Non-Thermal Plasma Application in Medicine—Focus on Reactive Species Involvement
Abstract
:1. Introduction
1.1. What Is the Plasma?
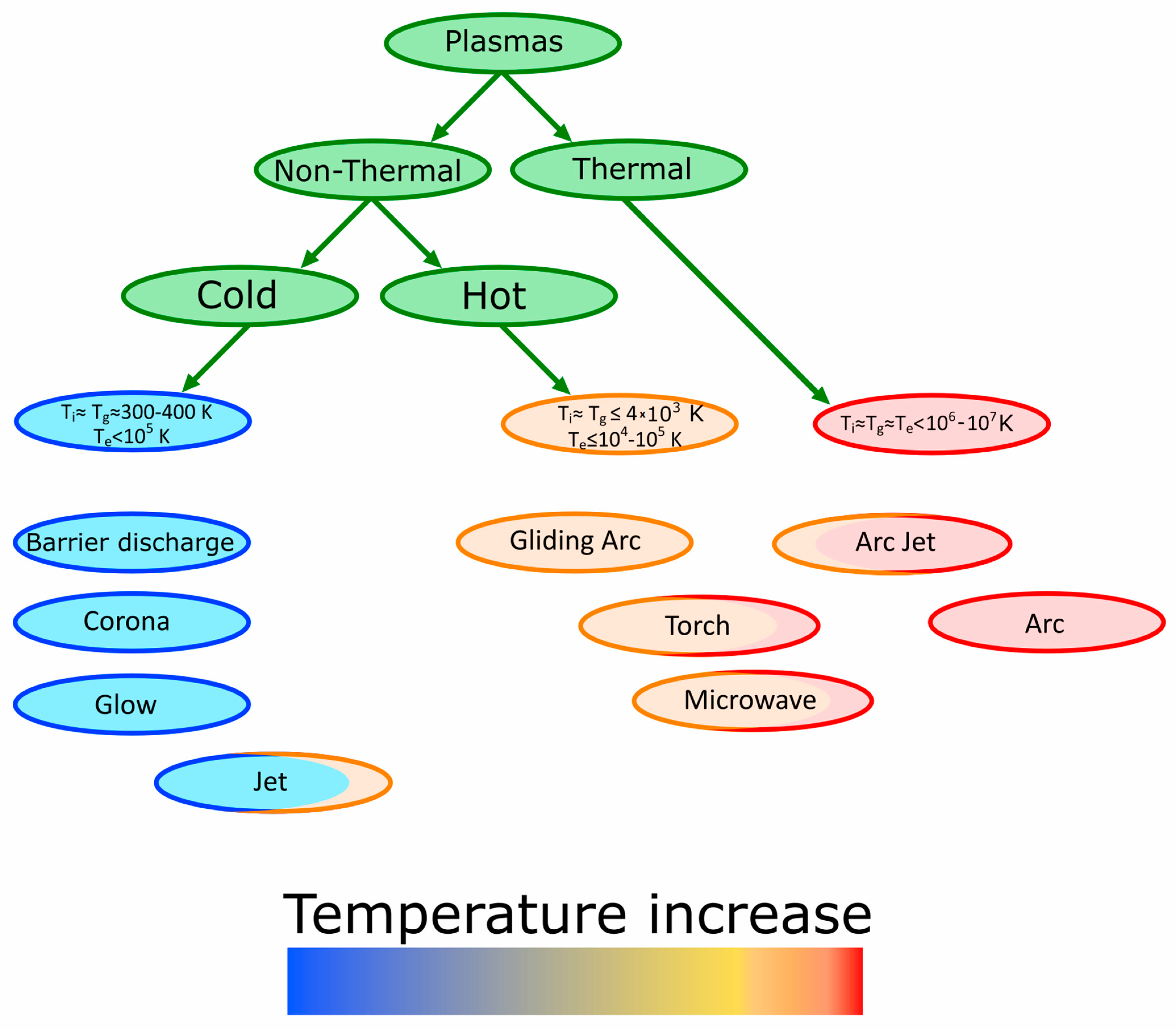
1.2. Types of Plasma
1.3. Reactive Forms in Non-Thermal Plasma
2. Plasma Antiseptic Properties
2.1. Sterilization and Decontamination
2.2. Hemostasis and Wound Healing
2.2.1. In Vitro Studies
2.2.2. In Vivo Studies
2.3. Anti-Viral Activity
3. Scarring and Skin Regeneration
4. Anti-Cancer Therapies
4.1. In Vitro Studies
4.2. In Vivo Studies
4.3. Selectivity
5. Surface Modifications
6. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eliezer, S.; Eliezer, Y. The Fourth State of Matter; Institute of Physics: Bristol, UK, 2001. [Google Scholar]
- Miao, Y.; Yokochi, A.; Jovanovic, G.; Zhang, S.; von Jouanne, A. Application-oriented non-thermal plasma in chemical reaction engineering: A review. Green Energy Resour. 2023, 1, 100004. [Google Scholar] [CrossRef]
- Zanini, M.; Poggio Fraccari, E.A.; Minotti, F.; Grondona, D. Scalable non-thermal plasma trielectrode reactor: A theoretical and experimental study on carbon monoxide removal in a nitrogen atmosphere. Chem. Eng. Sci. 2023, 280, 119014. [Google Scholar] [CrossRef]
- Xu, S.; Chen, H.; Fan, X. Rational design of catalysts for non-thermal plasma (NTP) catalysis: A reflective review. Catal. Today 2023, 419, 114144. [Google Scholar] [CrossRef]
- von Woedtke, T.; Schmidt, A.; Bekeschus, S.; Wende, K.; Weltmann, K.D. Plasma Medicine: A Field of Applied Redox Biology. In Vivo 2019, 33, 1011–1026. [Google Scholar] [CrossRef] [Green Version]
- Mumtaz, S.; Khan, R.; Rana, J.N.; Javed, R.; Iqbal, M.; Choi, E.H.; Han, I. Review on the Biomedical and Environmental Applications of Nonthermal Plasma. Catalysts 2023, 13, 685. [Google Scholar] [CrossRef]
- Pipliya, S.; Kumar, S.; Babar, N.; Srivastav, P.P. Recent trends in non-thermal plasma and plasma activated water: Effect on quality attributes, mechanism of interaction and potential application in food & agriculture. Food Chem. Adv. 2023, 2, 100249. [Google Scholar] [CrossRef]
- Reuter, S.; von Woedtke, T.; Weltmann, K.D. The kINPen—A review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J. Phys. D Appl. Phys. 2018, 51, 233001. [Google Scholar] [CrossRef] [Green Version]
- Samal, S.; Blanco, I. An overview of thermal plasma arc systems for treatment of various wastes in recovery of metals. Materials 2022, 15, 683. [Google Scholar] [CrossRef]
- Hussein, R.; Saifuddin, N.M. A Review of Microwave-Induced Plasma for Production of High Value Products from Waste Glycerol. Res. J. Pharm. Biol. Chem. Sci. 2018, 9, 156. [Google Scholar]
- Kaushik, N.K.; Kaushik, N.; Linh, N.N.; Ghimire, B.; Pengkit, A.; Sornsakdanuphap, J.; Lee, S.-J.; Choi, E.H. Plasma and Nanomaterials: Fabrication and Biomedical Applications. Nanomaterials 2019, 9, 98. [Google Scholar] [CrossRef] [Green Version]
- Rao, Y.; Shang, W.; Yang, Y.; Zhou, R.; Rao, X. Fighting Mixed-Species Microbial Biofilms with Cold Atmospheric Plasma. Front. Microbiol. 2020, 11, 1000. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Kaushik, N.; Moon, I.S.; Choi, E.H.; Kaushik, N.K. Utility of Reactive Species Generation in Plasma Medicine for Neuronal Development. Biomedicines 2020, 8, 348. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell. Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Yayci, A.; Baraibar, Á.G.; Krewing, M.; Fueyo, E.F.; Hollmann, F.; Alcalde, M.; Kourist, R.; Bandow, J.E. Plasma-Driven in Situ Production of Hydrogen Peroxide for Biocatalysis. ChemSusChem 2020, 13, 2072. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.C.; Andriantsitohaina, R. Reactive nitrogen species: Molecular mechanisms and potential significance in health and disease. Antioxid. Redox Signal. 2009, 11, 669–702. [Google Scholar] [CrossRef]
- Zhai, S.Y.; Kong, M.G.; Xia, Y.M. Cold Atmospheric Plasma Ameliorates Skin Diseases Involving Reactive Oxygen/Nitrogen Species-Mediated Functions. Front. Immunol. 2022, 26, 868386. [Google Scholar] [CrossRef]
- Yan, D.; Horkowitz, A.; Wang, Q.; Keidar, M. On the selective killing of cold atmospheric plasma cancer treatment: Status and beyond. Plasma Process. Polym. 2021, 18, 2100020. [Google Scholar] [CrossRef]
- Blázquez-Castro, A. Direct 1O2 optical excitation: A tool for redox biology. Redox Biol. 2017, 13, 39–59. [Google Scholar] [CrossRef]
- Saran, M.; Michel, C.; Bors, W. Reaction of NO with O2−. implications for the action of endothelium-derived relaxing factor (EDRF). Free. Radic. Res. Commun. 1990, 10, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Groh, M. Cancer cell death by cold atmospheric plasma gets a boost with nitrogen supplements. Scilight 2018, 2018, 420003. [Google Scholar] [CrossRef]
- Zver, M.; Dobnik, D.; Zaplotnik, R.; Mozetič, M.; Filipić, A.; Primc, G. Non-thermal plasma inactivation of viruses in water solutions. J. Water Process. Eng. 2023, 53, 103839. [Google Scholar] [CrossRef]
- Kaushik, N.; Mitra, S.; Baek, E.J.; Nguyen, L.N.; Bhartiya, P.; Kim, J.H.; Choi, E.H.; Kaushik, N.K. The inactivation and destruction of viruses by reactive oxygen species generated through physical and cold atmospheric plasma techniques: Current status and perspectives. J. Adv. Res. 2023, 43, 59–71. [Google Scholar] [CrossRef] [PubMed]
- von Woedtke, T.; Reuter, S.; Masur, K.; Weltmann, K.-D. Plasmas for medicine. Phys. Rep. 2013, 530, 291–320. [Google Scholar] [CrossRef]
- Weltmann, K.D.; Polak, M.; Masur, K.; von Woedtke, T.; Winter, J.; Reuter, S. Plasma Processes and Plasma Sources in Medicine. Contrib. Plasma Phys. 2012, 52, 644–654. [Google Scholar] [CrossRef]
- Fridman, G.; Peddinghaus, M.; Balasubramanian, M.; Ayan, H.; Fridman, A.; Gutsol, A.; Brooks, A. Blood Coagulation and Living Tissue Sterilization by Floating-Electrode Dielectric Barrier Discharge in Air. Plasma Chem. Plasma Process. 2006, 26, 425–442. [Google Scholar] [CrossRef]
- Sakudo, A.; Yagyu, Y.; Onodera, T. Disinfection and Sterilization Using Plasma Technology: Fundamentals and Future Perspectives for Biological Applications. Int. J. Mol. Sci. 2019, 20, 5216. [Google Scholar] [CrossRef] [Green Version]
- Mackinder, M.A.; Wang, K.; Zheng, B.; Shrestha, M.; Fan, Q.H. Magnetic field enhanced cold plasma sterilization. Clin. Plasma Med. 2020, 17–18, 100092. [Google Scholar] [CrossRef]
- Mendes-Oliveira, G.; Jensen, J.L.; Keener, K.M.; Campanella, O.S. Modeling the inactivation of Bacillus subtilis spores during cold plasma sterilization. Innov. Food Sci. Emerg. Technol. 2019, 52, 334–342. [Google Scholar] [CrossRef]
- Kim, J.Y.; Song, M.G.; Jeon, E.B.; Choi, E.H.; Lim, J.S.; Park, S.Y. Inactivation of Escherichia coli and Vibrio parahaemolyticus on polypropylene plastic container surfaces by non-thermal dielectric barrier discharge plasma. J. Food Eng. 2023, 338, 111253. [Google Scholar] [CrossRef]
- Patil, S.; Moiseev, T.; Misra, N.N.; Cullen, P.J.; Mosnier, J.P.; Keener, K.M.; Bourke, P. Influence of high voltage atmospheric cold plasma process parameters and role of relative humidity on inactivation of Bacillus atrophaeus spores inside a sealed package. J. Hosp. Infect. 2014, 88, 162–169. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Wang, Y.; Zhao, Y.; Duan, M.; Wang, H.; Dai, R.; Liu, Y.; Li, X.; Jia, F. Inactivation mechanisms of atmospheric pressure plasma jet on Bacillus cereus spores and its application on low-water activity foods. Food Res. Int. 2023, 169, 112867. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-S.; Kim, J.-G. An indoor air purification technology using a non-thermal plasma reactor with multiple-wire-to-wire type electrodes and a fiber air filter. J. Electrost. 2017, 86, 12–17. [Google Scholar] [CrossRef]
- Kyere-Yeboah, K.; Bique, I.K.; Qiao, X.C. Advances of non-thermal plasma discharge technology in degrading recalcitrant wastewater pollutants. A comprehensive review. Chemosphere 2023, 320, 138061. [Google Scholar] [CrossRef] [PubMed]
- Pramila Murugesan, P.; Evanjalin Monica, E.V.; Moses, J.A.; Anandharamakrishnan, C. Water decontamination using non-thermal plasma: Concepts, applications, and prospects. J. Environ. Chem. Eng. 2020, 8, 104377. [Google Scholar] [CrossRef]
- Laroussi, M.; Lu, X. Room-temperature atmospheric pressure plasma plume for biomedical applications. Appl. Phys. Lett. 2005, 87, 113902. [Google Scholar] [CrossRef] [Green Version]
- Bekeschus, S.; von Woedtke, T.; Emmert, S.; Schmidt, A. Medical gas plasma-stimulated wound healing: Evidence and mechanisms. Redox Biol. 2021, 46, 102116. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Bekeschus, S.; Poschkamp, B.; van der Linde, J. Medical gas plasma promotes blood coagulation via platelet activation. Biomaterials 2021, 278, 120433. [Google Scholar] [CrossRef]
- Ikehara, S.; Sakakita, H.; Ishikawy, K.; Akimoto, Y.; Yamaguchi, T.; Yamagishiego, M.; Kim, J.; Ueda, M.; Ikeda, J.I.; Nakanishi, H.; et al. Plasma Blood Coagulation without Involving the Activation of Platelets and Coagulation Factors. Plasma Process. Polym. 2015, 12, 1348–1353. [Google Scholar] [CrossRef]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied Plasma Medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Mohd Nasir, N.; Lee, B.K.; Yap, S.S.; Thong, K.L.; Yap, S.L. Cold plasma inactivation of chronic wound bacteria. Arch. Biochem. Biophys. 2016, 605, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Patinglag, L.; Melling, L.M.; Whitehead, K.A.; Sawtell, D.; Iles, A.; Shaw, K.J. Non-thermal plasma-based inactivation of bacteria in water using a microfluidic reactor. Water Res. 2021, 1, 117321. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.K.; Parab, S.; Alexander, A.; Agrawal, M.; Achalla, V.P.K.; Pal, U.N.; Pandey, M.M.; Kesharwani, P. Cold atmospheric plasma therapy in wound healing. Process Biochem. 2022, 112, 112–123. [Google Scholar] [CrossRef]
- Ferri, E.; Armato, E. Argon plasma coagulation versus cold dissection in pediatric tonsillectomy. Am. J. Otolaryngol. 2011, 32, 459–463. [Google Scholar] [CrossRef]
- Hung, Y.W.; Lee, L.T.; Peng, Y.C.; Chang, C.T.; Wong, Y.K.; Tung, K.C. Effect of a nonthermal-atmospheric pressure plasma jet on wound healing: An animal study. J. Chin. Med. Assoc. 2016, 79, 320–328. [Google Scholar] [CrossRef] [Green Version]
- Chatraie, M.; Torkaman, G.; Khani, M.; Salehi, H.; Shokri, B. In vivo study of non-invasive effects of non-thermal plasma in pressure ulcer treatment. Sci. Rep. 2018, 8, 5621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, P.; Liu, Y.; Li, J.; Zhang, N.; Zhou, M.; Li, Y.; Zhao, G.; Wang, N.; Wang, A.; Wang, Y.; et al. A novel atmospheric-pressure air plasma jet for wound healing. Int. Wound J. 2022, 19, 538–552. [Google Scholar] [CrossRef]
- Busco, G.; Robert, E.; Chettouh-Hammas, N.; Pouvesle, J.M.; Grillon, C. The emerging potential of cold atmospheric plasma in skin biology. Free Radic. Biol. Med. 2020, 161, 290–304. [Google Scholar] [CrossRef]
- Maho, T.; Binois, R.; Brulé-Morabito, F.; Demasure, M.; Douat, C.; Dozias, S.; Escot Bocanegra, P.; Goard, I.; Hocqueloux, L.; Le Helloco, C.; et al. Anti-Bacterial Action of Plasma Multi-Jets in the Context of Chronic Wound Healing. Appl. Sci. 2021, 11, 9598. [Google Scholar] [CrossRef]
- Lehmann, A.; Pietag, F.; Arnold, T. Human health risk evaluation of a microwave-driven atmospheric plasma jet as medical device. Clin. Plasma Med. 2017, 7, 16–23. [Google Scholar] [CrossRef]
- Lata, S.; Chakravorty, S.; Mitra, T.; Pradhan, P.K.; Mohanty, S.; Patel, P.; Jha, E.; Panda, P.K.; Verma, S.K.; Suar, M. Aurora Borealis in dentistry: The applications of cold plasma in biomedicine. Mater. Today Bio. 2021, 13, 100200. [Google Scholar] [CrossRef] [PubMed]
- Daeschlein, G.; Scholz, S.; Arnold, A.; von Podewils, S.; Haase, H.; Emmert, S.; von Woedtke, T.; Weltmann, K.-D.; Jünger, M. In Vitro Susceptibility of Important Skin and Wound Pathogens Against Low Temperature Atmospheric Pressure Plasma Jet (APPJ) and Dielectric Barrier Discharge Plasma (DBD). Plasma Process. Polym. 2012, 9, 380–389. [Google Scholar] [CrossRef]
- Eguiluz, R.P.; López-Callejas, R.; González-Arciniega, E.; Rodríguez-Méndez, B.G.; Mercado-Cabrera, A.; Guakil-Haber, A.; García, A.K.; Espinosa Mancilla, A.E.; Valencia-Alvarado, R. Non-thermal plasma wound healing after removal of a neck tumor in a patient with HIV: A case report. Otolaryngol. Case Rep. 2022, 22, 100391. [Google Scholar] [CrossRef]
- Assadi, I.; Guesmi, A.; Baaloudj, O.; Zeghioud, H.; Elfalleh, W.; Benhammadi, N.; Khezami, L.; Assadi, A.A. Review on inactivation of airborne viruses using non-thermal plasma technologies: From MS2 to coronavirus. Environ. Sci. Pollut. Res. 2021, 29, 4880–4892. [Google Scholar] [CrossRef] [PubMed]
- Daeschlein, G.; Scholz, S.; Ahmed, R.; von Woedtke, T.; Haase, H.; Niggemeier, M.; Kindel, E.; Brandenburg, R.; Weltmann, K.-D.; Juenger, M. Skin decontamination by low-temperature atmospheric pressure plasma jet and dielectric barrier discharge plasma. J. Hosp. Infect. 2012, 81, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Bong, C.; Lim, W.; Bae, P.K.; Abafogi, A.T.; Baek, S.H.; Shin, Y.-B.; Bak, M.S.; Park, S. Fast and Easy Disinfection of Coronavirus-Contaminated Face Masks Using Ozone Gas Produced by a Dielectric Barrier Discharge Plasma Generator. Environ. Sci. Technol. Lett. 2021, 19, 1c00089. [Google Scholar] [CrossRef]
- Han, I.; Mumtaz, S.; Choi, E.H. Nonthermal Biocompatible Plasma Inactivation of Coronavirus SARS-CoV-2: Prospects for Future Antiviral Applications. Viruses 2022, 14, 2685. [Google Scholar] [CrossRef]
- Metelmann, H.-R.; Vu, T.T.; Do, H.T.; Le, T.N.B.; Hoang, T.H.A.; Phi, T.T.T.; Luong, T.M.L.; Doan, V.T.; Nguyen, T.T.H.; Nguyen, T.L.; et al. Scar formation of laser skin lesions after cold atmospheric pressure plasma (CAP) treatment: A clinical long term observation. Clin. Plasma Med. 2013, 1, 30–35. [Google Scholar] [CrossRef]
- Vasilets, V.N.; Shekhter, A.B.; Guller, A.E.; Pekshev, A.V. Air plasma-generated nitric oxide in treatment of skin scars and articular musculoskeletal disorders: Preliminary review of observations. Clin. Plasma Med. 2015, 3, 32–39. [Google Scholar] [CrossRef]
- Rad, Z.S.; Davani, F.A. Measurements of the electrical parameters and wound area for investigation on the effect of different non-thermal atmospheric pressure plasma sources on wound healing time. Measurement 2020, 155, 107545. [Google Scholar] [CrossRef]
- Liu, P.; Wang, G.; Ruan, Q.; Tang, K.; Chu, P.K. Plasma-activated interfaces for biomedical engineering. Bioact Mater. 2021, 6, 2134–2143. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Sassning, S.; Lademann, O.; Darvin, M.E.; Schanzer, S.; Kramer, A.; Richter, H.; Sterry, W.; Lademann, J. In vivo skin treatment with tissue-tolerable plasma influences skin physiology and antioxidant profile in human stratum corneum. Exp. Dermatol. 2012, 21, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Athanasopoulos, D.K.; Svarnas, P.; Gerakis, A. Cold plasma bullet influence on the water contact angle of human skin surface. J. Electrost. 2019, 102, 103378. [Google Scholar] [CrossRef]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of pH on wound-healing: A new perspective for wound-therapy? Arch. Dermatol. Res. 2006, 298, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Brisset, J.L.; Benstaali, B.; Moussa, D.; Fanmoe, J.; Njoyim-Tamungang, E. Acidity control of plasma-chemical oxidation: Applications to dye removal, urban waste abatement and microbial inactivation. Plasma Sources Sci. Technol. 2011, 20, 034021. [Google Scholar] [CrossRef]
- Soleymani, T.; Lanoue, J.; Rahman, Z. A Practical Approach to Chemical Peels: A Review of Fundamentals and Step-by-step Algorithmic Protocol for Treatment. J. Clin. Aesthet Dermatol. 2018, 11, 21–28. [Google Scholar]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020; Available online: https://gco.iarc.fr/today (accessed on 6 July 2023).
- Thomas, G.; Eisenhauer, E.; Bristow, R.G.; Grau, C.; Hurkmans, C.; Ost, P.; Guckenberger, M.; Deutsch, E.; Lacombe, D.; Weber, D.C.; et al. The European Organisation for Research and Treatment of Cancer, State of Science in radiation oncology and priorities for clinical trials meeting report. Eur. J. Cancer 2020, 131, 76–88. [Google Scholar] [CrossRef]
- Sander Bekeschus, S. Medical gas plasma technology: Roadmap on cancer treatment and immunotherapy. Redox Biol. 2023, 65, 102798. [Google Scholar] [CrossRef]
- Gelbrich, N.; Miebach, L.; Berner, J.; Freund, E.; Saadati, F.; Schmidt, A.; Stope, M.; Zimmermann, U.; Burchardt, M.; Bekeschus, S. Medical gas plasma augments bladder cancer cell toxicity in preclinical models and patient-derived tumor tissues. J. Adv. Res. 2023, 47, 209–223. [Google Scholar] [CrossRef]
- Guo, B.; Pomicter, A.D.; Li, F.; Bhatt, S.; Chen, C.; Li, W.; Qi, M.; Huang, C.; Deininger, M.W.; Kong, M.G.; et al. Trident cold atmospheric plasma blocks three cancer survival pathways to overcome therapy resistance. Proc. Natl. Acad. Sci. USA 2021, 118, e2107220118. [Google Scholar] [CrossRef]
- Chen, B.; Jin, T.; Fu, Z.; Li, H.; Yang, J.; Liu, Y.; Han, Y.; Wang, X.; Wu, Z.; Xu, T. Non-thermal plasma-treated melatonin inhibits the biological activity of HCC cells by increasing intracellular ROS levels and reducing RRM2 expression. Heliyon 2023, 9, e15992. [Google Scholar] [CrossRef] [PubMed]
- Walk, R.M.; Snyder, J.A.; Srinivasan, P.; Kirsch, J.; Diaz, S.O.; Blanco, F.C.; Shashurin, A.; Keidar, M.; Sandler, A.D. Cold atmospheric plasma for the ablative treatment of neuroblastoma. J. Pediatr. Surg. 2013, 48, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Partecke, L.I.; Evert, K.; Haugk, J.; Doering, F.; Normann, L.; Diedrich, S.; Weiss, F.U.; Evert, M.; Huebner, N.O.; Guenther, C.; et al. Tissue tolerable plasma (TTP) induces apoptosis in pancreatic cancer cells in vitro and in vivo. BMC Cancer 2012, 12, 473. [Google Scholar] [CrossRef] [Green Version]
- Shakya, A.; Baniya, H.; Pradhan, S.; Basnet, N.; Adhikari, R.; Subedi, D.; Regmi, S. Cold Plasma as a Practical Approach to Cancer Treatment. Plasma Med. 2023, 12, 57–73. [Google Scholar] [CrossRef]
- Eto, K.; Ishinada, C.; Suemoto, T.; Hyakutake, K.; Tanaka, H.; Hori, M. A novel and distinctive mode of cell death revealed by using non-thermal atmospheric pressure plasma: The involvements of reactive oxygen species and the translation inhibitor Pdcd4. Chem Biol Interact. 2021, 1, 109403. [Google Scholar] [CrossRef]
- Attri, P.; Kaushik, N.K.; Kaushik, N.; Hammerschmid, D.; Privat-Maldonado, A.; De Backer, J.; Shiratani, M.; Choi, E.H.; Bogaerts, A. Plasma treatment causes structural modifications in lysozyme, and increases cytotoxicity towards cancer cells. Int. J. Biol. Macromol. 2021, 1, 1724–1736. [Google Scholar] [CrossRef] [PubMed]
- De Backer, J.; Lin, A.; Berghe, W.V.; Bogaerts, A.; Hoogewijs, D. Cytoglobin inhibits non-thermal plasma-induced apoptosis in melanoma cells through regulation of the NRF2-mediated antioxidant response. Redox Biol. 2022, 14, 102399. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Talbot, A.; Nourmohammadi, N.; Cheng, X.; Canady, J.; Sherman, J.; Keidar, M. Principles of using Cold Atmospheric Plasma Stimulated Media for Cancer Treatment. Sci. Rep. 2015, 5, 18339. [Google Scholar] [CrossRef] [Green Version]
- Vandamme, M.; Robert, E.; Dozias, S.; Sobilo, J.; Lerondel, S.; Le Pape, A.; Pouvesle, J.M. Response of Human Glioma U87 Xenografted on Mice to Non Thermal Plasma Treatment. Plasma Med. 2011, 1, 27–43. [Google Scholar] [CrossRef] [Green Version]
- Lin, A.; De Backer, J.; Quatannens, D.; Cuypers, B.; Verswyvel, H.; De La Hoz, E.C.; Ribbens, B.; Siozopoulou, V.; Van Audenaerde, J.; Marcq, E.; et al. The effect of local non-thermal plasma therapy on the cancer-immunity cycle in a melanoma mouse model. Bioeng. Transl. Med. 2022, 7, e10314. [Google Scholar] [CrossRef]
- Oliveri, V. Selective Targeting of Cancer Cells by Copper Ionophores: An Overview. Frontiers in Molecular Biosciences. 2022, 9, 841814. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, J.; Köritzer, J.; Boxhammer, V. Plasma in cancer treatment. Clin. Plasma Med. 2013, 1, 2–7. [Google Scholar] [CrossRef]
- Keidar, M.; Beilis, I.I. Chapter 7—Plasma Medicine. In Plasma Engineering, 2nd ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 455–539. [Google Scholar]
- Keidar, M.; Walk, R.; Shashurin, A.; Srinivasan, P.; Sandler, A.; Dasgupta, S.; Ravi, R.; Guerrero-Preston, R.; Trink, B. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br. J. Cancer 2011, 105, 1295–1301. [Google Scholar] [CrossRef]
- Leśniak-Ziółkowska, K.; Brodacz, K.; Babilas, D.; Dulski, M.; Blacha-Grzechnik, A.; Lu, X.; Kazek-Kęsik, A.; Simka, W. Fungistatic and bacteriostatic Mg-enriched oxide coatings on titanium formed via plasma electrolytic oxidation. Appl. Surf. Sci. 2013, 615, 156285. [Google Scholar] [CrossRef]
- Zheng, Z.; He, Y.; Long, L.; Gan, S.; Chen, S.; Zhang, M.; Xu, J.; Fu, R.; Liao, Y.; Zhu, Z.; et al. Involvement of PI3K/Akt signaling pathway in promoting osteogenesis on titanium implant surfaces modified with novel non-thermal atmospheric plasma. Front. Bioeng. Biotechnol. 2022, 16, 975840. [Google Scholar] [CrossRef] [PubMed]
- Finke, B.; Hempel, F.; Testrich, H.; Artemenko, A.; Rebl, H.; Kylián, O.; Meichsner, J.; Biederman, H.; Nebe, B.; Weltmann, K.-D.; et al. Plasma processes for cell-adhesive titanium surfaces based on nitrogen-containing coatings. Surf. Coat. Technol. 2011, 205, S520–S524. [Google Scholar] [CrossRef]
- Muro-Fraguas, I.; Sainz-García, A.; López, M.; Rojo-Bezares, B.; Múgica-Vidal, R.; Sainz-García, E.; Toledano, P.; Sáenz, Y.; González-Marcos, A.; Alba-Elías, F. Antibiofilm coatings through atmospheric pressure plasma for 3D printed surgical instruments. Surf. Coat. Technol. 2020, 399, 126163. [Google Scholar] [CrossRef]






| Lifetime | Reactive Form | Action |
|---|---|---|
| Short-lived | ·OH | Destroying of many vital molecules, including DNA, RNA, proteins, and phospholipids |
| ONOO− | Damaging the function of antioxidant enzymes and other molecules | |
| 1O2/·O2– | Cell proliferation or tissue regeneration | |
| Long-lived | H2O2 | Oxidation on many proteins and DNA |
| NO2–/NO3– | Increasing of ONOO− production | |
| O3 | It has a genotoxic and cytotoxic effect | |
| NO | Causes oxidative stress, disrupted energy metabolism, DNA damage, activation of poly(ADP-ribose) polymerase, or dysregulation of cytosolic calcium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moszczyńska, J.; Roszek, K.; Wiśniewski, M. Non-Thermal Plasma Application in Medicine—Focus on Reactive Species Involvement. Int. J. Mol. Sci. 2023, 24, 12667. https://doi.org/10.3390/ijms241612667
Moszczyńska J, Roszek K, Wiśniewski M. Non-Thermal Plasma Application in Medicine—Focus on Reactive Species Involvement. International Journal of Molecular Sciences. 2023; 24(16):12667. https://doi.org/10.3390/ijms241612667
Chicago/Turabian StyleMoszczyńska, Julia, Katarzyna Roszek, and Marek Wiśniewski. 2023. "Non-Thermal Plasma Application in Medicine—Focus on Reactive Species Involvement" International Journal of Molecular Sciences 24, no. 16: 12667. https://doi.org/10.3390/ijms241612667
APA StyleMoszczyńska, J., Roszek, K., & Wiśniewski, M. (2023). Non-Thermal Plasma Application in Medicine—Focus on Reactive Species Involvement. International Journal of Molecular Sciences, 24(16), 12667. https://doi.org/10.3390/ijms241612667








