Beyond the Epidermal-Melanin-Unit: The Human Scalp Anagen Hair Bulb Is Home to Multiple Melanocyte Subpopulations of Variable Melanogenic Capacity
Abstract
1. Introduction
2. Results
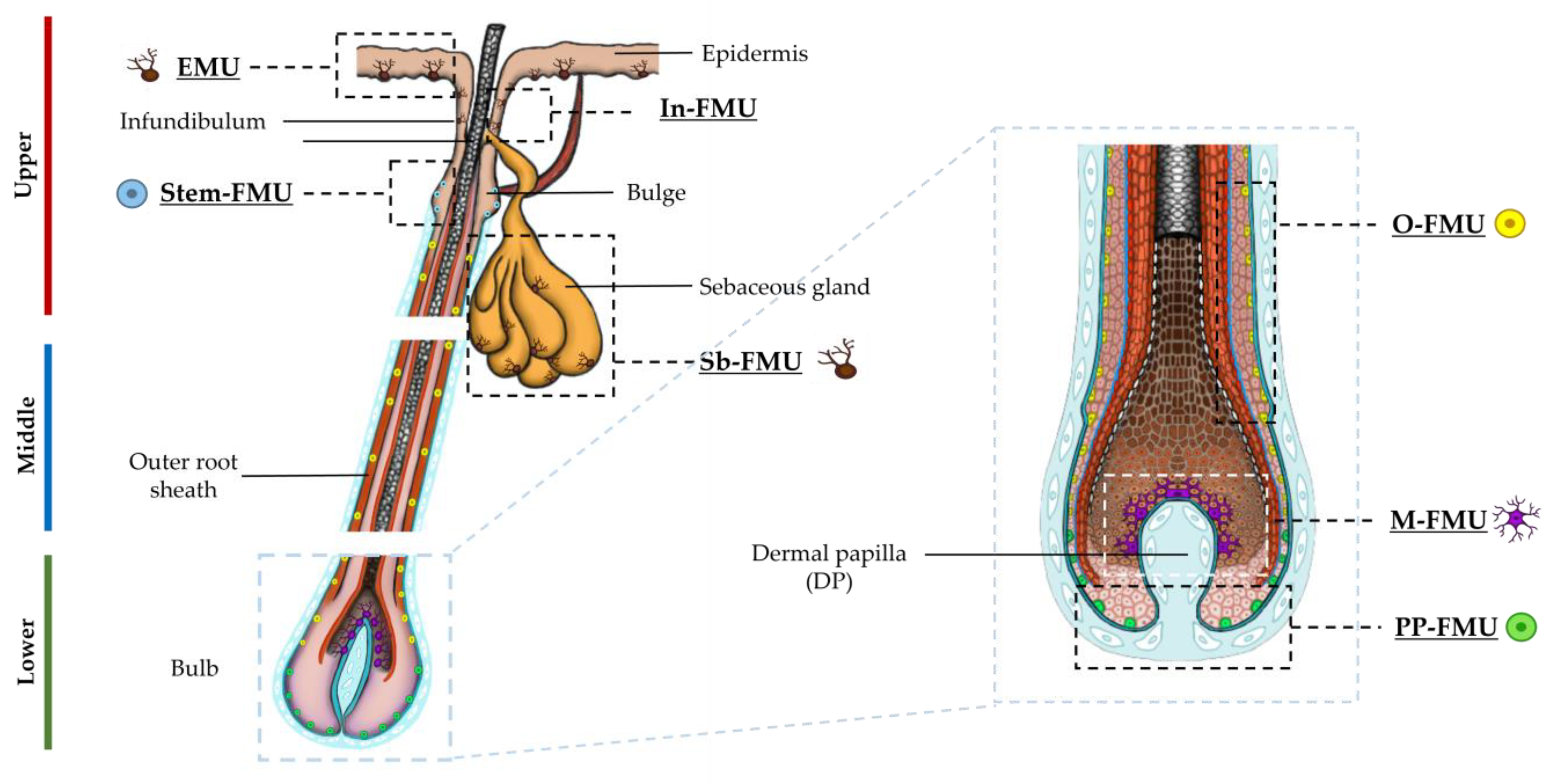
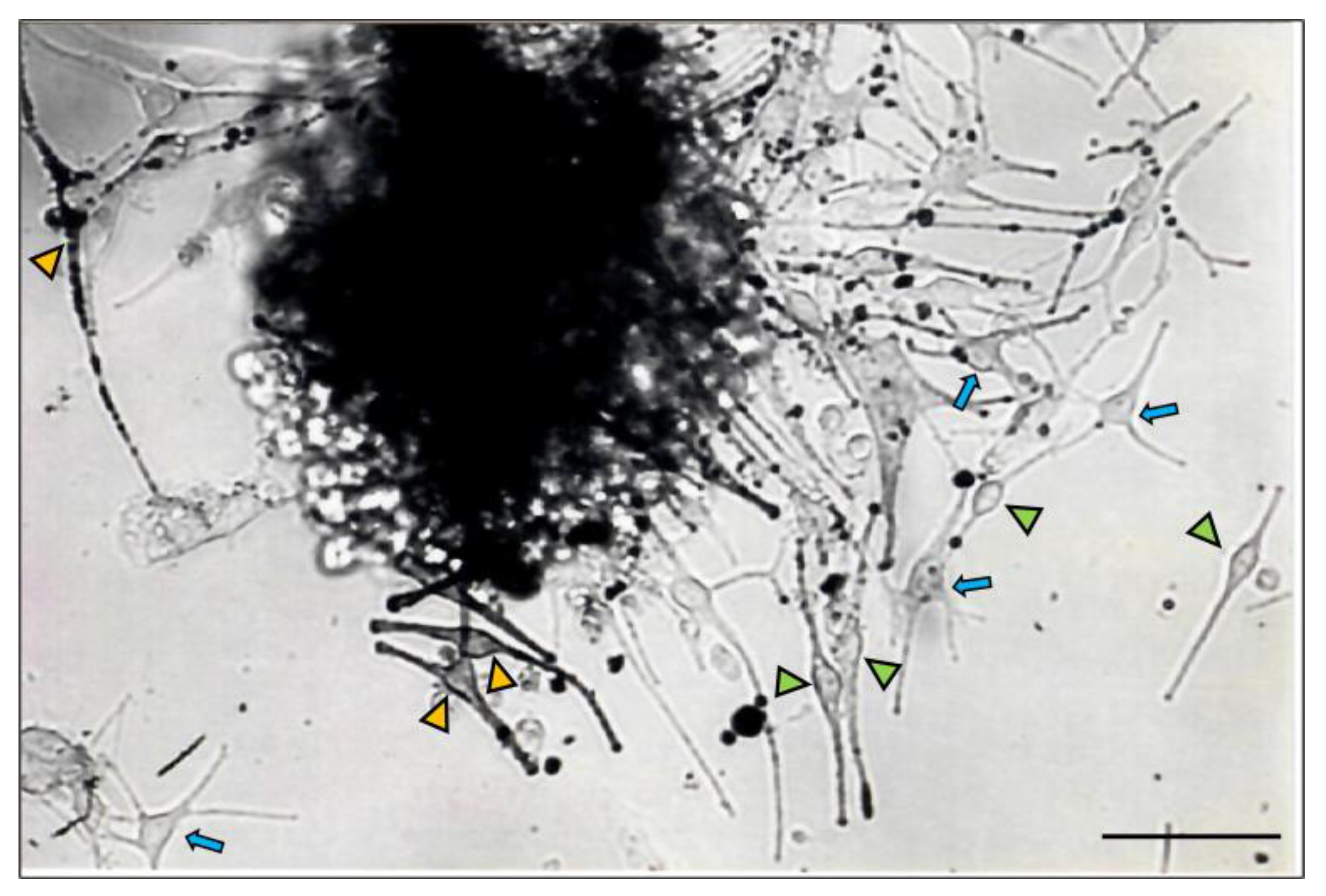
2.1. Distinct Melanocyte Subpopulations Can Be Isolated in Culture from the Adult Human Anagen Scalp Hair Bulb along a Differentiation/Maturation Spectrum
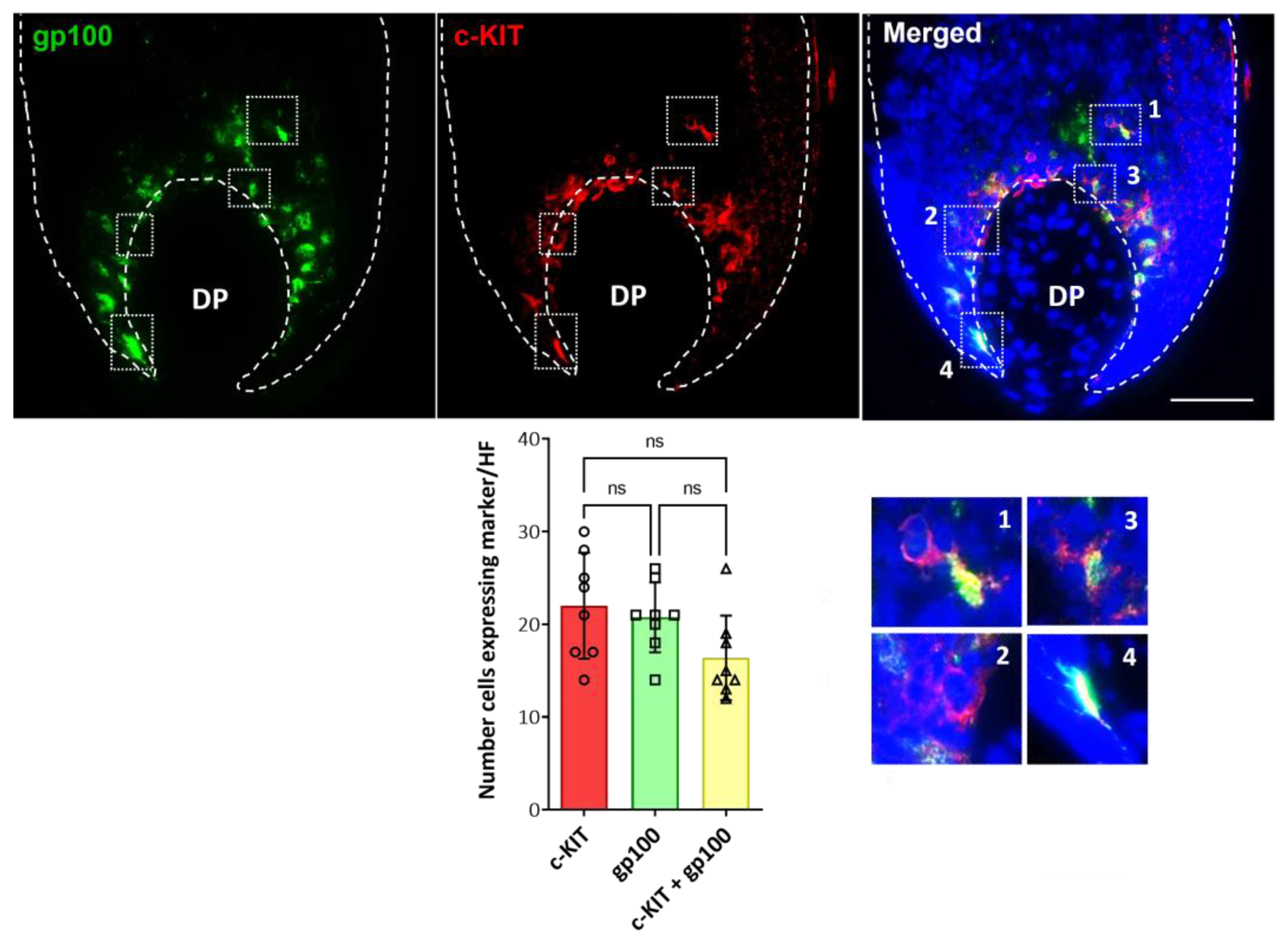
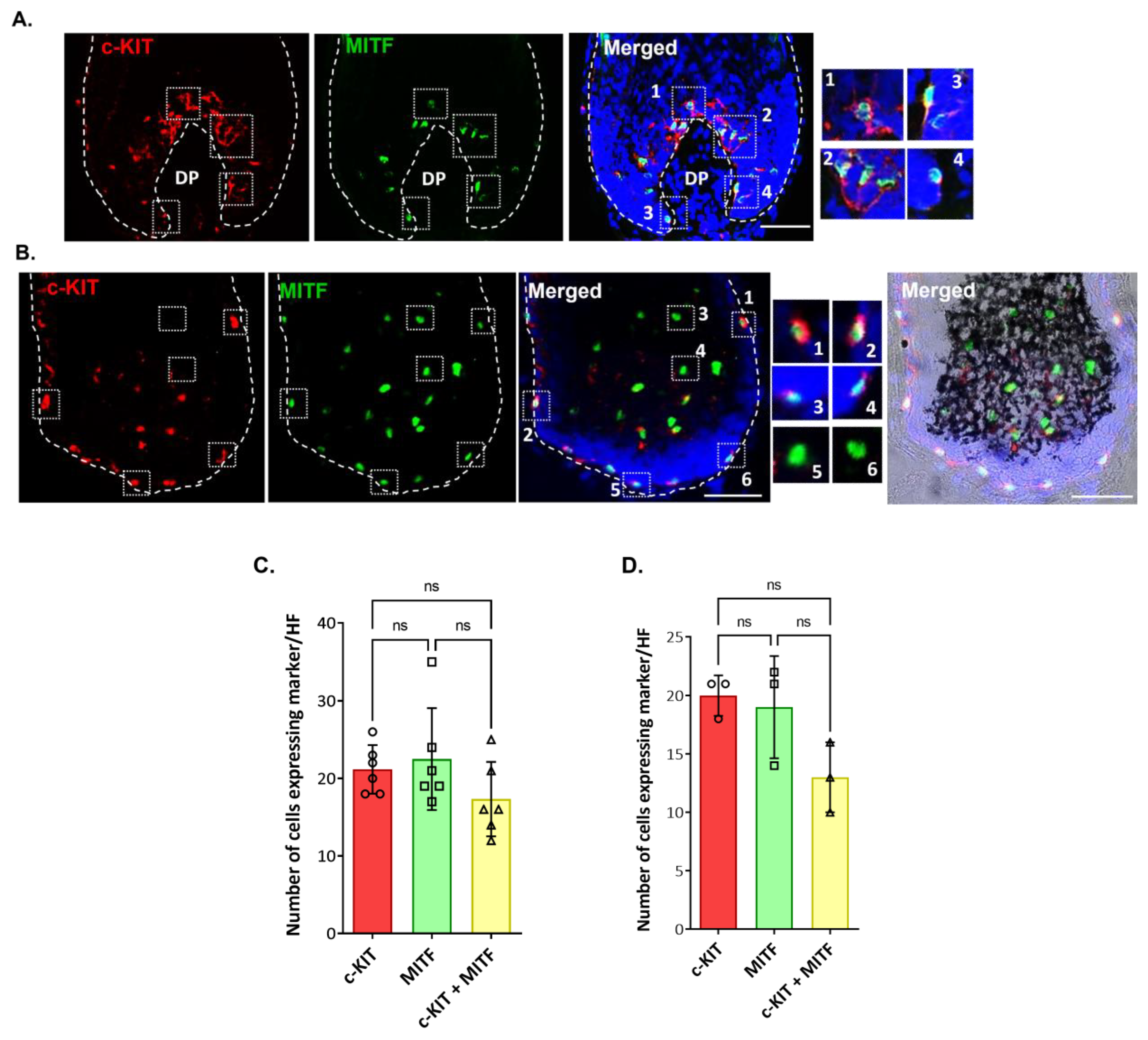
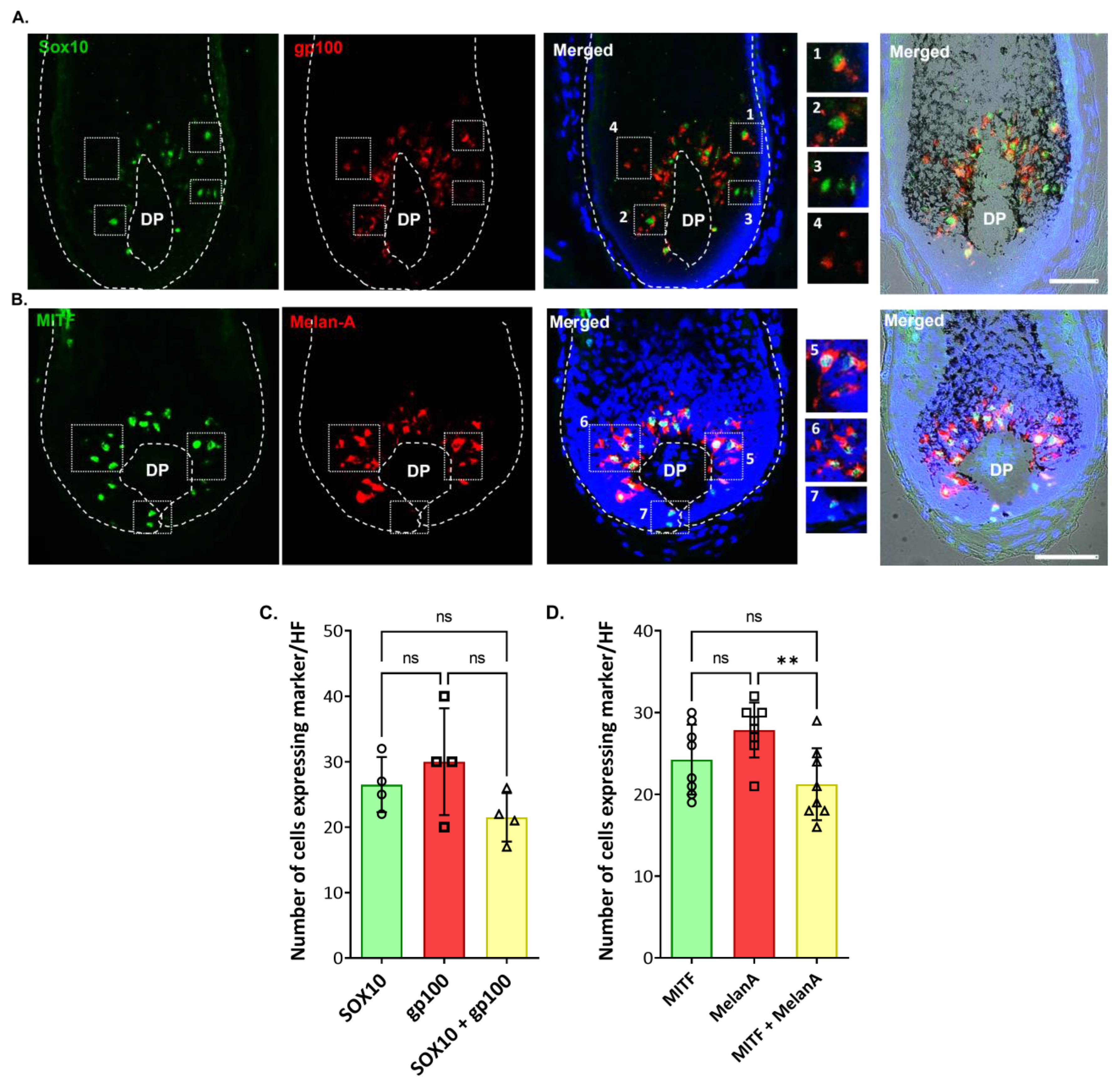
2.2. Localization and (Co)-Expression of Melanocyte Differentiation Markers in the Human Anagen Scalp Hair Bulb
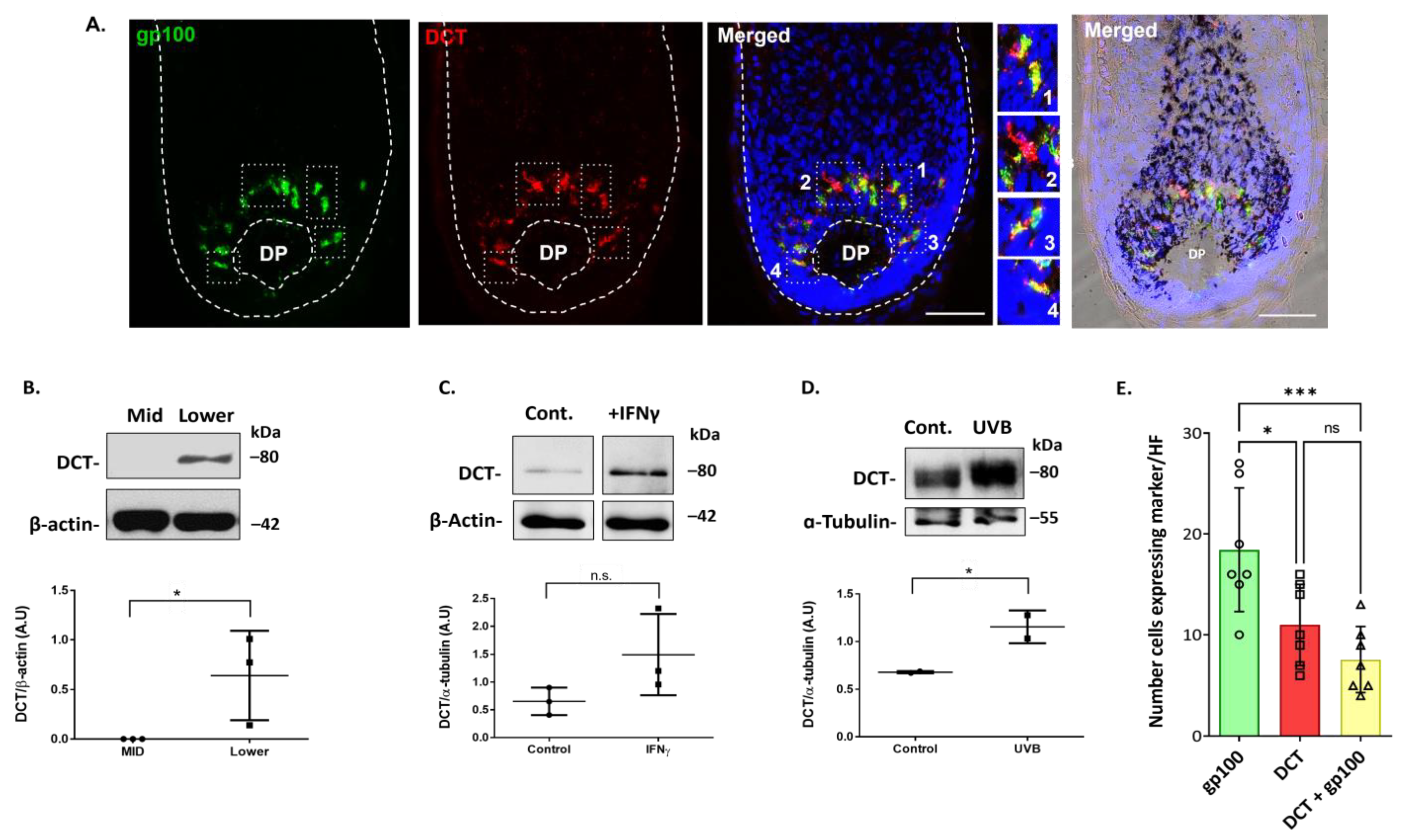
2.3. Dopachrome Tautomerase (DCT/TRP-2) Is Expressed by Melanocytes in the Human Scalp Hair Bulb, which Can Be Upregulated Further by Inflammatory (IFN-γ) and UVR Stressors
3. Discussion
4. Materials and Methods
4.1. Human HF Collection, Organ Histo-Culture and Follicular Melanocyte Isolation
4.2. Frozen Hair Sample Processing and Immunohistochemistry
4.3. Protein Extraction and Western-Blotting
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paus, R. Principles of Hair Cycle Control. J. Dermatol. 1998, 25, 793–802. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J.; Płonka, P.M.; Schallreuter, K.U.; Paus, R.; Tobin, D.J. Hair Follicle Pigmentation. J. Investig. Dermatol. 2005, 124, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Christoph, T.; Müller-Röver, S.; Audring, H.; Tobin, D.J.; Hermes, B.; Cotsarelis, G.; Rückert, R.; Paus, R. The Human Hair Follicle Immune System: Cellular Composition and Immune Privilege. Br. J. Dermatol. 2000, 142, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.J. The Cell Biology of Human Hair Follicle Pigmentation. Pigment. Cell Melanoma Res. 2010, 24, 75–88. [Google Scholar] [CrossRef]
- Slominski, A.; Paus, R. Melanogenesis Is Coupled to Murine Anagen: Toward New Concepts for the Role of Melanocytes and the Regulation of Melanogenesis in Hair Growth. J. Investig. Dermatol. 1993, 101, 90S–97S. [Google Scholar]
- Steingrímsson, E.; Copeland, N.G.; Jenkins, N.A. Melanocyte Stem Cell Maintenance and Hair Graying. Cell 2005, 121, 9–12. [Google Scholar] [CrossRef]
- Vandamme, N.; Berx, G. From Neural Crest Cells to Melanocytes: Cellular Plasticity during Development and Beyond. Cell. Mol. Life Sci. 2019, 76, 1919–1934. [Google Scholar] [CrossRef]
- Casalou, C.; Moreiras, H.; Mayatra, J.M.; Fabre, A.; Tobin, D.J. Loss of “Epidermal Melanin Unit” Integrity in Human Skin During Melanoma-Genesis. Front. Oncol. 2022, 12, 1318. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.D.B.; Nicu, C.; Picard, M.; Chéret, J.; Bedogni, B.; Tobin, D.J.; Paus, R. The Biology of Human Hair Greying. Biol. Rev. Camb. Philos. Soc. 2020, 96, 107–128. [Google Scholar] [CrossRef]
- Tobin, D.J.; Bystryn, J.-C. Different Populations of Melanocytes Are Present in Hair Follicles and Epidermis. Pigment. Cell Res. 1996, 9, 304–310. [Google Scholar] [CrossRef]
- Grichnik, J.M.; Ali, W.N.; Burch, J.A.; Byers, J.D.; Garcia, C.A.; Clark, R.E.; Shea, C.R. Kit expression reveals a population of precursor melanocytes in human skin. J. Investig. Dermatol. 1996, 106, 967–971. [Google Scholar] [CrossRef]
- Grichnik, J.M.; Burch, J.A.; Burchette, J.; Shea, C.R. The SCF/KIT Pathway Plays a Critical Role in the Control of Normal Human Melanocyte Homeostasis. J. Investig. Dermatol. 1998, 111, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.J.; Colen, S.R.; Bystryn, J.-C. Isolation and Long-Term Culture of Human Hair-Follicle Melanocytes. J. Investig. Dermatol. 1995, 104, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Kauser, S.; Thody, A.J.; Schallreuter, K.U.; Gummer, C.L.; Tobin, D.J. B-Endorphin as a Regulator of Human Hair Follicle Melanocyte Biology. J. Investig. Dermatol. 2004, 123, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Grützkau, A.; Radbruch, A. Small but Mighty: How the MACS®-Technology Based on Nanosized Superparamagnetic Particles Has Helped to Analyze the Immune System within the Last 20 Years. Cytom. Part A 2010, 77, 643–647. [Google Scholar] [CrossRef]
- Bashamboo, A.; Taylor, A.H.; Samuel, K.; Panthier, J.-J.; Whetton, A.D.; Forrester, L.M. The Survival of Differentiating Embryonic Stem Cells Is Dependent on The SCF-KIT Pathway. J. Cell Sci. 2006, 119, 3039–3046. [Google Scholar] [CrossRef] [PubMed]
- Lennartsson, J.; Rönnstrand, L. Stem cell factor receptor/c-kit: From basic science to clinical implications. Physiol. Rev. 2012, 92, 1619–1649. [Google Scholar] [CrossRef]
- Nishimura, E.K.; Jordan, S.A.; Oshima, H.; Yoshida, H.; Osawa, M.; Moriyama, M.; Jackson, I.J.; Barrandon, Y.; Miyachi, Y.; Nishikawa, S.-I. Dominant role of the niche in melanocyte stem-cell fate determination. Nature 2002, 416, 854–860. [Google Scholar] [CrossRef]
- Sun, Y.; Song, M.; Stevanović, S.; Jankowiak, C.; Paschen, A.; Rammensee, H.-G.; Schadendorf, D. Identification of a New HLA-A*0201-Restricted T-Cell Epitope from the Tyrosinase-Related Protein 2 (TRP2) Melanoma Antigen. Int. J. Cancer 2000, 87, 399–404. [Google Scholar] [CrossRef]
- Commo, S.; Gaillard, O.; Thibaut, S.; Bernard, B.A. Absence of TRP-2 in Melanogenic Melanocytes of Human Hair. Pigment. Cell Res. 2004, 17, 488–497. [Google Scholar] [CrossRef]
- Körner, A.M.; Pawelek, J.M. Dopachrome conversion: A possible control point in melanin biosynthesis. J. Investig. Dermatol. 1980, 75, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, A.; Fisher, D.E. Key Discoveries in Melanocyte Development. J. Investig. Dermatol. 2011, 131, E2–E4. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-P.; Booker, R.C.; Morrison, S.J.; Le, L.Q. Identification of Hair Shaft Progenitors That Create a Niche for Hair Pigmentation. Genes Dev. 2017, 31, 744–756. [Google Scholar] [CrossRef]
- Nishimura, E.K.; Granter, S.R.; Fisher, D.E. Mechanisms of Hair Graying: Incomplete Melanocyte Stem Cell Maintenance in the Niche. Science 2005, 307, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.J. Aging of the Hair Follicle Pigmentation System. Int. J. Trichology 2009, 1, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Kauser, S.; Westgate, G.E.; Green, M.R.; Tobin, D.J. Human Hair Follicle and Epidermal Melanocytes Exhibit Striking Differences in Their Aging Profile Which Involves Catalase. J. Investig. Dermatol. 2011, 131, 979–982. [Google Scholar] [CrossRef]
- Sharov, A.; Tobin, J.D.; Sharova, T.Y.; Atoyan, R.; Botchkarev, V.A. Changes in different melanocyte populations during hair follicle involution (catagen). J. Investig. Dermatol. 2005, 125, 1259–1267. [Google Scholar] [CrossRef]
- Polisetti, N.; Schlötzer-Schrehardt, U.; Reinhard, T.; Schlunck, G. Isolation and Enrichment of Melanocytes From Human Corneal Limbus Using CD117 (c-Kit) As Selection Marker. Sci. Rep. 2020, 10, 17588. [Google Scholar] [CrossRef]
- Li, S.; Zenkel, M.; Kruse, F.E.; Gießl, A.; Schlötzer-Schrehardt, U. Identification, Isolation, and Characterization of Melanocyte Precursor Cells in the Human Limbal Stroma. Int. J. Mol. Sci. 2022, 23, 3756. [Google Scholar] [CrossRef]
- Yoshida, H.; Kunisada, T.; Kusakabe, M.; Nishikawa, S.; Nishikawa, S.-I. Distinct stages of melanocyte differentiation revealed by analysis of nonuniform pigmentation patterns. Development 1996, 122, 1207–1214. [Google Scholar] [CrossRef]
- Botchkareva, N.V.; Khlgatian, M.; Jack Longley, B.; Botchkarev, V.A.; Gilchrest, B.A. Scf/C-Kit Signaling is required for cyclic regeneration of the hair pigmentation Unit. FASEB J. 2001, 15, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.M.J.; Tobin, D.J.; Botchkareva, N.; Maurer, M.; Paus, R. Migration of melanoblasts into the developing murine hair follicle is accompanied by transient c-Kit expression. J. Histochem. Cytochem. 2002, 50, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Kelsh, R.N. Sorting out Sox10 Functions in Neural Crest Development. BioEssays 2006, 28, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Cronin, J.C.; Watkins-Chow, D.E.; Incao, A.; Hasskamp, J.H.; Schönewolf, N.; Aoude, L.G.; Hayward, N.K.; Bastian, B.C.; Dummer, R.; Loftus, S.K.; et al. SOX10 Ablation Arrests Cell Cycle, Induces Senescence, and Suppresses Melanomagenesis. Cancer Res. 2013, 73, 5709–5718. [Google Scholar] [CrossRef] [PubMed]
- Shakhova, O.; Zingg, D.; Schaefer, S.M.; Hari, L.; Civenni, G.; Blunschi, J.; Claudinot, S.; Okoniewski, M.; Beermann, F.; Mihic-Probst, D.; et al. Sox10 Promotes the Formation and Maintenance of Giant Congenital Naevi and Melanoma. Nat. Cell Biol. 2012, 14, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.L.; Baxter, L.L.; Loftus, S.K.; Pavan, W.J. Sox Proteins in Melanocyte Development and Melanoma. Pigment. Cell Melanoma Res. 2010, 23, 496–513. [Google Scholar] [CrossRef]
- Michalak-Mićka, K.; Büchler, V.L.; Zapiórkowska-Blumer, N.; Biedermann, T.; Klar, A.S. Characterization of a Melanocyte Progenitor Population in Human Interfollicular Epidermis. Cell Rep. 2022, 38, 110419. [Google Scholar] [CrossRef]
- Kauser, S.; Thody, A.J.; Schallreuter, K.U.; Gummer, C.L.; Tobin, D.J. A Fully Functional Proopiomelanocortin/Melanocortin-1 Receptor System Regulates the Differentiation of Human Scalp Hair Follicle Melanocytes. Endocrinology 2005, 146, 532–543. [Google Scholar] [CrossRef]
- Potterf, S.B.; Furumura, M.; Dunn, K.J.; Arnheiter, H.; Pavan, W.J. Transcription Factor Hierarchy in Waardenburg Syndrome: Regulation of MITF Expression by SOX10 and PAX3. Hum. Genet. 2000, 107, 1–6. [Google Scholar] [CrossRef]
- Ludwig, A.; Rehberg, S.; Wegner, M. Melanocyte-Specific Expression of Dopachrome Tautomerase Is Dependent on Synergistic Gene Activation by the Sox10 and Mitf Transcription Factors. FEBS Lett. 2004, 556, 236–244. [Google Scholar] [CrossRef]
- Guyonneau, L.; Murisier, F.; Rossier, A.; Moulin, A.; Beermann, F. Melanocytes and Pigmentation Are Affected in Dopachrome Tautomerase Knockout Mice. Mol. Cell. Biol. 2004, 24, 3396–3403. [Google Scholar] [CrossRef]
- Seiberg, M. Age-Induced Hair Greying—The Multiple Effects of Oxidative Stress. Int. J. Cosmet. Sci. 2013, 35, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Michard, Q.; Commo, S.; Belaidi, J.-P.; Alleaume, A.-M.; Michelet, J.-F.; Daronnat, E.; Eilstein, J.; Duche, D.; Marrot, L.; Bernard, B.A. TRP-2 Specifically Decreases WM35 Cell Sensitivity to Oxidative Stress. Free. Radic. Biol. Med. 2008, 44, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Dilshat, R.; Nhung Vu, H.; Steingrímsson, E. Epigenetic regulation during melanocyte development and homeostasis. Exp. Dermatol. 2021, 30, 1033–1050. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casalou, C.; Mayatra, J.M.; Tobin, D.J. Beyond the Epidermal-Melanin-Unit: The Human Scalp Anagen Hair Bulb Is Home to Multiple Melanocyte Subpopulations of Variable Melanogenic Capacity. Int. J. Mol. Sci. 2023, 24, 12809. https://doi.org/10.3390/ijms241612809
Casalou C, Mayatra JM, Tobin DJ. Beyond the Epidermal-Melanin-Unit: The Human Scalp Anagen Hair Bulb Is Home to Multiple Melanocyte Subpopulations of Variable Melanogenic Capacity. International Journal of Molecular Sciences. 2023; 24(16):12809. https://doi.org/10.3390/ijms241612809
Chicago/Turabian StyleCasalou, Cristina, Jay M. Mayatra, and Desmond J. Tobin. 2023. "Beyond the Epidermal-Melanin-Unit: The Human Scalp Anagen Hair Bulb Is Home to Multiple Melanocyte Subpopulations of Variable Melanogenic Capacity" International Journal of Molecular Sciences 24, no. 16: 12809. https://doi.org/10.3390/ijms241612809
APA StyleCasalou, C., Mayatra, J. M., & Tobin, D. J. (2023). Beyond the Epidermal-Melanin-Unit: The Human Scalp Anagen Hair Bulb Is Home to Multiple Melanocyte Subpopulations of Variable Melanogenic Capacity. International Journal of Molecular Sciences, 24(16), 12809. https://doi.org/10.3390/ijms241612809






