The Histone Deacetylase HstD Regulates Fungal Growth, Development and Secondary Metabolite Biosynthesis in Aspergillus terreus
Abstract
:1. Introduction
2. Results
2.1. Bioinformatic Analysis of Sirtuin Deacetylases
2.2. Deletion and Complementation of hstD in A. terreus
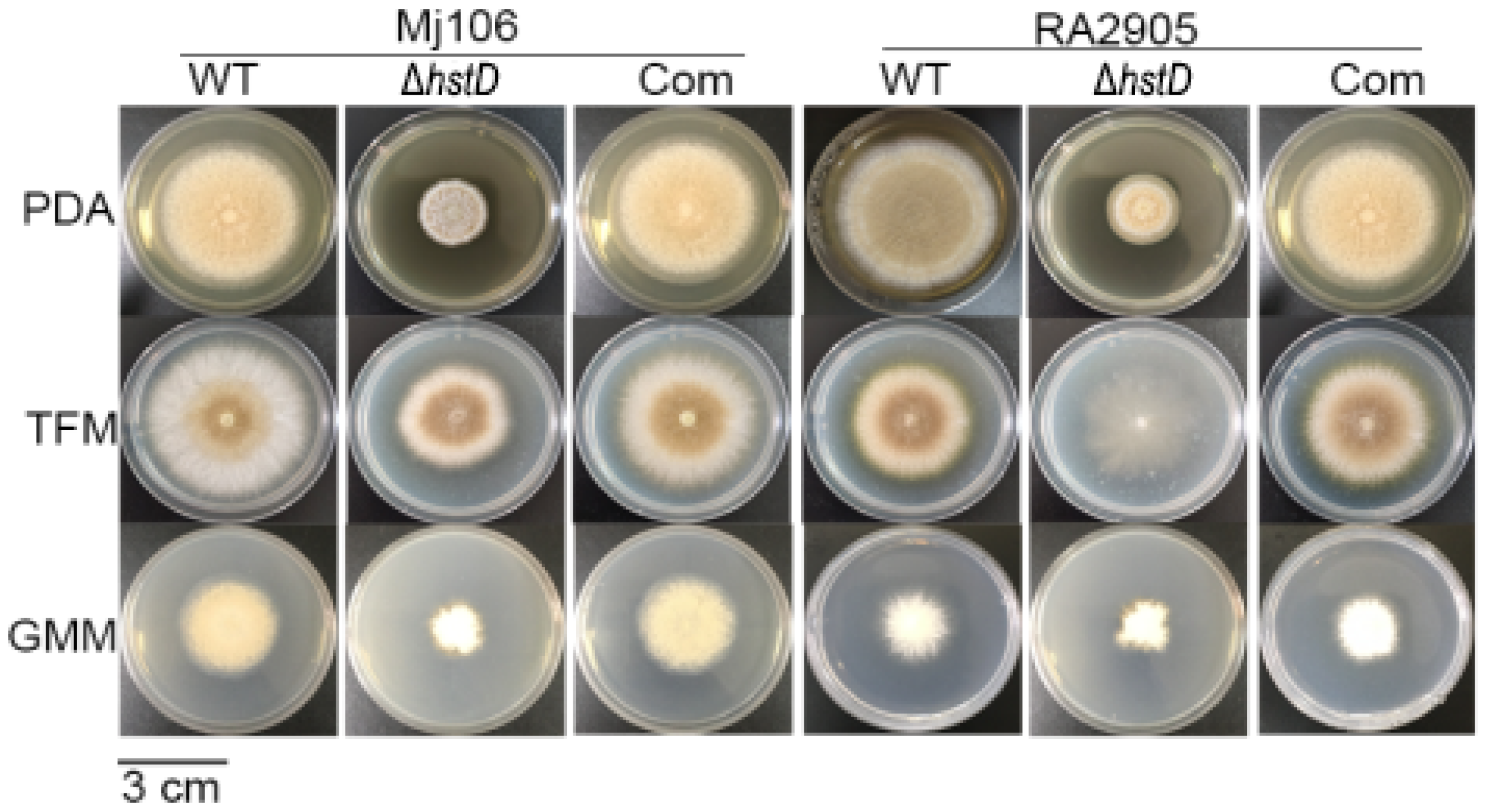
2.3. HstD Is Important for Mycelial Growth, Conidial Germination and Formation
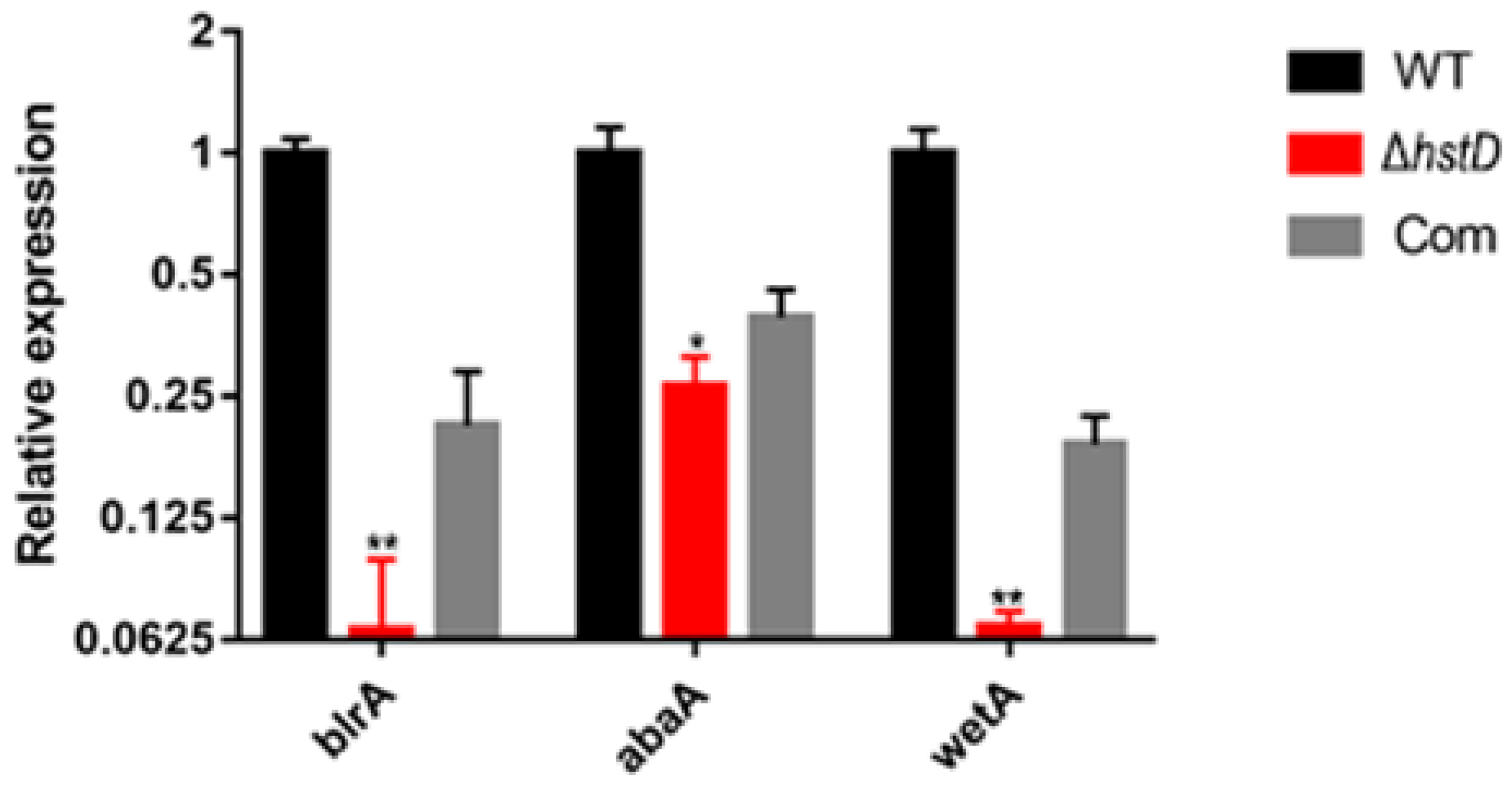
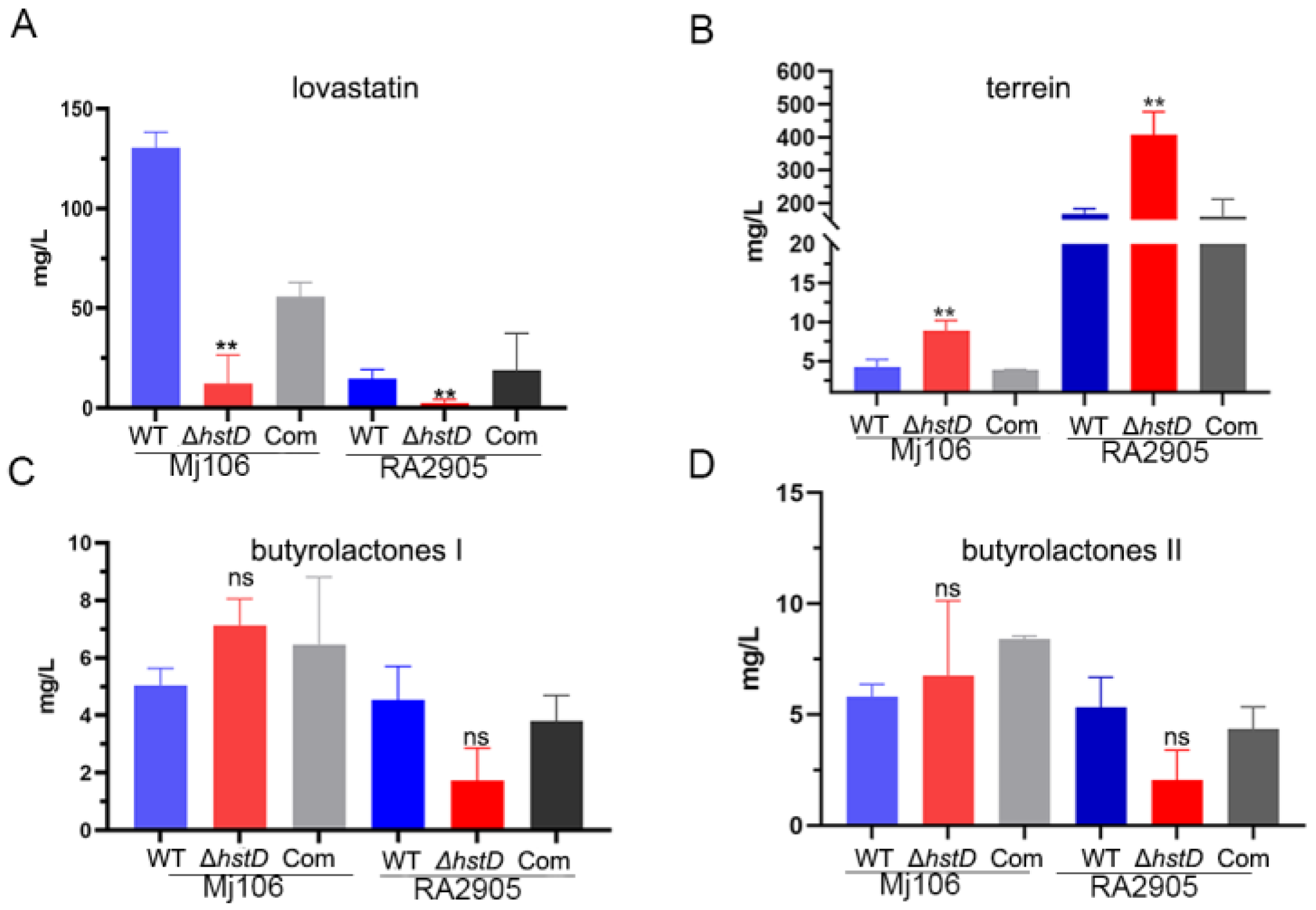
2.4. HstD Is Involved in the Regulation of Secondary Metabolism
2.5. Loss of HstD Does Not Sensitize A. terreus to DNA Damage Agents
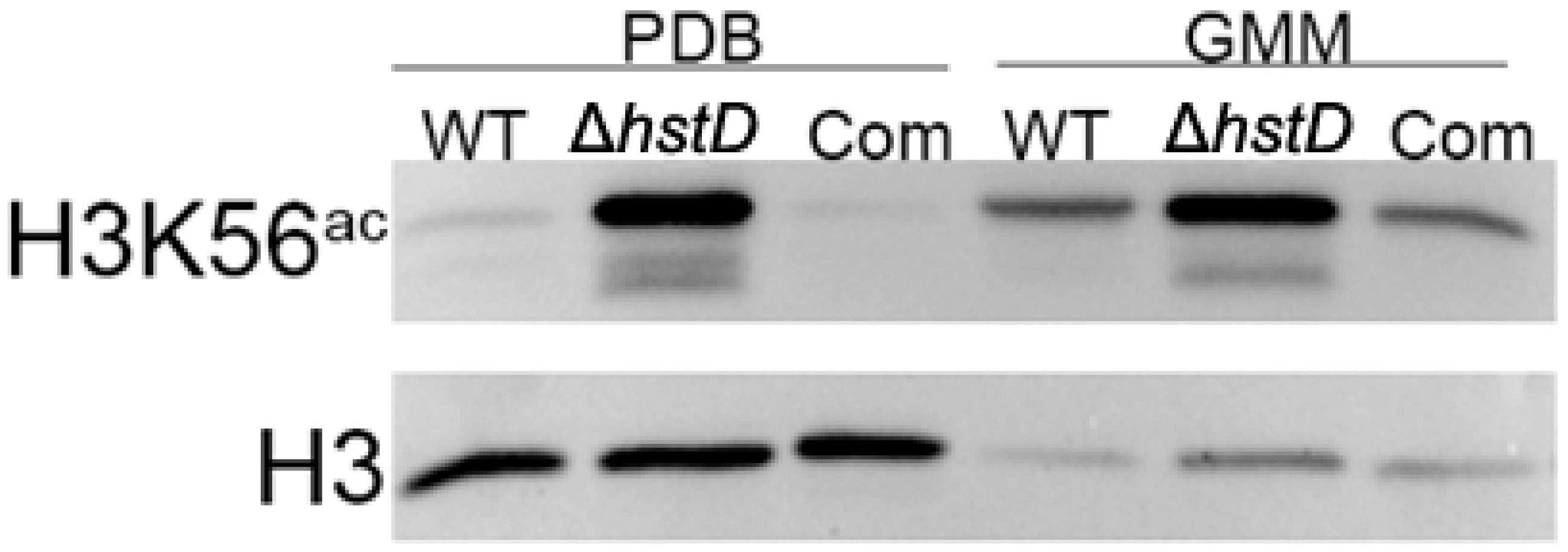
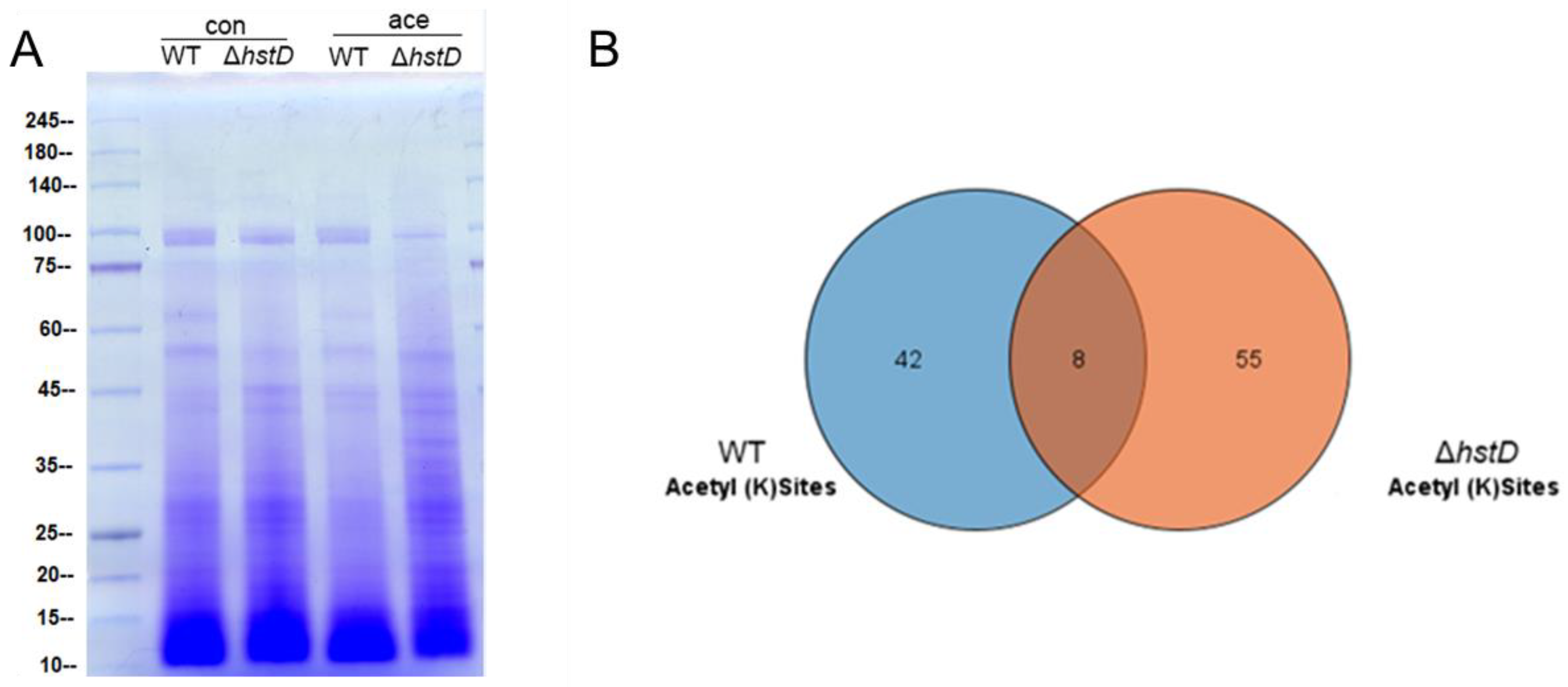
2.6. HstD Catalyzes the Deacetylation of Both Histone and Non-Histone Substances in A. terreus
3. Discussion
4. Materials and Methods
4.1. Bioinformatic Analysis
4.2. Strains and Media
4.3. Construction of hstD Deleted and Complemented Strains
4.4. Histone Acetylation Detection
4.5. Secondary Metabolite Analysis
4.6. RNA Extract, cDNA Synthesis, and Quantitative Real-Time-PCR
4.7. Lysine Acetylome
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mulder, K.C.; Mulinari, F.; Franco, O.L.; Soares, M.S.; Magalhaes, B.S.; Parachin, N.S. Lovastatin production: From molecular basis to industrial process optimization. Biotechnol. Adv. 2015, 33, 648–665. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Lee, H.K.; Park, S.H.; Lee, S.; Ryoo, I.J.; Kim, W.G.; Yoo, I.D.; Na, J.I.; Kwon, S.B.; Park, K.C. Terrein inhibits keratinocyte proliferation via ERK inactivation and G2/M cell cycle arrest. Exp. Dermatol. 2008, 17, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Lee, S.; Lee, H.K.; Park, S.H.; Ryoo, I.J.; Yoo, I.D.; Kwon, S.B.; Baek, K.J.; Na, J.I.; Park, K.C. The hypopigmentary action of KI-063 (a new tyrosinase inhibitor) combined with terrein. J. Pharm. Pharmacol. 2008, 60, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Mijiti, M.; Ding, W.; Song, J.; Yin, Y.; Sun, W.; Li, Z. (+)-Terrein inhibits human hepatoma Bel-7402 proliferation through cell cycle arrest. Oncol. Rep. 2015, 33, 1191–1200. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.Y.; Huang, T.C.; Chen, N.N.; Huang, H.C.; Juan, H.F. Combination therapy targeting ectopic ATP synthase and 26S proteasome induces ER stress in breast cancer cells. Cell Death Dis. 2014, 5, e1540. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Liu, Q.; Zang, X.; Yuan, S.; Bat-Erdene, U.; Nguyen, C.; Gan, J.; Zhou, J.; Jacobsen, S.E.; Tang, Y. Resistance-gene-directed discovery of a natural-product herbicide with a new mode of action. Nature 2018, 559, 415–418. [Google Scholar] [CrossRef]
- Wu, J.S.; Shi, X.H.; Yao, G.S.; Shao, C.L.; Fu, X.M.; Zhang, X.L.; Guan, H.S.; Wang, C.Y. New Thiodiketopiperazine and 3,4-Dihydroisocoumarin Derivatives from the Marine-Derived Fungus Aspergillus terreus. Mar. Drugs 2020, 18, 132. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.S.; Shi, X.H.; Zhang, Y.H.; Shao, C.L.; Fu, X.M.; Li, X.; Yao, G.S.; Wang, C.Y. Benzyl Furanones and Pyrones from the Marine-Derived Fungus Aspergillus terreus Induced by Chemical Epigenetic Modification. Molecules 2020, 25, 3927. [Google Scholar] [CrossRef]
- Pfannenstiel, B.T.; Keller, N.P. On top of biosynthetic gene clusters: How epigenetic machinery influences secondary metabolism in fungi. Biotechnol. Adv. 2019, 37, 107345. [Google Scholar] [CrossRef]
- Cichewicz, R.H. Epigenome manipulation as a pathway to new natural product scaffolds and their congeners. Nat. Prod. Rep. 2010, 27, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Shwab, E.K.; Bok, J.W.; Tribus, M.; Galehr, J.; Graessle, S.; Keller, N.P. Histone deacetylase activity regulates chemical diversity in Aspergillus. Eukaryot. Cell 2007, 6, 1656–1664. [Google Scholar] [CrossRef] [Green Version]
- Wen, M.; Lan, H.; Sun, R.; Chen, X.; Zhang, X.; Zhu, Z.; Tan, C.; Yuan, J.; Wang, S. Histone deacetylase SirE regulates development, DNA damage response and aflatoxin production in Aspergillus flavus. Environ. Microbiol. 2022, 24, 5596–5610. [Google Scholar] [CrossRef]
- Ding, Z.; Zhou, H.; Wang, X.; Huang, H.; Wang, H.; Zhang, R.; Wang, Z.; Han, J. Deletion of the Histone Deacetylase HdaA in Endophytic Fungus Penicillium chrysogenum Fes1701 Induces the Complex Response of Multiple Bioactive Secondary Metabolite Production and Relevant Gene Cluster Expression. Molecules 2020, 25, 3657. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Rusche, L.N. Sirtuins in Epigenetic Silencing and Control of Gene Expression in Model and Pathogenic Fungi. Annu. Rev. Microbiol. 2022, 76, 157–178. [Google Scholar] [CrossRef] [PubMed]
- Blander, G.; Guarente, L. The Sir2 family of protein deacetylases. Annu. Rev. Biochem. 2004, 73, 417–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillus, L.; Rine, J. SIR1 and the origin of epigenetic states in Saccharomyces cerevisiae. Cold Spring Harb. Symp. Quant. Biol. 2004, 69, 259–265. [Google Scholar] [CrossRef] [Green Version]
- Yao, G.; Bai, X.; Zhang, B.; Wang, L.; Chen, S.; Wang, Z. Enhanced production of terrein in marine-derived Aspergillus terreus by refactoring both global and pathway-specific transcription factors. Microb. Cell Fact. 2022, 21, 136. [Google Scholar] [CrossRef] [PubMed]
- Zaehle, C.; Gressler, M.; Shelest, E.; Geib, E.; Hertweck, C.; Brock, M. Terrein biosynthesis in Aspergillus terreus and its impact on phytotoxicity. Chem. Biol. 2014, 21, 719–731. [Google Scholar] [CrossRef] [Green Version]
- Haldar, D.; Kamakaka, R.T. Schizosaccharomyces pombe Hst4 functions in DNA damage response by regulating histone H3 K56 acetylation. Eukaryot. Cell 2008, 7, 800–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celic, I.; Masumoto, H.; Griffith, W.P.; Meluh, P.; Cotter, R.J.; Boeke, J.D.; Verreault, A. The sirtuins hst3 and Hst4p preserve genome integrity by controlling histone h3 lysine 56 deacetylation. Curr. Biol. 2006, 16, 1280–1289. [Google Scholar] [CrossRef] [Green Version]
- Masumoto, H.; Hawke, D.; Kobayashi, R.; Verreault, A. A role for cell-cycle-regulated histone H3 lysine 56 acetylation in the DNA damage response. Nature 2005, 436, 294–298. [Google Scholar] [CrossRef]
- Maas, N.L.; Miller, K.M.; DeFazio, L.G.; Toczyski, D.P. Cell cycle and checkpoint regulation of histone H3 K56 acetylation by Hst3 and Hst4. Mol. Cell 2006, 23, 109–119. [Google Scholar] [CrossRef]
- Henriksen, P.; Wagner, S.A.; Weinert, B.T.; Sharma, S.; Bacinskaja, G.; Rehman, M.; Juffer, A.H.; Walther, T.C.; Lisby, M.; Choudhary, C. Proteome-wide analysis of lysine acetylation suggests its broad regulatory scope in Saccharomyces cerevisiae. Mol. Cell. Proteom. 2012, 11, 1510–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Q.; Tian, L.; Xie, J.T.; Huang, Q.Y.; Feng, M.G.; Keyhani, N.O. A fungal sirtuin modulates development and virulence in the insect pathogen, Beauveria bassiana. Environ. Microbiol. 2021, 23, 5164–5183. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Masuo, S.; Fujita, T.; Doi, Y.; Kamimura, Y.; Takaya, N. Hydrolase controls cellular NAD, sirtuin, and secondary metabolites. Mol. Cell. Biol. 2012, 32, 3743–3755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, M.; Hou, X.; Fu, J.; Zhang, J.; Wang, J.; Zhao, Z.; Xu, D.; Lai, D.; Zhou, L. Recent Advances in Search of Bioactive Secondary Metabolites from Fungi Triggered by Chemical Epigenetic Modifiers. J. Fungi 2023, 9, 172. [Google Scholar] [CrossRef]
- Nogueiras, R.; Habegger, K.M.; Chaudhary, N.; Finan, B.; Banks, A.S.; Dietrich, M.O.; Horvath, T.L.; Sinclair, D.A.; Pfluger, P.T.; Tschop, M.H. Sirtuin 1 and sirtuin 3: Physiological modulators of metabolism. Physiol. Rev. 2012, 92, 1479–1514. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Zheng, Y.; Liu, B.; Gong, Y.; Shao, Y. Mrhst4 gene, coding for NAD+-dependent deacetylase is involved in citrinin production of Monascus ruber. J. Appl. Microbiol. 2023, 134, lxad042. [Google Scholar] [CrossRef]
- Kato, T.; Azegami, J.; Kano, M.; El Enshasy, H.A.; Park, E.Y. Effects of sirtuins on the riboflavin production in Ashbya gossypii. Appl. Microbiol. Biotechnol. 2021, 105, 7813–7823. [Google Scholar] [CrossRef]
- Ibarra, B.A.; Lohmar, J.M.; Satterlee, T.; McDonald, T.; Cary, J.W.; Calvo, A.M. The 14-3-3 Protein Homolog ArtA Regulates Development and Secondary Metabolism in the Opportunistic Plant Pathogen Aspergillus flavus. Appl. Environ. Microbiol. 2018, 84, e02241-17. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, Z.; Keyhani, N.O.; Peng, G.; Jin, K.; Xia, Y. The protein phosphatase gene MaPpt1 acts as a programmer of microcycle conidiation and a negative regulator of UV-B tolerance in Metarhizium acridum. Appl. Microbiol. Biotechnol. 2019, 103, 1351–1362. [Google Scholar] [CrossRef]
- Yao, G.; Chen, X.; Han, Y.; Zheng, H.; Wang, Z.; Chen, J. Development of versatile and efficient genetic tools for the marine-derived fungus Aspergillus terreus RA2905. Curr. Genet. 2022, 68, 153–164. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, G.; Han, N.; Zheng, H.; Wang, L. The Histone Deacetylase HstD Regulates Fungal Growth, Development and Secondary Metabolite Biosynthesis in Aspergillus terreus. Int. J. Mol. Sci. 2023, 24, 12569. https://doi.org/10.3390/ijms241612569
Yao G, Han N, Zheng H, Wang L. The Histone Deacetylase HstD Regulates Fungal Growth, Development and Secondary Metabolite Biosynthesis in Aspergillus terreus. International Journal of Molecular Sciences. 2023; 24(16):12569. https://doi.org/10.3390/ijms241612569
Chicago/Turabian StyleYao, Guangshan, Na Han, Huawei Zheng, and Lu Wang. 2023. "The Histone Deacetylase HstD Regulates Fungal Growth, Development and Secondary Metabolite Biosynthesis in Aspergillus terreus" International Journal of Molecular Sciences 24, no. 16: 12569. https://doi.org/10.3390/ijms241612569




