–2055. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.E. An experimental study of the regeneration of mammalian striped muscle. J. Anat. 1946, 80, 24–36. [Google Scholar]
- Liu, J.; Huang, Z.-P.; Nie, M.; Wang, G.; Silva, W.J.; Yang, Q.; Freire, P.P.; Hu, X.; Chen, H.; Deng, Z. Regulation of myonuclear positioning and muscle function by the skeletal muscle-specific CIP protein. Proc. Natl. Acad. Sci. USA 2020, 117, 19254–19265. [Google Scholar] [CrossRef]
- Wada, K.; Katsuta, S.; Soya, H. Formation process and fate of the nuclear chain after injury in regenerated myofiber. Anat. Rec. 2008, 291, 122–128. [Google Scholar] [CrossRef]
- Buckley, K.H.; Nestor-Kalinoski, A.L.; Pizza, F.X. Positional Context of Myonuclear Transcription During Injury-Induced Muscle Regeneration. Front. Physiol. 2022, 13, 845504. [Google Scholar] [CrossRef]
- Newlands, S.; Levitt, L.K.; Robinson, C.S.; Karpf, A.B.; Hodgson, V.R.; Wade, R.P.; Hardeman, E.C. Transcription occurs in pulses in muscle fibers. Genes. Dev. 1998, 12, 2748–2758. [Google Scholar] [CrossRef] [Green Version]
- Buckley, K.H.; Nestor-Kalinoski, A.L.; Pizza, F.X. Intercellular Adhesion Molecule-1 Enhances Myonuclear Transcription during Injury-Induced Muscle Regeneration. Int. J. Mol. Sci. 2022, 23, 7028. [Google Scholar] [CrossRef] [PubMed]
- Bruusgaard, J.C.; Liestol, K.; Gundersen, K. Distribution of myonuclei and microtubules in live muscle fibers of young, middle-aged, and old mice. J. Appl. Physiol. 2006, 100, 2024–2030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruusgaard, J.C.; Liestol, K.; Ekmark, M.; Kollstad, K.; Gundersen, K. Number and spatial distribution of nuclei in the muscle fibres of normal mice studied in vivo. J. Physiol. 2003, 551, 467–478. [Google Scholar] [CrossRef]
- Merrick, D.; Stadler, L.K.; Larner, D.; Smith, J. Muscular dystrophy begins early in embryonic development deriving from stem cell loss and disrupted skeletal muscle formation. Dis. Model. Mech. 2009, 2, 374–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whalen, R.G.; Harris, J.B.; Butler-Browne, G.S.; Sesodia, S. Expression of myosin isoforms during notexin-induced regeneration of rat soleus muscles. Dev. Biol. 1990, 141, 24–40. [Google Scholar] [CrossRef]
- Sartore, S.; Gorza, L.; Schiaffino, S. Fetal myosin heavy chains in regenerating muscle. Nature 1982, 298, 294–296. [Google Scholar] [CrossRef] [PubMed]
- d’Albis, A.; Couteaux, R.; Janmot, C.; Roulet, A.; Mira, J.C. Regeneration after cardiotoxin injury of innervated and denervated slow and fast muscles of mammals. Myosin isoform analysis. Eur. J. Biochem. 1988, 174, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jia, Y.; Zhao, J.; Lesner, N.P.; Menezes, C.J.; Shelton, S.D.; Venigalla, S.S.K.; Xu, J.; Cai, C.; Mishra, P. A mitofusin 2/HIF1alpha axis sets a maturation checkpoint in regenerating skeletal muscle. J. Clin. Investig. 2022, 132, e161638. [Google Scholar] [CrossRef]
- Fink, E.; Fortin, D.; Serrurier, B.; Ventura-Clapier, R.; Bigard, A.X. Recovery of contractile and metabolic phenotypes in regenerating slow muscle after notexin-induced or crush injury. J. Muscle Res. Cell Motil. 2003, 24, 421–429. [Google Scholar] [CrossRef]
- Harris, A.J. Embryonic growth and innervation of rat skeletal muscles. I. Neural regulation of muscle fibre numbers. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1981, 293, 257–277. [Google Scholar] [PubMed]
- Harris, A.J.; Fitzsimons, R.B.; McEwan, J.C. Neural control of the sequence of expression of myosin heavy chain isoforms in foetal mammalian muscles. Development 1989, 107, 751–769. [Google Scholar] [CrossRef]
- Goldspink, G. Changes in striated muscle fibres during contraction and growth with particular reference to myofibril splitting. J. Cell Sci. 1971, 9, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Goldspink, G. The proliferation of myofibrils during muscle fibre growth. J. Cell Sci. 1970, 6, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Roman, W.; Martins, J.P.; Carvalho, F.A.; Voituriez, R.; Abella, J.V.G.; Santos, N.C.; Cadot, B.; Way, M.; Gomes, E.R. Myofibril contraction and crosslinking drive nuclear movement to the periphery of skeletal muscle. Nat. Cell Biol. 2017, 19, 1189–1201. [Google Scholar] [CrossRef] [Green Version]
- Cramer, A.A.W.; Prasad, V.; Eftestol, E.; Song, T.; Hansson, K.A.; Dugdale, H.F.; Sadayappan, S.; Ochala, J.; Gundersen, K.; Millay, D.P. Nuclear numbers in syncytial muscle fibers promote size but limit the development of larger myonuclear domains. Nat. Commun. 2020, 11, 6287. [Google Scholar] [CrossRef]
- Williams, P.E.; Goldspink, G. Longitudinal growth of striated muscle fibres. J. Cell Sci. 1971, 9, 751–767. [Google Scholar] [CrossRef]
- Wada, K.I.; Katsuta, S.; Soya, H. Natural occurrence of myofiber cytoplasmic enlargement accompanied by decrease in myonuclear number. Jpn. J. Physiol. 2003, 53, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Gattazzo, F.; Laurent, B.; Relaix, F.; Rouard, H.; Didier, N. Distinct Phases of Postnatal Skeletal Muscle Growth Govern the Progressive Establishment of Muscle Stem Cell Quiescence. Stem Cell Rep. 2020, 15, 597–611. [Google Scholar] [CrossRef]
- Bachman, J.F.; Klose, A.; Liu, W.; Paris, N.D.; Blanc, R.S.; Schmalz, M.; Knapp, E.; Chakkalakal, J.V. Prepubertal skeletal muscle growth requires Pax7-expressing satellite cell-derived myonuclear contribution. Development 2018, 145, dev167197. [Google Scholar] [CrossRef] [Green Version]
- Huh, M.S.; Young, K.G.; Yan, K.; Price-O’Dea, T.; Picketts, D.J. Recovery from impaired muscle growth arises from prolonged postnatal accretion of myonuclei in Atrx mutant mice. PLoS ONE 2017, 12, e0186989. [Google Scholar] [CrossRef] [Green Version]
- White, R.B.; Bierinx, A.S.; Gnocchi, V.F.; Zammit, P.S. Dynamics of muscle fibre growth during postnatal mouse development. BMC Dev. Biol. 2010, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Cardasis, C.A.; Cooper, G.W. An analysis of nuclear numbers in individual muscle fibers during differentiation and growth: A satellite cell-muscle fiber growth unit. J. Exp. Zool. 1975, 191, 347–358. [Google Scholar] [CrossRef]
- Schultz, E. Satellite cell proliferative compartments in growing skeletal muscles. Dev. Biol. 1996, 175, 84–94. [Google Scholar] [CrossRef]
- Lash, J.W.; Holtzer, H.; Swift, H. Regeneration of mature skeletal muscle. Anat. Rec. 1957, 128, 679–697. [Google Scholar] [CrossRef] [PubMed]
- Grady, R.M.; Starr, D.A.; Ackerman, G.L.; Sanes, J.R.; Han, M. Syne proteins anchor muscle nuclei at the neuromuscular junction. Proc. Natl. Acad. Sci. USA 2005, 102, 4359–4364. [Google Scholar] [CrossRef]
- Capetanaki, Y.; Bloch, R.J.; Kouloumenta, A.; Mavroidis, M.; Psarras, S. Muscle intermediate filaments and their links to membranes and membranous organelles. Exp. Cell Res. 2007, 313, 2063–2076. [Google Scholar] [CrossRef]
- Waisman, A.; Norris, A.M.; Costa, M.E.; Kopinke, D. Automatic and unbiased segmentation and quantification of myofibers in skeletal muscle. Sci. Rep. 2021, 11, 11793. [Google Scholar] [CrossRef]
- Pichavant, C.; Pavlath, G.K. Incidence and severity of myofiber branching with regeneration and aging. Skelet. Muscle 2014, 4, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grubb, B.D.; Harris, J.B.; Schofield, I.S. Neuromuscular transmission at newly formed neuromuscular junctions in the regenerating soleus muscle of the rat. J. Physiol. 1991, 441, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Louboutin, J.P.; Fichter-Gagnepain, V.; Noireaud, J. Comparison of contractile properties between developing and regenerating soleus muscle: Influence of external calcium concentration upon the contractility. Muscle Nerve 1995, 18, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; Anderson, J.E.; Joya, J.E.; Head, S.I.; Pather, N.; Kee, A.J.; Gunning, P.W.; Hardeman, E.C. Aged skeletal muscle retains the ability to fully regenerate functional architecture. Bioarchitecture 2013, 3, 25–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duguez, S.; Feasson, L.; Denis, C.; Freyssenet, D. Mitochondrial biogenesis during skeletal muscle regeneration. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E802–E809. [Google Scholar] [CrossRef]
- Wagner, K.R.; Max, S.R.; Grollman, E.M.; Koski, C.L. Glycolysis in skeletal muscle regeneration. Exp. Neurol. 1976, 52, 40–48. [Google Scholar] [CrossRef]
- McArdle, A.; Edwards, R.H.; Jackson, M.J. Release of creatine kinase and prostaglandin E2 from regenerating skeletal muscle fibers. J. Appl. Physiol. 1994, 76, 1274–1278. [Google Scholar] [CrossRef]
- Gregorevic, P.; Plant, D.R.; Stupka, N.; Lynch, G.S. Changes in contractile activation characteristics of rat fast and slow skeletal muscle fibres during regeneration. J. Physiol. 2004, 558, 549–560. [Google Scholar] [CrossRef] [Green Version]
- de Lima, J.E.; Bonnin, M.A.; Bourgeois, A.; Parisi, A.; Le Grand, F.; Duprez, D. Specific pattern of cell cycle during limb fetal myogenesis. Dev. Biol. 2014, 392, 308–323. [Google Scholar] [CrossRef]
- Stickland, N.C. Muscle development in the human fetus as exemplified by m. sartorius: A quantitative study. J. Anat. 1981, 132, 557–579. [Google Scholar] [PubMed]
- Mantilla, C.B.; Sill, R.V.; Aravamudan, B.; Zhan, W.Z.; Sieck, G.C. Developmental effects on myonuclear domain size of rat diaphragm fibers. J. Appl. Physiol. 2008, 104, 787–794. [Google Scholar] [CrossRef] [Green Version]
- Pawlikowski, B.; Pulliam, C.; Betta, N.D.; Kardon, G.; Olwin, B.B. Pervasive satellite cell contribution to uninjured adult muscle fibers. Skelet. Muscle 2015, 5, 42. [Google Scholar] [CrossRef] [Green Version]
- Gokhin, D.S.; Ward, S.R.; Bremner, S.N.; Lieber, R.L. Quantitative analysis of neonatal skeletal muscle functional improvement in the mouse. J. Exp. Biol. 2008, 211, 837–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachman, J.F.; Chakkalakal, J.V. Insights into muscle stem cell dynamics during postnatal development. FEBS J. 2022, 289, 2710–2722. [Google Scholar] [CrossRef]
- Keefe, A.C.; Lawson, J.A.; Flygare, S.D.; Fox, Z.D.; Colasanto, M.P.; Mathew, S.J.; Yandell, M.; Kardon, G. Muscle stem cells contribute to myofibres in sedentary adult mice. Nat. Commun. 2015, 6, 7087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaucage, K.L.; Pollmann, S.I.; Sims, S.M.; Dixon, S.J.; Holdsworth, D.W. Quantitative in vivo micro-computed tomography for assessment of age-dependent changes in murine whole-body composition. Bone Rep. 2016, 5, 70–80. [Google Scholar] [CrossRef] [Green Version]
- Somerville, J.M.; Aspden, R.M.; Armour, K.E.; Armour, K.J.; Reid, D.M. Growth of C57BL/6 mice and the material and mechanical properties of cortical bone from the tibia. Calcif. Tissue Int. 2004, 74, 469–475. [Google Scholar] [CrossRef]
- Burkholder, T.J.; Fingado, B.; Baron, S.; Lieber, R.L. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J. Morphol. 1994, 221, 177–190. [Google Scholar] [CrossRef]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007, 204, 1057–1069. [Google Scholar] [CrossRef]
- Bates, P.C.; Millward, D.J. Characteristics of skeletal muscle growth and protein turnover in a fast-growing rat strain. Br. J. Nutr. 1981, 46, 7–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millward, D.J.; Garlick, P.J.; Stewart, R.J.; Nnanyelugo, D.O.; Waterlow, J.C. Skeletal-muscle growth and protein turnover. Biochem. J. 1975, 150, 235–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrany, M.J.; Swoboda, C.O.; Sun, C.; Chetal, K.; Chen, X.; Weirauch, M.T.; Salomonis, N.; Millay, D.P. Single-nucleus RNA-seq identifies transcriptional heterogeneity in multinucleated skeletal myofibers. Nat. Commun. 2020, 11, 6374. [Google Scholar] [CrossRef]
- Proud, C.G. Regulation of mammalian translation factors by nutrients. Eur. J. Biochem. 2002, 269, 5338–5349. [Google Scholar] [CrossRef] [PubMed]
- von Walden, F. Ribosome biogenesis in skeletal muscle: Coordination of transcription and translation. J. Appl. Physiol. 2019, 127, 591–598. [Google Scholar] [CrossRef]
- Kim, M.; Franke, V.; Brandt, B.; Lowenstein, E.D.; Schowel, V.; Spuler, S.; Akalin, A.; Birchmeier, C. Single-nucleus transcriptomics reveals functional compartmentalization in syncytial skeletal muscle cells. Nat. Commun. 2020, 11, 6375. [Google Scholar] [CrossRef]
- Plant, D.R.; Colarossi, F.E.; Lynch, G.S. Notexin causes greater myotoxic damage and slower functional repair in mouse skeletal muscles than bupivacaine. Muscle Nerve 2006, 34, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Griffin, C.A.; Kafadar, K.A.; Pavlath, G.K. MOR23 promotes muscle regeneration and regulates cell adhesion and migration. Dev. Cell 2009, 17, 649–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simionescu-Bankston, A.; Leoni, G.; Wang, Y.; Pham, P.P.; Ramalingam, A.; DuHadaway, J.B.; Faundez, V.; Nusrat, A.; Prendergast, G.C.; Pavlath, G.K. The N-BAR domain protein, Bin3, regulates Rac1- and Cdc42-dependent processes in myogenesis. Dev. Biol. 2013, 382, 160–171. [Google Scholar] [CrossRef] [Green Version]
- Goh, Q.; Song, T.; Petrany, M.J.; Cramer, A.A.; Sun, C.; Sadayappan, S.; Lee, S.J.; Millay, D.P. Myonuclear accretion is a determinant of exercise-induced remodeling in skeletal muscle. eLife 2019, 8, e44876. [Google Scholar] [CrossRef]
- Englund, D.A.; Figueiredo, V.C.; Dungan, C.M.; Murach, K.A.; Peck, B.D.; Petrosino, J.M.; Brightwell, C.R.; Dupont, A.M.; Neal, A.C.; Fry, C.S.; et al. Satellite Cell Depletion Disrupts Transcriptional Coordination and Muscle Adaptation to Exercise. Function 2021, 2, zqaa033. [Google Scholar] [CrossRef]
- Egner, I.M.; Bruusgaard, J.C.; Gundersen, K. Satellite cell depletion prevents fiber hypertrophy in skeletal muscle. Development 2016, 143, 2898–2906. [Google Scholar] [CrossRef] [Green Version]
- Goh, Q.; Millay, D.P. Requirement of myomaker-mediated stem cell fusion for skeletal muscle hypertrophy. eLife 2017, 6, e20007. [Google Scholar] [CrossRef]
- Biressi, S.; Molinaro, M.; Cossu, G. Cellular heterogeneity during vertebrate skeletal muscle development. Dev. Biol. 2007, 308, 281–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stockdale, F.E. Myogenic cell lineages. Dev. Biol. 1992, 154, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Chal, J.; Pourquie, O. Making muscle: Skeletal myogenesis in vivo and in vitro. Development 2017, 144, 2104–2122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biressi, S.; Tagliafico, E.; Lamorte, G.; Monteverde, S.; Tenedini, E.; Roncaglia, E.; Ferrari, S.; Ferrari, S.; Cusella-De Angelis, M.G.; Tajbakhsh, S.; et al. Intrinsic phenotypic diversity of embryonic and fetal myoblasts is revealed by genome-wide gene expression analysis on purified cells. Dev. Biol. 2007, 304, 633–651. [Google Scholar] [CrossRef] [Green Version]
- Hutcheson, D.A.; Zhao, J.; Merrell, A.; Haldar, M.; Kardon, G. Embryonic and fetal limb myogenic cells are derived from developmentally distinct progenitors and have different requirements for beta-catenin. Genes. Dev. 2009, 23, 997–1013. [Google Scholar] [CrossRef] [Green Version]
- Lepper, C.; Fan, C.M. Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis 2010, 48, 424–436. [Google Scholar] [CrossRef]
- Relaix, F.; Rocancourt, D.; Mansouri, A.; Buckingham, M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature 2005, 435, 948–953. [Google Scholar] [CrossRef] [Green Version]
- Duxson, M.J.; Usson, Y.; Harris, A.J. The origin of secondary myotubes in mammalian skeletal muscles: Ultrastructural studies. Development 1989, 107, 743–750. [Google Scholar] [CrossRef]
- Dennis, M.J.; Ziskind-Conhaim, L.; Harris, A.J. Development of neuromuscular junctions in rat embryos. Dev. Biol. 1981, 81, 266–279. [Google Scholar] [CrossRef]
- Harris, A.J. Embryonic growth and innervation of rat skeletal muscles. III. Neural regulation of junctional and extra-junctional acetylcholine receptor clusters. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1981, 293, 287–314. [Google Scholar]
- Ross, J.J.; Duxson, M.J.; Harris, A.J. Neural determination of muscle fibre numbers in embryonic rat lumbrical muscles. Development 1987, 100, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Ontell, M.; Dunn, R.F. Neonatal muscle growth: A quantitative study. Am. J. Anat. 1978, 152, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Ontell, M. Neonatal muscle: An electron microscopic study. Anat. Rec. 1977, 189, 669–690. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Garry, G.A.; Li, S.; Bezprozvannaya, S.; Sanchez-Ortiz, E.; Chen, B.; Shelton, J.M.; Jaichander, P.; Bassel-Duby, R.; Olson, E.N. A Twist2-dependent progenitor cell contributes to adult skeletal muscle. Nat. Cell Biol. 2017, 19, 202–213. [Google Scholar] [CrossRef] [Green Version]
- Kuang, S.; Charge, S.B.; Seale, P.; Huh, M.; Rudnicki, M.A. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J. Cell Biol. 2006, 172, 103–113. [Google Scholar] [CrossRef]
- Davis, C.E.; Harris, J.B.; Nicholson, L.V. Myosin isoform transitions and physiological properties of regenerated and re-innervated soleus muscles of the rat. Neuromuscul. Disord. 1991, 1, 411–421. [Google Scholar] [CrossRef]
- Bassaglia, Y.; Gautron, J. Fast and slow rat muscles degenerate and regenerate differently after whole crush injury. J. Muscle Res. Cell Motil. 1995, 16, 420–429. [Google Scholar] [CrossRef]
- Tamaki, T.; Akatsuka, A. Appearance of complex branched fibers following repetitive muscle trauma in normal rat skeletal muscle. Anat. Rec. 1994, 240, 217–224. [Google Scholar] [CrossRef]
- Murach, K.A.; Dungan, C.M.; Peterson, C.A.; McCarthy, J.J. Muscle Fiber Splitting Is a Physiological Response to Extreme Loading in Animals. Exerc. Sport. Sci. Rev. 2019, 47, 108–115. [Google Scholar] [CrossRef]
- Jorgenson, K.W.; Phillips, S.M.; Hornberger, T.A. Identifying the Structural Adaptations that Drive the Mechanical Load-Induced Growth of Skeletal Muscle: A Scoping Review. Cells 2020, 9, 1658. [Google Scholar] [CrossRef]
- Lovering, R.M.; Michaelson, L.; Ward, C.W. Malformed mdx myofibers have normal cytoskeletal architecture yet altered EC coupling and stress-induced Ca2+ signaling. Am. J. Physiol. Cell Physiol. 2009, 297, C571–C580. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.; Head, S.I. The role of branched fibres in the pathogenesis of Duchenne muscular dystrophy. Exp. Physiol. 2011, 96, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Hurme, T.; Kalimo, H.; Lehto, M.; Jarvinen, M. Healing of skeletal muscle injury: An ultrastructural and immunohistochemical study. Med. Sci. Sports Exerc. 1991, 23, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Jenniskens, G.J.; Hafmans, T.; Veerkamp, J.H.; van Kuppevelt, T.H. Spatiotemporal distribution of heparan sulfate epitopes during myogenesis and synaptogenesis: A study in developing mouse intercostal muscle. Dev. Dyn. 2002, 225, 70–79. [Google Scholar] [CrossRef] [Green Version]
- Patton, B.L.; Miner, J.H.; Chiu, A.Y.; Sanes, J.R. Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. J. Cell Biol. 1997, 139, 1507–1521. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, J.A.; Jones, D.A.; Round, J.M. Injury to skeletal muscles of mice by forced lengthening during contractions. Q. J. Exp. Physiol. 1989, 74, 661–670. [Google Scholar] [CrossRef] [Green Version]
- McCully, K.K.; Faulkner, J.A. Injury to skeletal muscle fibers of mice following lengthening contractions. J. Appl. Physiol. 1985, 59, 119–126. [Google Scholar] [CrossRef]
- Brooks, S.V.; Faulkner, J.A. Contraction-induced injury: Recovery of skeletal muscles in young and old mice. Am. J. Physiol. 1990, 258, C436–C442. [Google Scholar] [CrossRef]
- Paul, T.A.; Macpherson, P.C.; Janetzke, T.L.; Davis, C.S.; Jackson, M.J.; McArdle, A.; Brooks, S.V. Older mice show decreased regeneration of neuromuscular junctions following lengthening contraction-induced injury. Geroscience 2023, 45, 1899–1912. [Google Scholar] [CrossRef]
- Carpenter, S.; Karpati, G. Segmental necrosis and its demarcation in experimental micropuncture injury of skeletal muscle fibers. J. Neuropathol. Exp. Neurol. 1989, 48, 154–170. [Google Scholar] [CrossRef]
- Novak, M.L.; Weinheimer-Haus, E.M.; Koh, T.J. Macrophage activation and skeletal muscle healing following traumatic injury. J. Pathol. 2014, 232, 344–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, D.; Oron, U. Repair mechanisms involved in muscle regeneration following partial excision of the rat gastrocnemius muscle. Exp. Cell Biol. 1985, 53, 107–114. [Google Scholar] [CrossRef]
- Aarimaa, V.; Kaariainen, M.; Vaittinen, S.; Tanner, J.; Jarvinen, T.; Best, T.; Kalimo, H. Restoration of myofiber continuity after transection injury in the rat soleus. Neuromuscul. Disord. 2004, 14, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Kaariainen, M.; Kaariainen, J.; Jarvinen, T.L.; Nissinen, L.; Heino, J.; Jarvinen, M.; Kalimo, H. Integrin and dystrophin associated adhesion protein complexes during regeneration of shearing-type muscle injury. Neuromuscul. Disord. 2000, 10, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Robertson, T.A.; Grounds, M.D.; Papadimitriou, J.M. Elucidation of aspects of murine skeletal muscle regeneration using local and whole body irradiation. J. Anat. 1992, 181, 265–276. [Google Scholar]
- Turner, N.J.; Badylak, S.F. Regeneration of skeletal muscle. Cell Tissue Res. 2012, 347, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.E.; Han, W.M.; Srinivasa, V.; Mohiuddin, M.; Ruehle, M.A.; Moon, J.Y.; Shin, E.; San Emeterio, C.L.; Ogle, M.E.; Botchwey, E.A.; et al. Determination of a Critical Size Threshold for Volumetric Muscle Loss in the Mouse Quadriceps. Tissue Eng. Part. C Methods 2019, 25, 59–70. [Google Scholar] [CrossRef]
- Sicari, B.M.; Rubin, J.P.; Dearth, C.L.; Wolf, M.T.; Ambrosio, F.; Boninger, M.; Turner, N.J.; Weber, D.J.; Simpson, T.W.; Wyse, A.; et al. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci. Transl. Med. 2014, 6, 234ra58. [Google Scholar] [CrossRef] [Green Version]
- Quarta, M.; Cromie, M.; Chacon, R.; Blonigan, J.; Garcia, V.; Akimenko, I.; Hamer, M.; Paine, P.; Stok, M.; Shrager, J.B.; et al. Bioengineered constructs combined with exercise enhance stem cell-mediated treatment of volumetric muscle loss. Nat. Commun. 2017, 8, 15613. [Google Scholar] [CrossRef] [Green Version]
- Papadimitriou, J.M.; Robertson, T.A.; Mitchell, C.A.; Grounds, M.D. The process of new plasmalemma formation in focally injured skeletal muscle fibers. J. Struct. Biol. 1990, 103, 124–134. [Google Scholar] [CrossRef]
- Menetrey, J.; Kasemkijwattana, C.; Fu, F.H.; Moreland, M.S.; Huard, J. Suturing versus immobilization of a muscle laceration. A morphological and functional study in a mouse model. Am. J. Sports Med. 1999, 27, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Pizza, F.X.; Peterson, J.M.; Baas, J.H.; Koh, T.J. Neutrophils contribute to muscle injury and impair its resolution after lengthening contractions in mice. J. Physiol. 2005, 562, 899–913. [Google Scholar] [CrossRef] [PubMed]
- Lovering, R.M.; McMillan, A.B.; Gullapalli, R.P. Location of myofiber damage in skeletal muscle after lengthening contractions. Muscle Nerve 2009, 40, 589–594. [Google Scholar] [CrossRef] [Green Version]
- Lovering, R.M.; Roche, J.A.; Bloch, R.J.; De Deyne, P.G. Recovery of function in skeletal muscle following 2 different contraction-induced injuries. Arch. Phys. Med. Rehabil. 2007, 88, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Lieber, R.L.; Schmitz, M.C.; Mishra, D.K.; Friden, J. Contractile and cellular remodeling in rabbit skeletal muscle after cyclic eccentric contractions. J. Appl. Physiol. 1994, 77, 1926–1934. [Google Scholar] [CrossRef]
- Friden, J.; Lieber, R.L. Segmental muscle fiber lesions after repetitive eccentric contractions. Cell Tissue Res. 1998, 293, 165–171. [Google Scholar] [CrossRef]
- Keller, H.L.; St Pierre Schneider, B.; Eppihimer, L.A.; Cannon, J.G. Association of IGF-I and IGF-II with myofiber regeneration in vivo. Muscle Nerve 1999, 22, 347–354. [Google Scholar] [CrossRef]
- Snow, M.H. Satellite cell response in rat soleus muscle undergoing hypertrophy due to surgical ablation of synergists. Anat. Rec. 1990, 227, 437–446. [Google Scholar] [CrossRef]
- Wanek, L.J.; Snow, M.H. Activity-induced fiber regeneration in rat soleus muscle. Anat. Rec. 2000, 258, 176–185. [Google Scholar] [CrossRef]
- Armstrong, R.B.; Ogilvie, R.W.; Schwane, J.A. Eccentric exercise-induced injury to rat skeletal muscle. J. Appl. Physiol. 1983, 54, 80–93. [Google Scholar] [CrossRef]
- Krippendorf, B.B.; Riley, D.A. Temporal changes in sarcomere lesions of rat adductor longus muscles during hindlimb reloading. Anat. Rec. 1994, 238, 304–310. [Google Scholar] [CrossRef]
- Thompson, J.L.; Balog, E.M.; Fitts, R.H.; Riley, D.A. Five myofibrillar lesion types in eccentrically challenged, unloaded rat adductor longus muscle—A test model. Anat. Rec. 1999, 254, 39–52. [Google Scholar] [CrossRef]
- Tsivitse, S.K.; McLoughlin, T.J.; Peterson, J.M.; Mylona, E.; McGregor, S.J.; Pizza, F.X. Downhill running in rats: Influence on neutrophils, macrophages, and MyoD+ cells in skeletal muscle. Eur. J. Appl. Physiol. 2003, 90, 633–638. [Google Scholar] [CrossRef]
- Smith, H.K.; Plyley, M.J.; Rodgers, C.D.; McKee, N.H. Skeletal muscle damage in the rat hindlimb following single or repeated daily bouts of downhill exercise. Int. J. Sports Med. 1997, 18, 94–100. [Google Scholar] [CrossRef]
- Frenette, J.; St-Pierre, M.; Cote, C.H.; Mylona, E.; Pizza, F.X. Muscle impairment occurs rapidly and precedes inflammatory cell accumulation after mechanical loading. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R351–R357. [Google Scholar] [CrossRef]
- Marino, J.S.; Tausch, B.J.; Dearth, C.L.; Manacci, M.V.; McLoughlin, T.J.; Rakyta, S.J.; Linsenmayer, M.P.; Pizza, F.X. Beta2-integrins contribute to skeletal muscle hypertrophy in mice. Am. J. Physiol. Cell Physiol. 2008, 295, C1026–C1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuipers, H.; Drukker, J.; Frederik, P.M.; Geurten, P.; van Kranenburg, G. Muscle degeneration after exercise in rats. Int. J. Sports Med. 1983, 4, 45–51. [Google Scholar] [CrossRef]
- Koh, T.J.; Brooks, S.V. Lengthening contractions are not required to induce protection from contraction-induced muscle injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R155–R161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasilaki, A.; Pollock, N.; Giakoumaki, I.; Goljanek-Whysall, K.; Sakellariou, G.K.; Pearson, T.; Kayani, A.; Jackson, M.J.; McArdle, A. The effect of lengthening contractions on neuromuscular junction structure in adult and old mice. Age 2016, 38, 259–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathbone, C.R.; Wenke, J.C.; Warren, G.L.; Armstrong, R.B. Importance of satellite cells in the strength recovery after eccentric contraction-induced muscle injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R1490–R1495. [Google Scholar] [CrossRef]
- Kawashima, M.; Kawanishi, N.; Tominaga, T.; Suzuki, K.; Miyazaki, A.; Nagata, I.; Miyoshi, M.; Miyakawa, M.; Sakuraya, T.; Sonomura, T.; et al. Icing after eccentric contraction-induced muscle damage perturbs the disappearance of necrotic muscle fibers and phenotypic dynamics of macrophages in mice. J. Appl. Physiol. 2021, 130, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Rader, E.P.; Faulkner, J.A. Effect of aging on the recovery following contraction-induced injury in muscles of female mice. J. Appl. Physiol. 2006, 101, 887–892. [Google Scholar] [CrossRef] [Green Version]
- Dearth, C.L.; Goh, Q.; Marino, J.S.; Cicinelli, P.A.; Torres-Palsa, M.J.; Pierre, P.; Worth, R.G.; Pizza, F.X. Skeletal muscle cells express ICAM-1 after muscle overload and ICAM-1 contributes to the ensuing hypertrophic response. PLoS ONE 2013, 8, e58486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, J.R.; Mula, J.; Kirby, T.J.; Fry, C.S.; Lee, J.D.; Ubele, M.F.; Campbell, K.S.; McCarthy, J.J.; Peterson, C.A.; Dupont-Versteegden, E.E. Satellite cell depletion does not inhibit adult skeletal muscle regrowth following unloading-induced atrophy. Am. J. Physiol. Cell Physiol. 2012, 303, C854–C861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondesen, B.A.; Mills, S.T.; Pavlath, G.K. The COX-2 pathway regulates growth of atrophied muscle via multiple mechanisms. Am. J. Physiol. Cell Physiol. 2006, 290, C1651–C1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, D.; Barash, I.A.; Burdi, M.; Yuan, P.S.; Mathew, L.; Friden, J.; Lieber, R.L. Asynchronous functional, cellular and transcriptional changes after a bout of eccentric exercise in the rat. J. Physiol. 2003, 553, 947–957. [Google Scholar] [CrossRef]
- Beaton, L.J.; Tarnopolsky, M.A.; Phillips, S.M. Variability in estimating eccentric contraction-induced muscle damage and inflammation in humans. Can. J. Appl. Physiol. 2002, 27, 516–526. [Google Scholar] [CrossRef]
- Fielding, R.A.; Manfredi, T.J.; Ding, W.; Fiatarone, M.A.; Evans, W.J.; Cannon, J.G. Acute phase response in exercise. III. Neutrophil and IL-1 beta accumulation in skeletal muscle. Am. J. Physiol. 1993, 265, R166–R172. [Google Scholar] [CrossRef]
- Paulsen, G.; Crameri, R.; Benestad, H.B.; Fjeld, J.G.; Morkrid, L.; Hallen, J.; Raastad, T. Time course of leukocyte accumulation in human muscle after eccentric exercise. Med. Sci. Sports Exerc. 2010, 42, 75–85. [Google Scholar] [CrossRef]
- Paulsen, G.; Egner, I.M.; Drange, M.; Langberg, H.; Benestad, H.B.; Fjeld, J.G.; Hallen, J.; Raastad, T. A COX-2 inhibitor reduces muscle soreness, but does not influence recovery and adaptation after eccentric exercise. Scand. J. Med. Sci. Sports 2010, 20, e195–e207. [Google Scholar] [CrossRef]
- Crameri, R.M.; Aagaard, P.; Qvortrup, K.; Langberg, H.; Olesen, J.; Kjaer, M. Myofibre damage in human skeletal muscle: Effects of electrical stimulation versus voluntary contraction. J. Physiol. 2007, 583, 365–380. [Google Scholar] [CrossRef]
- Mackey, A.L.; Rasmussen, L.K.; Kadi, F.; Schjerling, P.; Helmark, I.C.; Ponsot, E.; Aagaard, P.; Durigan, J.L.; Kjaer, M. Activation of satellite cells and the regeneration of human skeletal muscle are expedited by ingestion of nonsteroidal anti-inflammatory medication. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2016, 30, 2266–2281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackey, A.L.; Kjaer, M. The breaking and making of healthy adult human skeletal muscle in vivo. Skelet. Muscle 2017, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- De Micheli, A.J.; Laurilliard, E.J.; Heinke, C.L.; Ravichandran, H.; Fraczek, P.; Soueid-Baumgarten, S.; De Vlaminck, I.; Elemento, O.; Cosgrove, B.D. Single-Cell Analysis of the Muscle Stem Cell Hierarchy Identifies Heterotypic Communication Signals Involved in Skeletal Muscle Regeneration. Cell Rep. 2020, 30, 3583–3595.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKellar, D.W.; Walter, L.D.; Song, L.T.; Mantri, M.; Wang, M.F.Z.; De Vlaminck, I.; Cosgrove, B.D. Large-scale integration of single-cell transcriptomic data captures transitional progenitor states in mouse skeletal muscle regeneration. Commun. Biol. 2021, 4, 1280. [Google Scholar] [CrossRef]
- Young, L.V.; Wakelin, G.; Cameron, A.W.R.; Springer, S.A.; Ross, J.P.; Wolters, G.; Murphy, J.P.; Arsenault, M.G.; Ng, S.; Collao, N.; et al. Muscle injury induces a transient senescence-like state that is required for myofiber growth during muscle regeneration. FASEB J. 2022, 36, e22587. [Google Scholar] [CrossRef] [PubMed]



| Myotube | Regenerating Myofiber | |
|---|---|---|
| Central Myonuclei | Yes | Yes |
| Number of Myonuclei | 2–20 | Hundreds |
| Number of Myofibrils | Few | Many |
| Radial Size | Small | Small to Large |
| Embryonic Myosin | Yes | Yes, transient |
| 1st Author | Ref # | Muscle | Species | # Contractions | Recovery Time Point (Weeks) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 6 | 8 | |||||
| Keller | [197] | SOL | Mice | 450 | ↑ # | |||||
| Koh | [208] | EDL | Mice | 75 | 15% | 8% | ||||
| Lovering | [194] | TA | Rat | 150 | 5% | 40% | 10% | |||
| Lovering | [193] | TA | Rat | 15 | 40% | |||||
| Paul | [179] | EDL | Mice | 175–225 | 30% | |||||
| Pizza | [192] | EDL | Mice | 75 | 5% | 18% | ||||
| Rader | [212] | GAST | Mice | 225 | 60% | 60% | ||||
| Rathbone | [210] | TA | Mice | 150 | ↑ # | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizza, F.X.; Buckley, K.H. Regenerating Myofibers after an Acute Muscle Injury: What Do We Really Know about Them? Int. J. Mol. Sci. 2023, 24, 12545. https://doi.org/10.3390/ijms241612545
Pizza FX, Buckley KH. Regenerating Myofibers after an Acute Muscle Injury: What Do We Really Know about Them? International Journal of Molecular Sciences. 2023; 24(16):12545. https://doi.org/10.3390/ijms241612545
Chicago/Turabian StylePizza, Francis X., and Kole H. Buckley. 2023. "Regenerating Myofibers after an Acute Muscle Injury: What Do We Really Know about Them?" International Journal of Molecular Sciences 24, no. 16: 12545. https://doi.org/10.3390/ijms241612545






