Protective Effects of Lanostane Triterpenoids from Chaga Mushroom in Human Keratinocytes, HaCaT Cells, against Inflammatory and Oxidative Stresses
Abstract
1. Introduction
2. Results
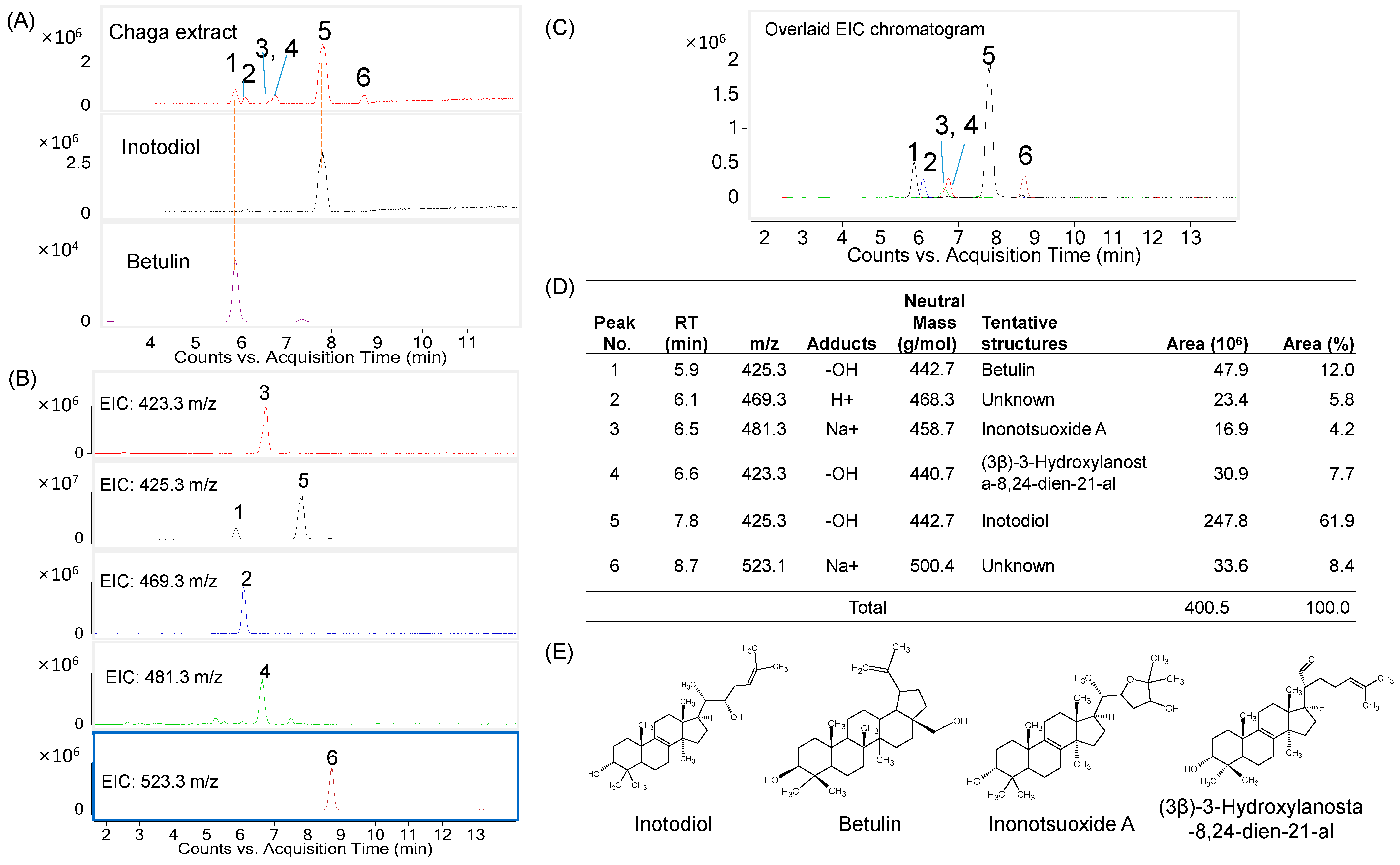
2.1. Determination of Major Triterpenes in the Inotodiol Concentrate
2.2. Evaluation of Cytotoxic Effect of Inotodiol and Inotodiol Concentrate
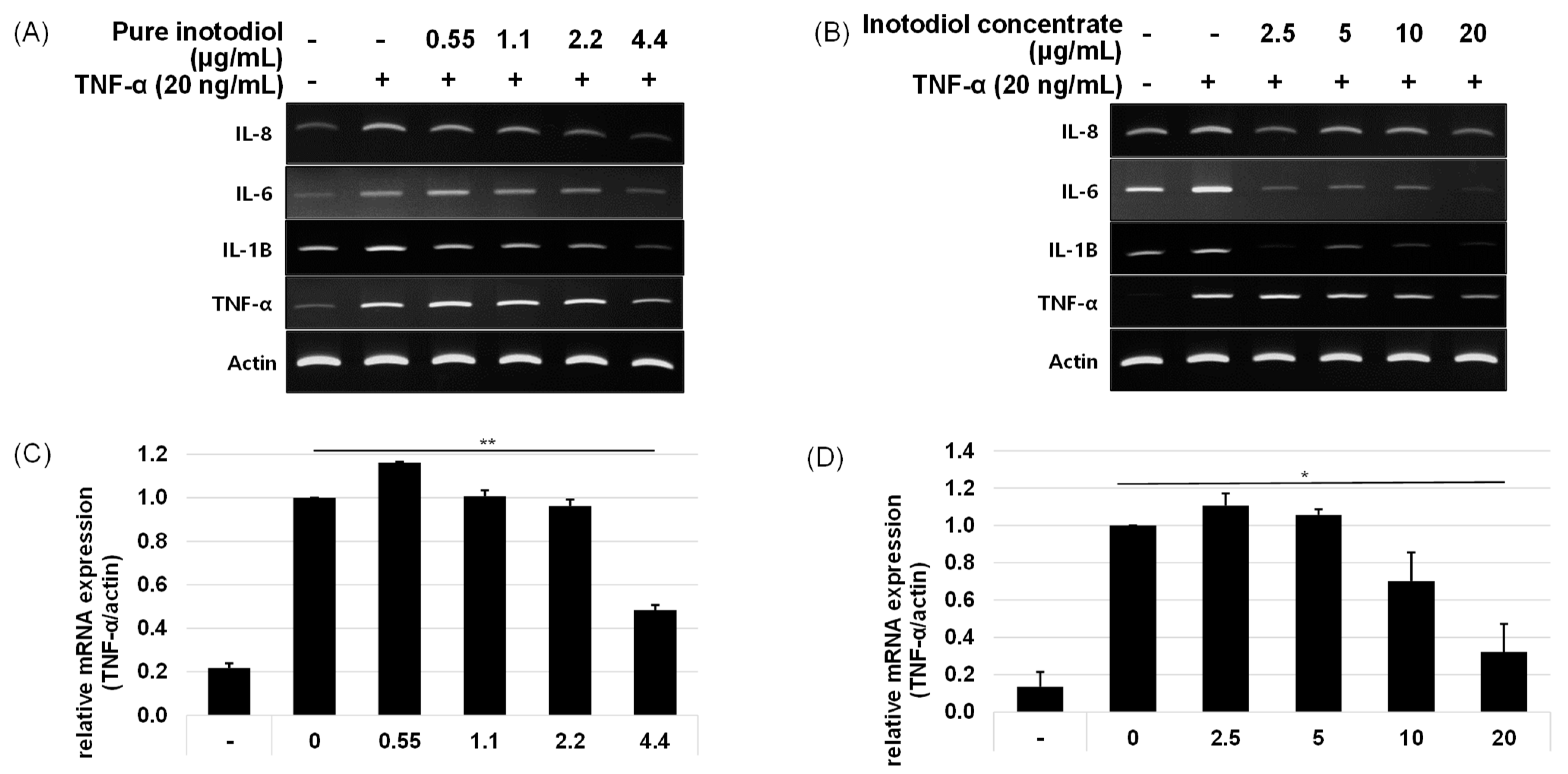
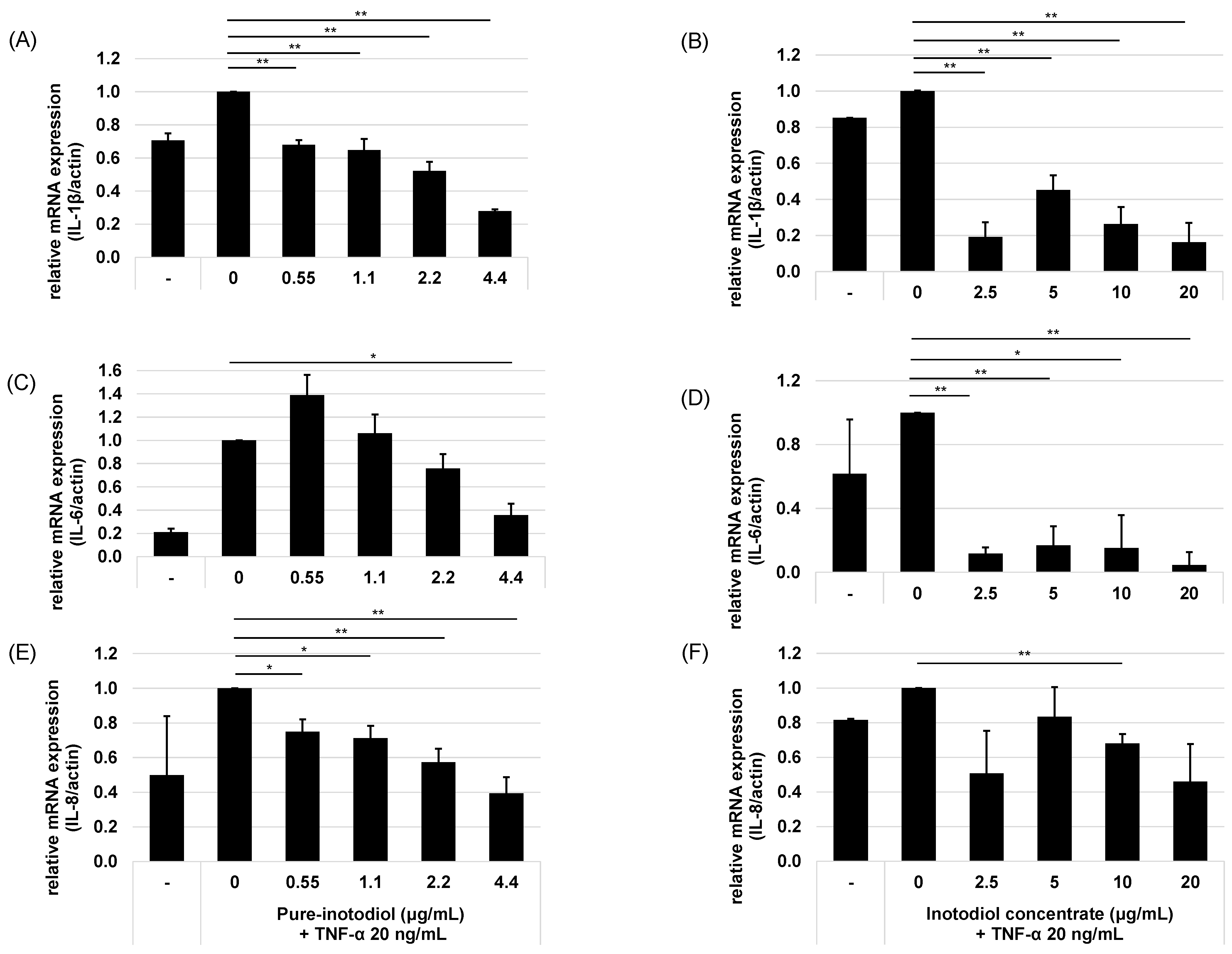
2.3. Impact of Inotodiol and Inotodiol Concentrate on Pro-Inflammatory Cytokine Expression after Treatment with TNF-α in HaCaT Cells
2.4. Impact of Inotodiol and Inotodiol Concentrate on Pro-Inflammatory Cytokine Expression after Treatment with UV in HaCaT Cells
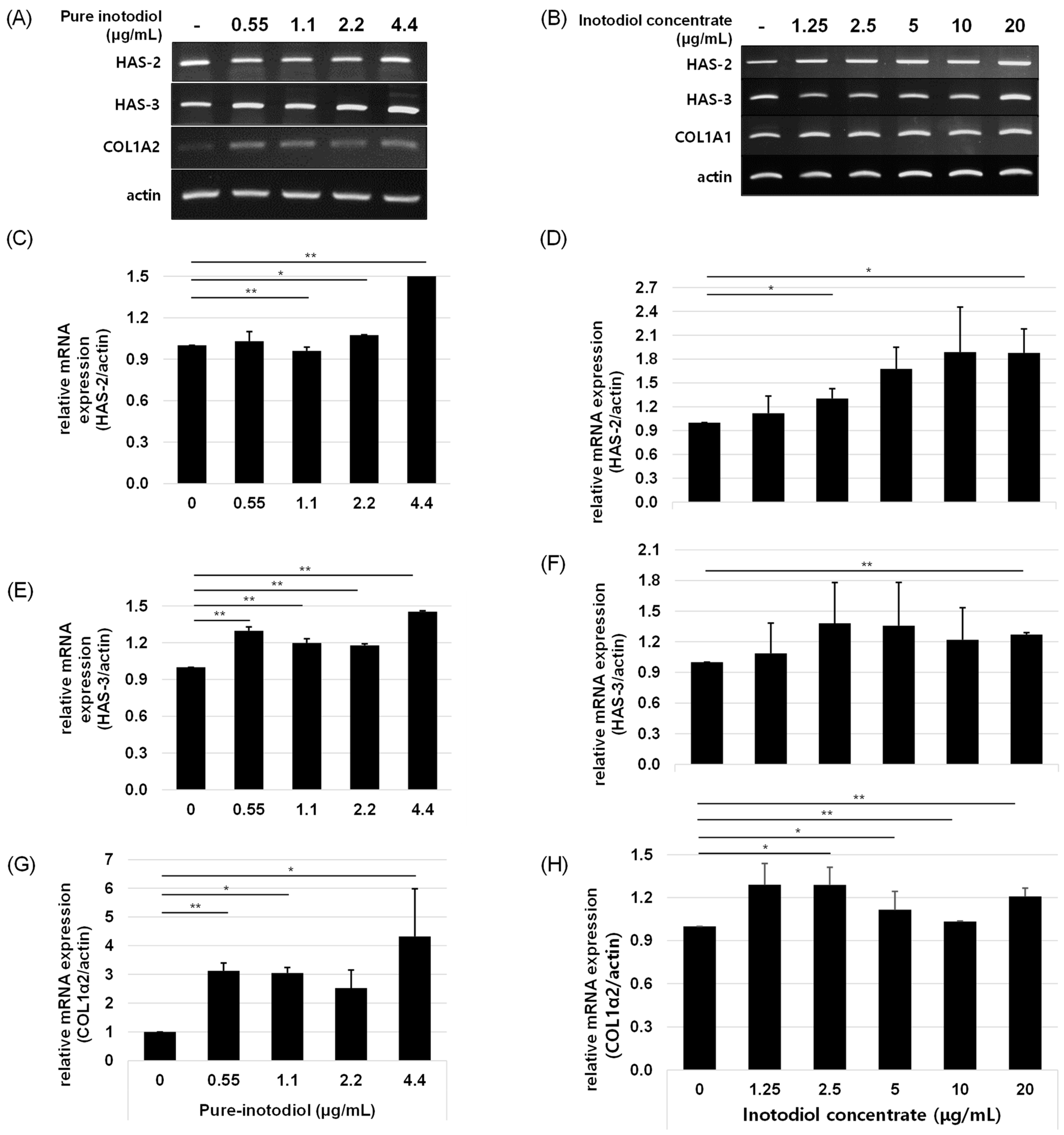
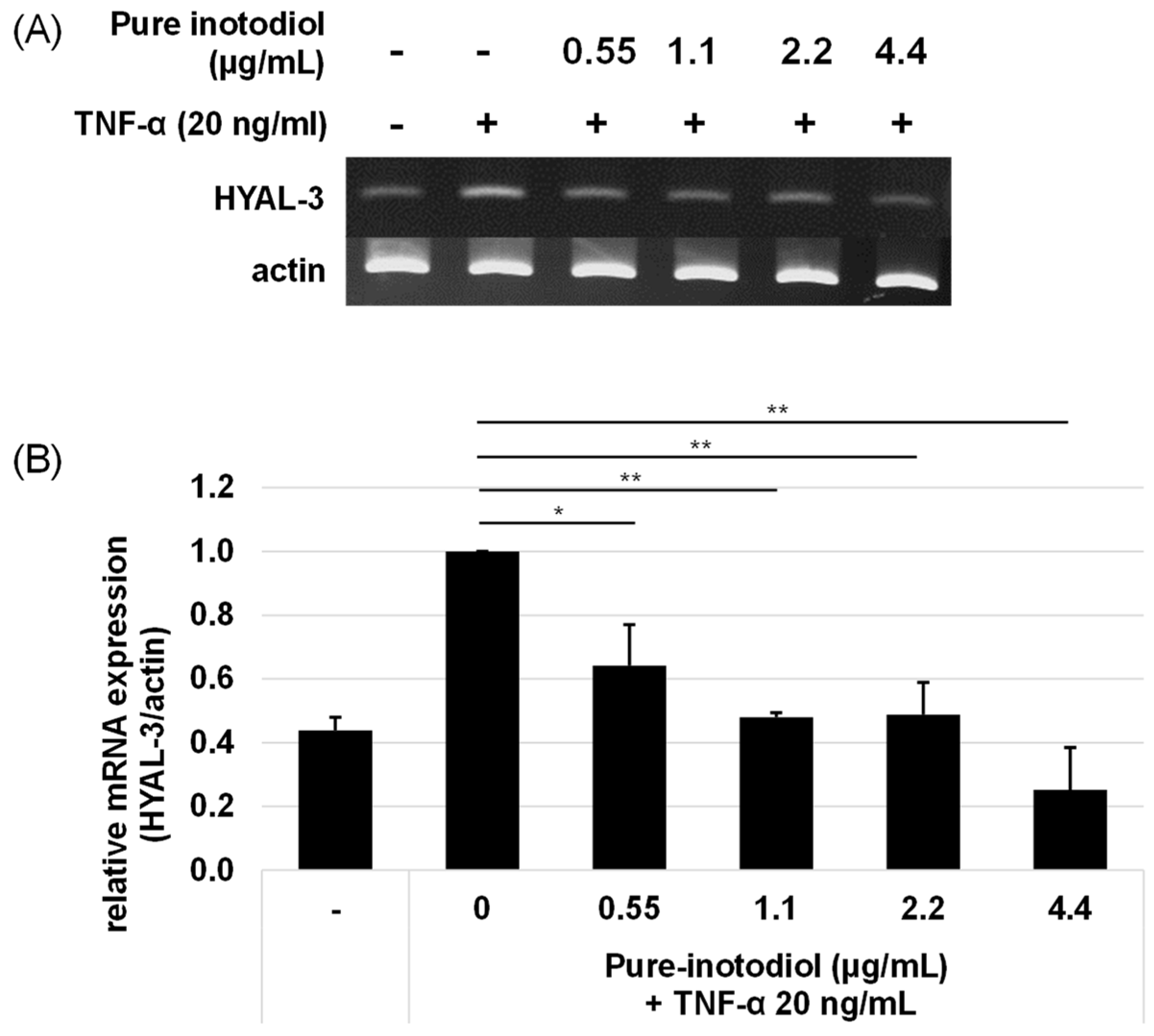
2.5. Impact of Inotodiol/Inotodiol Concentrate on Hyaluronan Synthesis and Collagen
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Inotodiol Concentrate and High Purity Inotodiol
4.3. HPLC-MS/MS Analyses of Inotodiol Concentrate and High Purity Inotodiol
4.4. Cell Culture
4.5. Cell Viability Assay
4.6. UVB Irradiation and TNF-α Treatment
4.7. Quantitative Polymerase Chain Reaction (qPCR)
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhandapani, S.; Wang, R.; Hwang, K.C.; Kim, H.; Kim, Y.-J. Enhanced skin anti-inflammatory and moisturizing action of gold nanoparticles produced utilizing Diospyros kaki fruit extracts. Arab. J. Chem. 2023, 16, 104551. [Google Scholar] [CrossRef]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From clinical significance to quantification. Adv. Sci. 2021, 8, e2004433. [Google Scholar] [CrossRef]
- Fernandes, A.; Rodrigues, P.; Pintado, M.; Tavaria, F. A systematic review of natural products for skin applications: Targeting inflammation, wound healing, and photo-aging. Phytomedicine 2023, 115, 154824. [Google Scholar] [CrossRef] [PubMed]
- Alves, G.D.A.D.; De Souza, R.O.; Rogez, H.L.G.; Masaki, H.; Fonseca, M.J.V. Cecropia obtusa extract and chlorogenic acid exhibit anti aging effect in human fibroblasts and keratinocytes cells exposed to UV radiation. PLoS ONE 2019, 14, e0216501. [Google Scholar] [CrossRef] [PubMed]
- Valeri, M.; Raffatellu, M. Cytokines IL-17 and IL-22 in the host response to infection. Pathog. Dis. 2016, 74. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Pawelec, G.; Khalil, A.; Cohen, A.A.; Hirokawa, K.; Witkowski, J.M.; Franceschi, C. Immunology of Aging: The Birth of Inflammaging. Clin. Rev. Allergy Immunol. 2021, 64, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Koppula, S.; Akther, M.; Haque, M.E. Potential nutrients from natural and synthetic sources targeting inflammaging-a review of literature. Clin. Data Pat. 2021, 13, 4058. [Google Scholar]
- Pająk, J.; Nowicka, D.; Szepietowski, J.C. Inflammaging and Immunosenescence as Part of Skin Aging—A Narrative Review. Int. J. Mol. Sci. 2023, 24, 7784. [Google Scholar] [CrossRef]
- Yap, W.N. Tocotrienol-rich fraction attenuates UV-induced inflammaging: A bench to bedside study. J. Cosmet. Dermatol. 2017, 17, 555–565. [Google Scholar] [CrossRef]
- Park, Y.-M.; Won, J.-H.; Kim, Y.-H.; Choi, J.-W.; Park, H.-J.; Lee, K.-T. In vivo and in vitro anti-inflammatory and anti-nociceptive effects of the methanol extract of Inonotus obliquus. J. Ethnopharmacol. 2005, 101, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Yoon, D.H.; Kim, C.H.; Shrestha, B.; Chang, W.C.; Lim, S.Y.; Lee, W.H.; Han, S.G.; Lee, J.O.; Lim, M.H.; et al. Ethanol extract of Inonotus obliquus inhibits lipopolysaccharide-induced inflammation in RAW 264.7 mac-rophage cells. J. Med. Food 2007, 10, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Choi, M.; Park, S.; Kang, J.; Chung, E.H.; Park, J.T.; Kim, Y.M. Inotodiol suppresses allergic inflammation in allergic rhinitis mice. Int. Forum Allergy Rhinol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.C.; Nguyen, M.T.T.; Truong, B.T.; Kim, D.R.; Shin, S.; Kim, J.E.; Park, K.B.; Park, J.H.; Tran, P.L.; Ban, S.Y.; et al. Isolation, physicochemical characterization, and biological properties of inotodiol, the potent pharmaceutical oxysterol from Chaga mushroom. Antioxidants 2023, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, W.; Chen, C.; Zhang, C.; Duan, J.; Yao, H.; Wei, Q.; Meng, A.; Shi, J. Inotodiol protects PC12 cells against injury induced by oxygen and glucose deprivation/restoration through inhibiting oxidative stress and apoptosis. J. Appl. Biomed. 2018, 16, 126–132. [Google Scholar] [CrossRef]
- Lee, S.H.; Won, G.-W.; Choi, S.-H.; Kim, M.-Y.; Oh, C.-H.; Park, J.-T. Antiaging effect of inotodiol on oxidative stress in human dermal fibroblasts. Biomed. Pharmacother. 2022, 153, 113311. [Google Scholar] [CrossRef]
- Nguyen, T.M.N.; Ban, S.-Y.; Park, K.-B.; Lee, C.-K.; Lee, S.-W.; Lee, Y.-J.; Baek, S.-M.; Park, J.-K.; Nguyen, M.T.T.; Kim, J.; et al. Evaluation of Toxicity and Efficacy of Inotodiol as an Anti-Inflammatory Agent Using Animal Model. Molecules 2022, 27, 4704. [Google Scholar] [CrossRef]
- Podgórski, R.; Wojasiński, M.; Ciach, T. Nanofibrous materials affect the reaction of cytotoxicity assays. Sci. Rep. 2022, 12, 9047. [Google Scholar] [CrossRef]
- Bosch, R.; Philips, N.; Suárez-Pérez, J.A.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; González, S. Mechanisms of Photoaging and Cutaneous Photocarcinogenesis, and Photoprotective Strategies with Phytochemicals. Antioxidants 2015, 4, 248–268. [Google Scholar] [CrossRef]
- Sze, J.H.; Brownlie, J.C.; Love, C.A. Biotechnological production of hyaluronic acid: A mini review. 3 Biotech 2016, 6, 67. [Google Scholar] [CrossRef]
- Usui, T.; Amano, S.; Oshika, T.; Suzuki, K.; Miyata, K.; Araie, M.; Heldin, P.; Yamashita, H. Expression regulation of hyaluronan synthase in corneal endothelial cells. Investig. Opthalmology Vis. Sci. 2000, 41, 3261–3267. [Google Scholar]
- Krupkova, O.; Greutert, H.; Boos, N.; Lemcke, J.; Liebscher, T.; Wuertz-Kozak, K. Expression and activity of hyaluronidases HYAL-1, HYAL-2 and HYAL-3 in the human intervertebral disc. Eur. Spine J. 2019, 29, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Trentini, M.; Zanolla, I.; Zanotti, F.; Tiengo, E.; Licastro, D.; Dal Monego, S.; Lovatti, L.; Zavan, B. Apple derived exosomes improve collagen type I production and decrease MMPs during aging of the skin through downregulation of the NF-κB pathway as mode of action. Cells 2022, 11, 3950. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.N.; Le, H.S.; Le, B.V.; Kim, Y.H.; Hwang, I. Anti-allergic effect of inotodiol, a lanostane triterpenoid from Chaga mushroom, via selective inhibition of mast cell function. Int. Immunopharmacol. 2020, 81, 106244. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Gao, D.; Cho, C.W.; Hwang, I.; Kim, H.M.; Kang, J.S. A novel bioanalytical method for determination of inotodiol isolated from Inonotus obliquus and its application to pharmacokinetic atudy. Plants 2021, 10, 1631. [Google Scholar] [CrossRef]
- Nomura, M.; Gao, D.; Cho, C.W.; Hwang, I.; Kim, H.M.; Kang, J.S. Inotodiol, a lanostane triterpenoid, from Inonotus obliquus inhibits cell proliferation through caspa-se-3-dependent apoptosis. Anticancer Res. 2008, 28, 2691–2696. [Google Scholar]
- Duru, K.C.; Kovaleva, E.G.; Danilova, I.G.; van der Bijl, P. The pharmacological potential and possible molecular mechanisms of action of Inonotus obliquus from preclinical studies. Phytother. Res. 2019, 33, 1966–1980. [Google Scholar] [CrossRef] [PubMed]
- Glamočlija, J.; Ćirić, A.; Nikolić, M.; Fernandes, Â.; Barros, L.; Calhelha, R.C.; Ferreira, I.C.; Soković, M.; van Griensven, L.J. Chemical characterization and biological activity of Chaga (Inonotus obliquus), a medicinal “mushroom”. J. Ethnopharmacol. 2015, 162, 323–332. [Google Scholar] [CrossRef]
- Zhang, X.; Bao, C.; Zhang, J. Inotodiol suppresses proliferation of breast cancer in rat model of type 2 diabetes mellitus via downregulation of β-catenin signaling. Biomed. Pharmacother. 2018, 99, 142–150. [Google Scholar] [CrossRef]
- Hu, L.; Nomura, S.; Sato, Y.; Takagi, K.; Ishii, T.; Honma, Y.; Watanabe, K.; Mizukami, Y.; Muto, J. Anti-inflammatory effects of differential molecular weight hyaluronic acids on UVB-induced calprotectin-mediated keratinocyte inflammation. J. Dermatol. Sci. 2022, 107, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Lee, T.H.; Wahedi, H.M.; Baek, S.H.; Kim, S.Y. Resveratrol-enriched rice attenuates UVB-ROS-induced skin aging via downregulation of inflammatory cascades. Oxid. Med. Cell. Longev. 2017, 2017, 8379539. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Kim, D.-S.; Park, K.-C. Antioxidant effect of Inonotus obliquus. J. Ethnopharmacol. 2005, 96, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.K.; Kim, Y.S.; Jang, Y.W.; Jung, J.Y.; Yun, B.S. New antioxidant polyphenols from the medicinal mushroom Inonotus obliquus. Bioorg. Med. Chem. Lett. 2007, 17, 6678–6681. [Google Scholar] [CrossRef] [PubMed]
- Nová, R.; Pavlík, V. Hyaluronan: A key player or just a bystander in skin photoaging? Exp. Dermatol. 2022, 31, 442–458. [Google Scholar]
- Jeon, Y.J.; Kim, B.H.; Kim, S.; Oh, I.; Lee, S.; Shin, J.; Kim, T.Y. Rhododendrin ameliorates skin inflammation through inhibition of NF-κB, MAPK, and PI3K/Akt signaling. Eur. J. Pharmacol. 2013, 714, 7–14. [Google Scholar] [CrossRef]
- Kristensen, M.; Chu, C.Q.; Eedy, D.J.; Feldmann, M.; Brennan, F.M.; Breathnach, S.M. Localization of tumour necrosis factor-alpha (TNF-α) and its receptors in normal and psoriatic skin: Epidermal cells express the 55-kD but not the 75-kD TNF receptor. Clin. Exp. Immunol. 1993, 94, 354–362. [Google Scholar] [CrossRef]
- Qin, Z.; Okubo, T.; Voorhees, J.J.; Fisher, G.J.; Quan, T. Elevated cysteine-rich protein 61 (CCN1) promotes skin aging via upregulation of IL-1β in chronically sun-exposed human skin. Age 2013, 36, 353–364. [Google Scholar] [CrossRef]


| Gene | Forward | Reverse |
|---|---|---|
| GAPDH | AAGTGGATATTGTTGCCATC | ACTGTGGTCATGAGTCCTTC |
| IL-6 | ATGAACTCCTTCTCCACAAGC | GTTTTCTGCCAGTGCCTCTTTG |
| IL-8 | TCTGTGTGAAGGTGCAGTT | AGCCCTCTTCAAAAACTTCT |
| IL-1β | AAACAGATGAAGTGCTCCTTCCAGG | TGGAGAACACCACTTGTTGCTCCA |
| TNF-α | GAGCTGAGAGATAACCAGCTGGTG | CAGATAGATGGGCTCATACCAGGG |
| HAS-2 | TGGGTGTGTTCAGTGCAT | GCATTGTACAGCCATTCTCG |
| HAS-3 | CCCAGCCAGATTTGTTGATG | AGTGGTCACGGGTTTCTTCC |
| COL1A1 | AGCCAGCAGATCGAGAACAT | TCTTGTCCTTGGGGTTCTTG |
| COL1A2 | TCAAGGTTTCCAAGGACCTG | GTGTCCCCTAATGCCTTTGA |
| HYAL-3 | CAGTCCATTGGTGTGAGTG | CACAGGTGTAGAAAGGCTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Nguyen, T.M.N.; Park, H.-a.; Nguyen, M.T.T.; Lee, N.-y.; Ban, S.-y.; Park, K.-b.; Lee, C.-k.; Kim, J.; Park, J.-T. Protective Effects of Lanostane Triterpenoids from Chaga Mushroom in Human Keratinocytes, HaCaT Cells, against Inflammatory and Oxidative Stresses. Int. J. Mol. Sci. 2023, 24, 12803. https://doi.org/10.3390/ijms241612803
Park J, Nguyen TMN, Park H-a, Nguyen MTT, Lee N-y, Ban S-y, Park K-b, Lee C-k, Kim J, Park J-T. Protective Effects of Lanostane Triterpenoids from Chaga Mushroom in Human Keratinocytes, HaCaT Cells, against Inflammatory and Oxidative Stresses. International Journal of Molecular Sciences. 2023; 24(16):12803. https://doi.org/10.3390/ijms241612803
Chicago/Turabian StylePark, Jihyun, Thi Minh Nguyet Nguyen, Hyun-ah Park, My Tuyen Thi Nguyen, Nan-young Lee, So-young Ban, Kyu-been Park, Chang-kyu Lee, Jaehan Kim, and Jong-Tae Park. 2023. "Protective Effects of Lanostane Triterpenoids from Chaga Mushroom in Human Keratinocytes, HaCaT Cells, against Inflammatory and Oxidative Stresses" International Journal of Molecular Sciences 24, no. 16: 12803. https://doi.org/10.3390/ijms241612803
APA StylePark, J., Nguyen, T. M. N., Park, H.-a., Nguyen, M. T. T., Lee, N.-y., Ban, S.-y., Park, K.-b., Lee, C.-k., Kim, J., & Park, J.-T. (2023). Protective Effects of Lanostane Triterpenoids from Chaga Mushroom in Human Keratinocytes, HaCaT Cells, against Inflammatory and Oxidative Stresses. International Journal of Molecular Sciences, 24(16), 12803. https://doi.org/10.3390/ijms241612803







