The Neglected Uterine NK Cells/Hamperl Cells/Endometrial Stromal Granular Cell, or K Cells: A Narrative Review from History through Histology and to Medical Education
Abstract
1. Introduction
2. Historical Overview of the Uterine Natural Killer (uNK) Cell’s Discovery
3. Terminological Confusions around uNK Cells
4. Current Histological Knowledge and Immunohistochemistry of uNK Cells
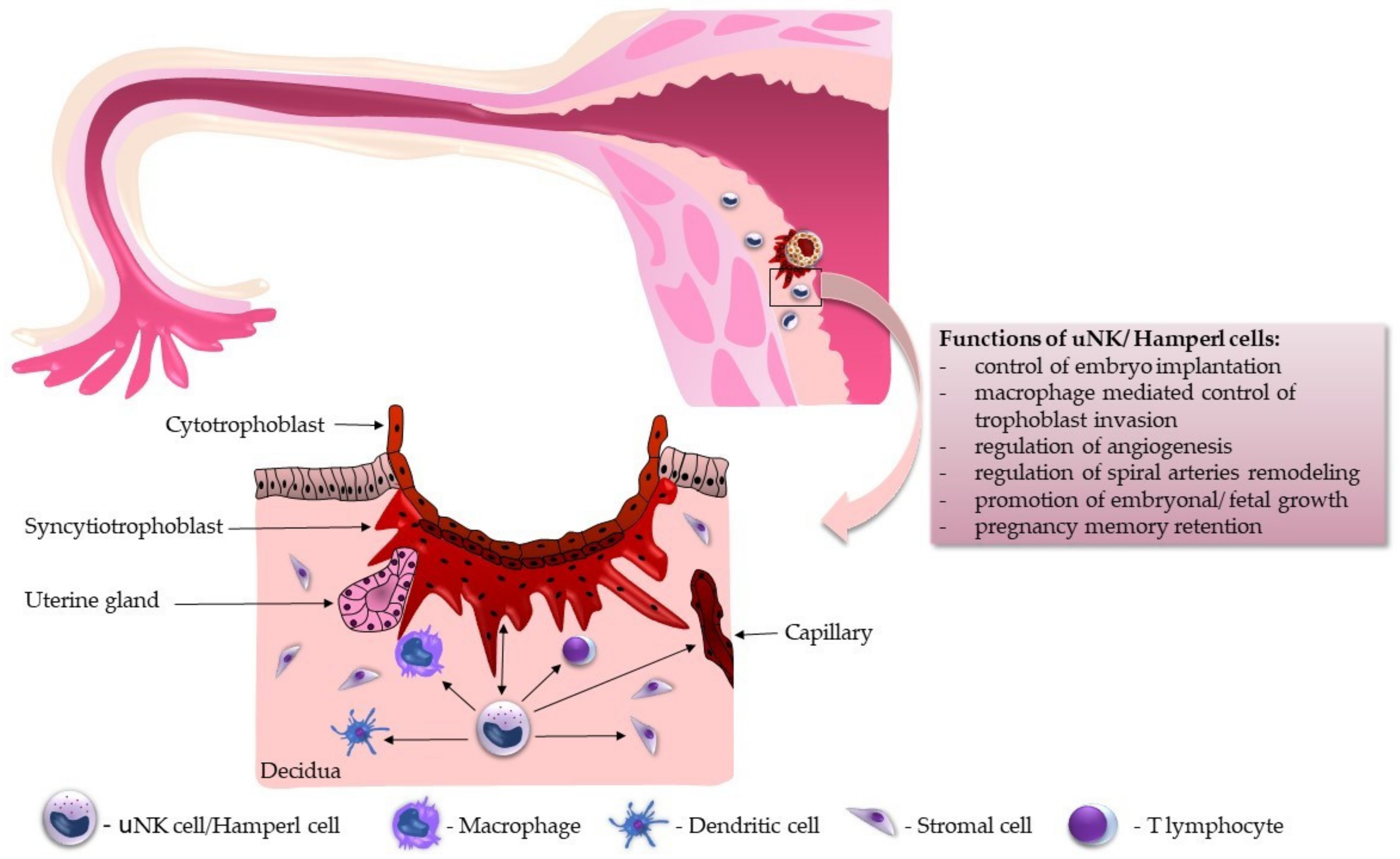
5. Functional Overview of uNK Cells during Implantation
6. Current Knowledge of uNK Cells in Histology and Embryology Textbooks
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maxwell, A.J.; You, Y.; Aldo, P.B.; Zhang, Y.; Ding, J.; Mor, G. The role of immune system during pregnancy: General concepts. In Reproductive Immunology; Mor, G., Ed.; Elsevier Academic Press: London, UK, 2021; Volume 1, pp. 1–21. [Google Scholar]
- Lamps, L.W.; Quick, C.M.; Chang, A.; McKenney, J.K.; Cox, R.M. Diagnostic Pathology: Normal Histology, 1st ed.; Lamps, L.W., Ed.; Amirsys Publishing, Inc.: Salt Lake City, UT, USA, 2013. [Google Scholar]
- Flynn, L.; Byrne, B.; Carton, J.; Kelehan, P.; O’Herlihy, C.; O’Farrelly, C. Menstrual cycle dependent fluctuations in NK and T-lymphocyte subsets from non-pregnant human endometrium. Am. J. Reprod. Immunol. 2000, 43, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Wei, H. Natural killer cells in reproduction: Before, during and after pregnancy. In Reproductive Immunology; Mor, G., Ed.; Elsevier Academic Press: London, UK, 2021; Volume 1, pp. 55–72. [Google Scholar]
- Kanter, J.R.; Mani, S.; Gordon, S.M.; Mainigi, M. Uterine natural killer cell biology and role in early pregnancy establishment and outcomes. FS Rev. 2021, 2, 265–286. [Google Scholar] [CrossRef] [PubMed]
- Co, E.C.; Gormley, M.; Kapidzic, M.; Rosen, D.B.; Scott, M.A.; Stolp, H.A.; McMaster, M.; Lanier, L.L.; Bárcena, A.; Fisher, S.J. Maternal decidual macrophages inhibit NK cell killing of invasive cytotrophoblasts during human pregnancy. Biol. Reprod. 2013, 88, 155. [Google Scholar] [CrossRef]
- Crespo, A.C.; Strominger, J.L.; Tilburgs, T. Expression of KIR2DS1 by Decidual Natural Killer Cells Increases Their Ability to Control Placental HCMV Infection. Proc. Natl. Acad. Sci. USA 2016, 113, 15072–15077. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Su, X.; Zhao, Y.; Tang, L.; Chen, J.; Zhong, G. Innate Lymphoid Cells Are Required for Endometrial Resistance to Chlamydia trachomatis Infection. Infect. Immun. 2020, 88, e00152-20. [Google Scholar] [CrossRef]
- Kitazawa, J.; Kimura, F.; Nakamura, A.; Morimune, A.; Takahashi, A.; Takashima, A.; Amano, T.; Tsuji, S.; Kaku, S.; Kasahara, K.; et al. Endometrial Immunity for Embryo Implantation and Pregnancy Establishment. Tohoku J. Exp. Med. 2020, 250, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Goldman-Wohl, D.; Gamliel, M.; Mandelboim, O.; Yagel, S. Learning from experience: Cellular and molecular bases for improved outcome in subsequent pregnancies. Am. J. Obstet. Gynecol. 2019, 221, 183–193. [Google Scholar] [CrossRef]
- Male, V.; Moffett, A. Natural Killer Cells in the Human Uterine Mucosa. Annu. Rev. Immunol. 2023, 41, 127–151. [Google Scholar] [CrossRef]
- Gross, D.; Kaiser, S.; Gräf, C.; Uhlendahl, H.; Schmidt, M. Between fiction and reality: Herwig Hamperl (1899–1976) and the Third Reich as reflected in his autobiography. Pathol. Res. Pract. 2019, 215, 832–841. [Google Scholar] [CrossRef]
- Haitinger, M.; Hamperl, H. Die Anwendung des Fluoreszenzmikroskops zur Untersuchung tierischer Gewebe. Ztschr. Mikro. Anat. 1933, 33, 193–221. [Google Scholar]
- Hamperl, H. Über fluorescierende Körnchenzellen (“Fluorocyten”). Virchows Arch. Pathol. Anat. Physiol. Klin. Med. 1950, 318, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Marchand, F. On the so-called decidual tumors following normal childbirth, hydatidiform mole and ex-trauterine pregnancy. Obstet. Gynecol. 1895, 1, 419. [Google Scholar]
- Weill, P. Etudes sur les leucocytes.I.Les cellules des muqueuses intestinale et uterines. Arch. Anat. Microsc. 1921, 17, 77–82. [Google Scholar]
- Hellweg, G. Über körnchenhaltige Zellen im menschlichen und tierischen Endometrium (endometriale Körnchenzellen, metachromasierende Zellen). Z. Zellforsch. Mikrosk. Anat. 1959, 49, 555–568. [Google Scholar] [CrossRef]
- Hamperl, H. Über endometriale Granulocyten (endometriale Körnchenzellen). Klin. Wochenschr. 1954, 32, 665–668. [Google Scholar] [CrossRef]
- Hellweg, G. Untersuchungen zur Charakterisierung der Granula in endometrialen Körnchenzellen. Virchows Arch. Pathol. Anat. Physiol. Klin. Med. 1956, 329, 111–120. [Google Scholar] [CrossRef]
- Hamperl, H.; Hellweg, G. Granular endometrial stroma cells. Obstet. Gynecol. Surv. 1958, 13, 891–893. [Google Scholar] [CrossRef]
- Bulmer, J.N.; Pace, D.; Ritson, A. Immunoregulatory cells in human decidua: Morphology, immunohistochemistry and function. Reprod. Nutr. Dev. 1988, 28, 1599–1614. [Google Scholar] [CrossRef]
- Starkey, P.M.; Sargent, I.L.; Redman, C.W. Cell populations in human early pregnancy decidua: Characterization and isolation of large granular lymphocytes by flow cytometry. Immunology 1988, 65, 129–134. [Google Scholar]
- King, A.; Wellings, V.; Gardner, L.; Loke, Y.W. Immunocytochemical characterization of the unusual large granular lymphocytes in human endometrium throughout the menstrual cycle. Hum. Immunol. 1989, 24, 195–205. [Google Scholar] [CrossRef]
- Starkey, P.M.; Clover, L.M.; Rees, M.C. Variation during the menstrual cycle of immune cell populations in human endometrium. Eur. J. Obstet. Gynecol. Reprod. Biol. 1991, 39, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Bulmer, J.N.; Morrison, L.; Longfellow, M.; Ritson, A.; Pace, D. Granulated lymphocytes in human endometrium: Histochemical and immunohistochemical studies. Hum. Reprod. 1991, 6, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Croy, B.A.; Waterfield, A.; Wood, W.; King, G.J. Normal murine and porcine embryos recruit NK cells to the uterus. Cell. Immunol. 1988, 115, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Parr, E.L.; Parr, M.B.; Zheng, L.M.; Young, J.D. Mouse granulated metrial gland cells originate by local activation of uterine natural killer lymphocytes. Biol. Reprod. 1991, 44, 834–841. [Google Scholar] [CrossRef]
- King, A.; Balendran, N.; Wooding, P.; Carter, N.P.; Loke, Y.W. CD3− leukocytes present in the human uterus during early placentation: Phenotypic and morphologic characterization of the CD56++ population. Dev. Immunol. 1991, 1, 169–190. [Google Scholar] [CrossRef]
- Burdan, F.; Dworzański, W.; Cendrowska-Pinkosz, M.; Burdan, M.; Dworzańska, A. Anatomical eponyms—unloved names in medical terminology. Folia Morphol. 2016, 75, 413–438. [Google Scholar] [CrossRef]
- Winkelmann, A. Should we teach Abernethy and Zuckerkandl? Clin. Anat. 2012, 25, 241–245. [Google Scholar] [CrossRef]
- Fadare, O.; Roma, A.A. Atlas of Uterine Pathology; Springer Nature: Berlin/Heidelberg, Germany, 2019; p. 269. [Google Scholar]
- Young, B.; O’Dowd, G.; Woodford, P. Wheaters’s Functional Histology, 6th ed.; Elsevier Churchill Livingstone: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Mills, S.E. (Ed.) Histology for Pathologists, 5th ed.; Wolters Kluwer: Tokyo, Japan, 2020. [Google Scholar]
- FIPAT. Terminologia Histologica: International Terms for Human Cytology and Histology, 1st ed.; Lippincott Raven: Philadelphia, PA, USA, 2008. [Google Scholar]
- Moore, K.L.; Persaud, T.V.N.; Torchia, M.G. The developing human. In Clinically Oriented Embryology, 11th ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Male, V.; Sharkey, A.; Masters, L.; Kennedy, P.R.; Farrell, L.E.; Moffett, A. The effect of pregnancy on the uterine NK cell KIR repertoire. Eur. J. Immunol. 2011, 41, 3017–3027. [Google Scholar] [CrossRef]
- Xie, M.; Li, Y.; Meng, Y.Z.; Xu, P.; Yang, Y.G.; Dong, S.; He, J.; Hu, Z. Uterine Natural Killer Cells: A Rising Star in Human Pregnancy Regulation. Front. Immunol. 2022, 13, 918550. [Google Scholar] [CrossRef]
- Huhn, O.; Zhao, X.; Esposito, L.; Moffett, A.; Colucci, F.; Sharkey, A.M. How Do Uterine Natural Killer and Innate Lymphoid Cells Contribute to Successful Pregnancy? Front. Immunol. 2021, 12, 607669. [Google Scholar] [CrossRef]
- Bulmer, J.N.; Lash, G.E. The Role of Uterine NK Cells in Normal Reproduction and Reproductive Disorders. Adv. Exp. Med. Biol. 2015, 868, 95–126. [Google Scholar] [PubMed]
- Pace, D.; Morrison, L.; Bulmer, J.N. Proliferative activity in endometrial stromal granulocytes throughout menstrual cycle and early pregnancy. J. Clin. Pathol. 1989, 42, 35–39. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Loke, Y.W. Human trophoblast and JEG choriocarcinoma cells are sensitive to lysis by IL-2-stimulated decidual NK cells. Cell. Immunol. 1990, 129, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Croy, B.A.; Kiso, Y. Granulated metrial gland cells: A natural killer cell subset of the pregnant murine uterus. Microsc. Res. Tech. 1993, 25, 189–200. [Google Scholar] [CrossRef]
- Goodridge, J.P.; Jacobs, B.; Saetersmoen, M.L.; Clement, D.; Hammer, Q.; Clancy, T.; Skarpen, E.; Brech, A.; Landskron, J.; Grimm, C.; et al. Remodeling of secretory lysosomes during education tunes functional potential in NK cells. Nat. Commun. 2019, 10, 514. [Google Scholar] [CrossRef]
- Huhn, O.; Ivarsson, M.A.; Gardner, L.; Hollinshead, M.; Stinchcombe, J.C.; Chen, P.; Shreeve, N.; Chazara, O.; Farrell, L.E.; Theorell, J.; et al. Distinctive phenotypes and functions of innate lymphoid cells in human decidua during early pregnancy. Nat. Commun. 2020, 11, 381. [Google Scholar] [CrossRef]
- Sojka, D.K.; Tian, Z.; Yokoyama, W.M. Tissue-resident natural killer cells and their potential diversity. Semin. Immunol. 2014, 26, 127–131. [Google Scholar] [CrossRef]
- Haugstøyl, M.E.; Cornillet, M.; Strand, K.; Stiglund, N.; Sun, D.; Lawrence-Archer, L.; Hjellestad, I.D.; Busch, C.; Mellgren, G.; Björkström, N.K.; et al. Phenotypic diversity of human adipose tissue-resident NK cells in obesity. Front. Immunol. 2023, 14, 1130370. [Google Scholar] [CrossRef]
- Bulmer, J.N.; Sunderland, C.A. Immunohistological characterization of lymphoid cell populations in the early human placental bed. Immunology 1984, 52, 349–357. [Google Scholar]
- Bulmer, J.N.; Hollings, D.; Ritson, A. Immunocytochemical evidence that endometrial stromal granulocytes are granulated lymphocytes. J. Pathol. 1987, 153, 281–288. [Google Scholar] [CrossRef]
- Bulmer, J.N.; Lunny, D.P.; Hagin, S.V. Immunohistochemical characterization of stromal leucocytes in non-pregnant human endometrium. Am. J. Reprod. Immunol. Microbiol. 1988, 17, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Ritson, A.; Bulmer, J.N. Endometrial granulocytes in human decidua react with a natural-killer (NK) cell marker, NKH1. Immunology 1987, 62, 329–331. [Google Scholar]
- Manaster, I.; Mandelboim, O. The unique properties of uterine NK cells. Am. J. Reprod. Immunol. 2010, 63, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Vento-Tormo, R.; Efremova, M.; Botting, R.A.; Turco, M.Y.; Vento-Tormo, M.; Meyer, K.B.; Park, J.E.; Stephenson, E.; Polański, K.; Goncalves, A.; et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 2018, 563, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Croy, B.A.; Zhang, J.; Tayade, C.; Colucci, F.; Yadi, H.; Yamada, A.T. Analysis of uterine natural killer cells in mice. Methods Mol. Biol. 2010, 612, 465–503. [Google Scholar] [PubMed]
- Kusakabe, K.; Okada, T.; Sasaki, F.; Kiso, Y. Cell death of uterine natural killer cells in murine placenta during placentation and preterm periods. J. Vet. Med. Sci. 1999, 61, 1093–1100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lapides, L.; Klein, M.; Belušáková, V.; Csöbönyeiová, M.; Varga, I.; Babál, P. Uterine Natural Killer Cells in the Context of Implantation: Immunohistochemical Analysis of Endometrial Samples from Women with Habitual Abortion and Recurrent Implantation Failure. Physiol. Res. 2022, 71, S99–S105. [Google Scholar] [CrossRef] [PubMed]
- Lapides, L.; Varga, I.; Klein, M.; Rybánska, L.; Belušáková, V.; Babál, P. When Less Is More—Pipelle Endometrial Sampling for Quantification of Uterine Natural Killer Cells in Patients With Recurrent Implantation Failure or Habitual Abortion. Physiol. Res. 2022, 71, S65–S73. [Google Scholar] [CrossRef]
- Kuon, R.J.; Weber, M.; Heger, J.; Santillán, I.; Vomstein, K.; Bär, C.; Strowitzki, T.; Markert, U.R.; Toth, B. Uterine natural killer cells in patients with idiopathic recurrent miscarriage. Am. J. Reprod. Immunol. 2017, 78, e12721. [Google Scholar] [CrossRef]
- Alfer, J.; Fattahi, A.; Bleisinger, N.; Antoniadis, S.; Krieg, J.; Dittrich, R.; Beckmann, M.W.; Hartmann, A.; Popovici, R.M.; Tremellen, K. Individual dynamics of uterine natural killer cells in natural and stimulated cycles monitored using a new endometrial dating method. Am. J. Reprod. Immunol. 2022, 88, e13620. [Google Scholar]
- Yang, W.J.; Chen, X.; Zhao, Y.; Cheung, W.C.; Hsiao, S.Y.; Liu, Y.; Law, T.S.M.; Chung, J.P.W.; Li, T.C. A comparison of uterine natural killer cell density in the peri-implantation period between natural cycles and hormone replacement therapy cycles. Am. J. Reprod. Immunol. 2019, 82, e13156. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Man, G.C.W.; Wang, J.; Liu, Y.; Kwong, J.; Zhang, T.; Chung, J.P.W.; Wang, C.C.; Chen, X.; Li, T.C. The identification of endometrial immune cell densities and clustering analysis in the mid-luteal phase as predictor for pregnancy outcomes after IVF-ET treatment. J. Reprod. Immunol. 2021, 148, 103431. [Google Scholar] [CrossRef] [PubMed]
- Sudoma, I.; Goncharova, Y.; Dons’koy, B.; Mykytenko, D. Immune phenotype of the endometrium in patients with recurrent implantation failures after the transfer of genetically tested embryos in assisted reproductive technology programs. J. Reprod. Immunol. 2023, 157, 103943. [Google Scholar] [CrossRef] [PubMed]
- Marron, K.; Harrity, C. Potential utility of a non-invasive menstrual blood immunophenotype analysis in reproductive medicine. Reprod. Fertil. 2022, 3, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, S.; Huang, C.; Lian, R.; Chen, C.; Liu, S.; Li, L.; Diao, L.; Markert, U.R.; Zeng, Y. Evaluation of peripheral and uterine immune status of chronic endometritis in patients with recurrent reproductive failure. Fertil. Steril. 2020, 113, 187–196.e1. [Google Scholar] [CrossRef]
- Marron, K.; Walsh, D.; Harrity, C. Detailed endometrial immune assessment of both normal and adverse reproductive outcome populations. J. Assist. Reprod. Genet. 2019, 36, 199–210. [Google Scholar] [CrossRef]
- Tohma, Y.A.; Musabak, U.; Gunakan, E.; Akilli, H.; Onalan, G.; Zeyneloglu, H.B. The Role of Analysis of NK Cell Subsets in Peripheral Blood and Uterine Lavage Samples in Evaluation of Patients with Recurrent Implantation Failure. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101793. [Google Scholar] [CrossRef]
- Lai, Z.-Z.; Wang, Y.; Zhou, W.-J.; Liang, Z.; Shi, J.-W.; Yang, H.-L.; Xie, F.; Chen, W.-D.; Zhu, R.; Zhang, C.; et al. Single-cell transcriptome profiling of the human endometrium of patients with recurrent implantation failure. Theranostics 2022, 12, 6527–6547. [Google Scholar] [CrossRef]
- Bajpai, K.; Acharya, N.; Prasad, R.; Wanjari, M.B. Endometrial Receptivity During the Preimplantation Period: A Narrative Review. Cureus 2023, 15, e37753. [Google Scholar]
- Mahajan, D.; Sharma, N.R.; Kancharla, S.; Kolli, P.; Tripathy, A.; Sharma, A.K.; Singh, S.; Kumar, S.; Mohanty, A.K.; Jena, M.K. Role of Natural Killer Cells during Pregnancy and Related Complications. Biomolecules 2022, 12, 68. [Google Scholar] [CrossRef]
- Gong, H.; Chen, Y.; Xu, J.; Xie, X.; Yu, D.; Yang, B.; Kuang, H. The regulation of ovary and conceptus on the uterine natural killer cells during early pregnancy. Reprod. Biol. Endocrinol. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Abrahams, V.M.; Mor, G.; Guller, S. Placental Hofbauer cells and complications of pregnancy. Ann. N. Y. Acad. Sci. 2011, 1221, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, M.; Lee, S.K. Role of endometrial immune cells in implantation. Clin. Exp. Reprod. Med. 2011, 38, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Lédée, N.; Petitbarat, M.; Prat-Ellenberg, L.; Dray, G.; Cassuto, G.N.; Chevrier, L.; Kazhalawi, A.; Vezmar, K.; Chaouat, G. The uterine immune profile: A method for individualizing the management of women who have failed to implant an embryo after IVF/ICSI. J. Reprod. Immunol. 2020, 142, 103207. [Google Scholar]
- Sacks, G. Enough! Stop the arguments and get on with the science of natural killer cell testing. Hum. Reprod. 2015, 30, 1526–1531. [Google Scholar] [CrossRef]
- Robson, A.; Harris, L.K.; Innes, B.A.; Lash, G.E.; Aljunaidy, M.M.; Aplin, J.D.; Baker, P.N.; Robson, S.C.; Bulmer, J.N. Uterine natural killer cells initiate spiral artery remodeling in human pregnancy. FASEB J. 2012, 26, 4876–4885. [Google Scholar] [CrossRef]
- Ross, M.H.; Pawlina, W. Histology with Correlated Cell and Molecular Biology, 7th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2016. [Google Scholar]
- Ovalle, W.K.; Nahirney, P.C. Netter’s Essential Histology, 2nd ed.; Elsevier Saunders: Philadelphia, PA, USA, 2013. [Google Scholar]
- Mescher, A.L. Junqueira’s Basic Histology, 14th ed.; McGraw Hill Education: New York, NY, USA, 2016. [Google Scholar]
- Gartner, L.P. Textbook of Histology, 4th ed.; Elsevier: Philadelphia, PA, USA, 2017. [Google Scholar]
- Lowe, J.S.; Anderson, P.G.; Anderson, S.I. Stevens & Lowe’s Human Histology, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Sadler, T.W. Langman’s Medical Embryology, 14th ed.; Wolters Kluwer: Tokyo, Japan, 2019. [Google Scholar]
- Carlson, B.M. Human Embryology and Developmental Biology, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Schoenwolf, G.C.; Bleyl, S.B.; Brauer, P.R.; Francis-West, P.H. Larsen’s Human Embryology, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Balko, J.; Tonar, Z.; Varga, I. Memorix Histology, 1st ed.; Triton: Prague, Czech Republic, 2018. [Google Scholar]
- Kierszenbaum, A.L.; Tres, L.L. Histology and Cell Biology: An Introduction to Pathology, 4th ed.; Elsevier: Philadelphia, PA, USA, 2016. [Google Scholar]
- Von Woon, E.; Greer, O.; Shah, N.; Nikolaou, D.; Johnson, M.; Male, V. Number and function of uterine natural killer cells in recurrent miscarriage and implantation failure: A systematic review and meta-analysis. Hum. Reprod. Update 2022, 28, 548–582. [Google Scholar] [CrossRef]
- Kanter, J.; Gordon, S.M.; Mani, S.; Sokalska, A.; Park, J.Y.; Senapati, S.; Huh, D.D.; Mainigi, M. Hormonal stimulation reduces numbers and impairs function of human uterine natural killer cells during implantation. Hum. Reprod. 2023, 38, 1047–1059. [Google Scholar] [CrossRef]
- Balen, A. Infertility in Practice, 5th ed.; Reproductive Medicine and Assisted Reproductive Techniques Series; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Benkhalifa, M.; Joao, F.; Duval, C.; Montjean, D.; Bouricha, M.; Cabry, R.; Bélanger, M.C.; Bahri, H.; Miron, P.; Benkhalifa, M. Endometrium Immunomodulation to Prevent Recurrent Implantation Failure in Assisted Reproductive Technology. Int. J. Mol. Sci. 2022, 23, 12787. [Google Scholar] [CrossRef]
- Ban, Y.; Yang, X.; Xing, Y.; Que, W.; Yu, Z.; Gui, W.; Chen, Y.; Liu, X. Intrauterine Infusion of Leukocyte-Poor Platelet-Rich Plasma Is an Effective Therapeutic Protocol for Patients with Recurrent Implantation Failure: A Retrospective Cohort Study. J. Clin. Med. 2023, 12, 2823. [Google Scholar]
- Kumar, P.; Marron, K.; Harrity, C. Intralipid therapy and adverse reproductive outcome: Is there any evidence? Reprod. Fertil. 2021, 2, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Arefi, S.; Fazeli, E.; Esfahani, M.; Borhani, N.; Yamini, N.; Hosseini, A.; Farifteh, F. Granulocyte-colony stimulating factor may improve pregnancy outcome in patients with history of unexplained recurrent implantation failure: An RCT. Int. J. Reprod. Biomed. 2018, 16, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Eapen, A.; Joing, M.; Kwon, P.; Tong, J.; Maneta, E.; De Santo, C.; Mussai, F.; Lissauer, D.; Carter, D.; RESPONSE Study Group. Recombinant human granulocyte- colony stimulating factor in women with unexplained recurrent pregnancy losses: A randomized clinical trial. Hum. Reprod. 2019, 34, 424–432. [Google Scholar] [PubMed]
- Sun, Y.; Cui, L.; Lu, Y.; Tan, J.; Dong, X.; Ni, T.; Yan, J.; Guan, Y.; Hao, G.; Liu, J.Y.; et al. Prednisone vs Placebo and Live Birth in Patients With Recurrent Implantation Failure Undergoing In Vitro Fertilization: A Randomized Clinical Trial. JAMA 2023, 329, 1460–1468. [Google Scholar] [CrossRef]
- Lucas, E.S.; Dyer, N.P.; Fishwick, K.; Ott, S.; Brosens, J.J. Success after failure: The role of endometrial stem cells in recurrent miscarriage. Reproduction 2016, 152, R159–R166. [Google Scholar] [CrossRef] [PubMed]
- Sfakianoudis, K.; Rapani, A.; Grigoriadis, S.; Pantou, A.; Maziotis, E.; Kokkini, G.; Tsirligkani, C.; Bolaris, S.; Nikolettos, K.; Chronopoulou, M.; et al. The Role of Uterine Natural Killer Cells on Recurrent Miscarriage and Recurrent Implantation Failure: From Pathophysiology to Treatment. Biomedicines 2021, 9, 1425. [Google Scholar] [CrossRef]
- Cooper, S.; Laird, S.M.; Mariee, N.; Li, T.C.; Metwally, M. The effect of prednisolone on endometrial uterine NK cell concentrations and pregnancy outcome in women with reproductive failure. A retrospective cohort study. J. Reprod. Immunol. 2019, 131, 1–6. [Google Scholar] [CrossRef]
- Michimata, T.; Ogasawara, M.S.; Tsuda, H.; Suzumori, K.; Aoki, K.; Sakai, M.; Fujimura, M.; Nagata, K.; Nakamura, M.; Saito, S. Distributions of endometrial NK cells, B cells, T cells, and Th2/Tc2 cells fail to predict pregnancy outcome following recurrent abortion. Am. J. Reprod. Immunol. 2002, 47, 196–202. [Google Scholar] [CrossRef]
- Seshadri, S.; Sunkara, S.K. Natural killer cells in female infertility and recurrent miscarriage: A systematic review and meta-analysis. Hum. Reprod. Update 2014, 20, 429–438. [Google Scholar] [CrossRef]
| PubMed and Medline | Google Scholar | Web of Science | |
|---|---|---|---|
| Uterine NK cells | 182 results | 5110 results | 392 results |
| Decidual NK cells | 182 results | 6630 results | 324 results |
| Endometrial granular cells | 9 results | 79 results | 3 results |
| Endometrial NK cell | 33 results | 823 results | 13 results |
| Title of Textbook | Authors | Description |
|---|---|---|
| The Developing Human. 11th edition. Year 2020, Page No. 108. | Moore K.L., Persaud T.V.N., Torchia M.G. [35] | In addition to averting T cells, extravillous trophoblast cells must also shield themselves from potential attack by NK lymphocytes. Maternal lymphocytes within the pregnancy-associated decidua include a high portion (65–70%) of NK cells and a low portion (10–12%) of T cells. Decidual or uterine NK cells are distinct from peripheral blood NK cells’ phenotype and function in having poor cytotoxicity for extravillous trophoblast cells. |
| Wheater’s Functional Histology. Sixth Edition. Year 2014, Page No. 356. | Young B., O’Dowd G., Woodford P. [32] | Secretory endometrium: Endometrial stromal granulocytes, which are probably large granular lymphocytes, are found in the stroma at this stage. |
| Memorix Histology. 1st edition. Year 2018, Page No. 397. | Balko J., Tonar Z., Varga I. [83] | Endometrial granular cells and Hamperl cells have a T lymphocyte of a spherical shape and markedly lobular nucleus. Present mainly in the secretory phase of the menstrual cycle. Realizing cytokines, which affect the growth and multiplication of vessels in the mucosa. |
| Histology and Cell Biology. Fourth Edition. Year 2016, Page No. 699. | Kierszenbaum A.L., Tres L.L. [84] | The decidual reaction involves the production of immunosuppressive substances (mainly prostaglandins) by decidual cells to inhibit the activation of natural killer cells at the implantation site. |
| Histology for Pathologists. Fifth edition. Year 2020, Page No. 1080. | Mills S.E. (Ed). [33] | A second prominent cellular constituent (after stromal cells), particularly in the late secretory phase and during pregnancy, is what historically has been referred to as the “stromal granulocyte” but is now known to be a uterine NK cell. These are rounded cells with bilobed nuclei and pale cytoplasm containing eosinophilic granules. Their immune profile differs from that of blood NK cells. Their number appears to be positively correlated with the degree of predecidualization or decidualization in the surrounding endometrium. Indeed, the number of such cells was used by Noyes as a dating criterion. This close association has suggested to some workers that uterine NK cells play a role in the control of trophoblast invasion and spiral artery remodeling and may be important in initiating and maintaining decidualization. Alternatively, the death of uterine NK cells might be an early event in the onset of endometrial breakdown at menstruation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapides, L.; Varga, I.; Csöbönyeiová, M.; Klein, M.; Pavlíková, L.; Visnyaiová, K.; Babál, P.; Mikušová, R. The Neglected Uterine NK Cells/Hamperl Cells/Endometrial Stromal Granular Cell, or K Cells: A Narrative Review from History through Histology and to Medical Education. Int. J. Mol. Sci. 2023, 24, 12693. https://doi.org/10.3390/ijms241612693
Lapides L, Varga I, Csöbönyeiová M, Klein M, Pavlíková L, Visnyaiová K, Babál P, Mikušová R. The Neglected Uterine NK Cells/Hamperl Cells/Endometrial Stromal Granular Cell, or K Cells: A Narrative Review from History through Histology and to Medical Education. International Journal of Molecular Sciences. 2023; 24(16):12693. https://doi.org/10.3390/ijms241612693
Chicago/Turabian StyleLapides, Lenka, Ivan Varga, Mária Csöbönyeiová, Martin Klein, Lada Pavlíková, Kristína Visnyaiová, Pavel Babál, and Renáta Mikušová. 2023. "The Neglected Uterine NK Cells/Hamperl Cells/Endometrial Stromal Granular Cell, or K Cells: A Narrative Review from History through Histology and to Medical Education" International Journal of Molecular Sciences 24, no. 16: 12693. https://doi.org/10.3390/ijms241612693
APA StyleLapides, L., Varga, I., Csöbönyeiová, M., Klein, M., Pavlíková, L., Visnyaiová, K., Babál, P., & Mikušová, R. (2023). The Neglected Uterine NK Cells/Hamperl Cells/Endometrial Stromal Granular Cell, or K Cells: A Narrative Review from History through Histology and to Medical Education. International Journal of Molecular Sciences, 24(16), 12693. https://doi.org/10.3390/ijms241612693







