Endocytosis and Endocytic Motifs across the Connexin Gene Family
Abstract
:1. Introduction
2. Results
2.1. Connexin Family Members Contain Both Canonical Tyrosine- and Di-Leucine-Based Endocytic Motifs
2.2. Non-Canonical Tyrosine and Di-Leucine-Based Endocytic Motifs in Connexins
2.3. Location of Endocytic Motifs in Connexins
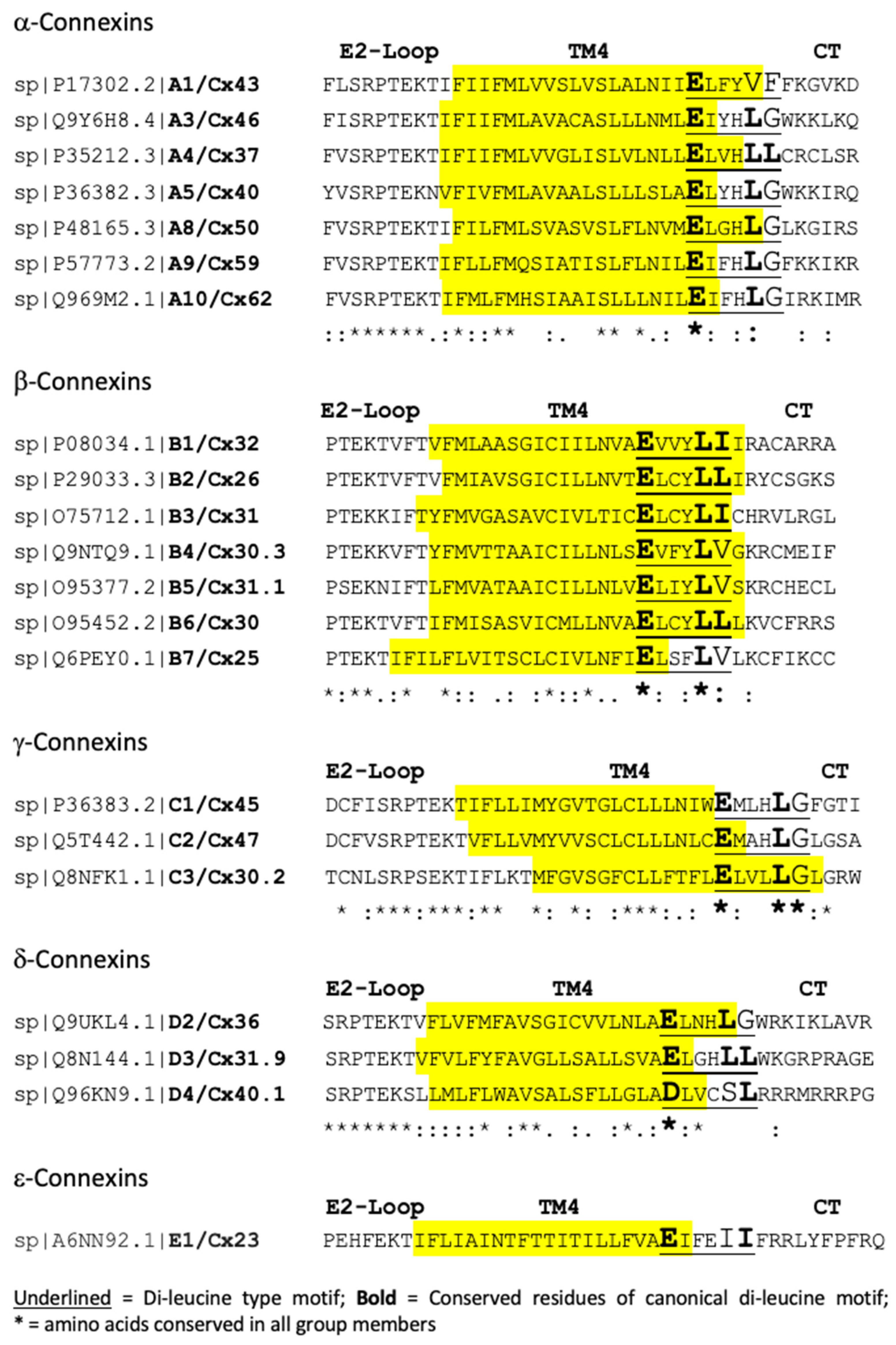
2.4. Conservation of Endocytic Motifs
2.5. Phylogenetic Relation of Endocytic Motifs
2.6. Conserved Canonical and Non-Canonical Di-Leucine-Type Motifs Located in the TM4/CT Transition Zone
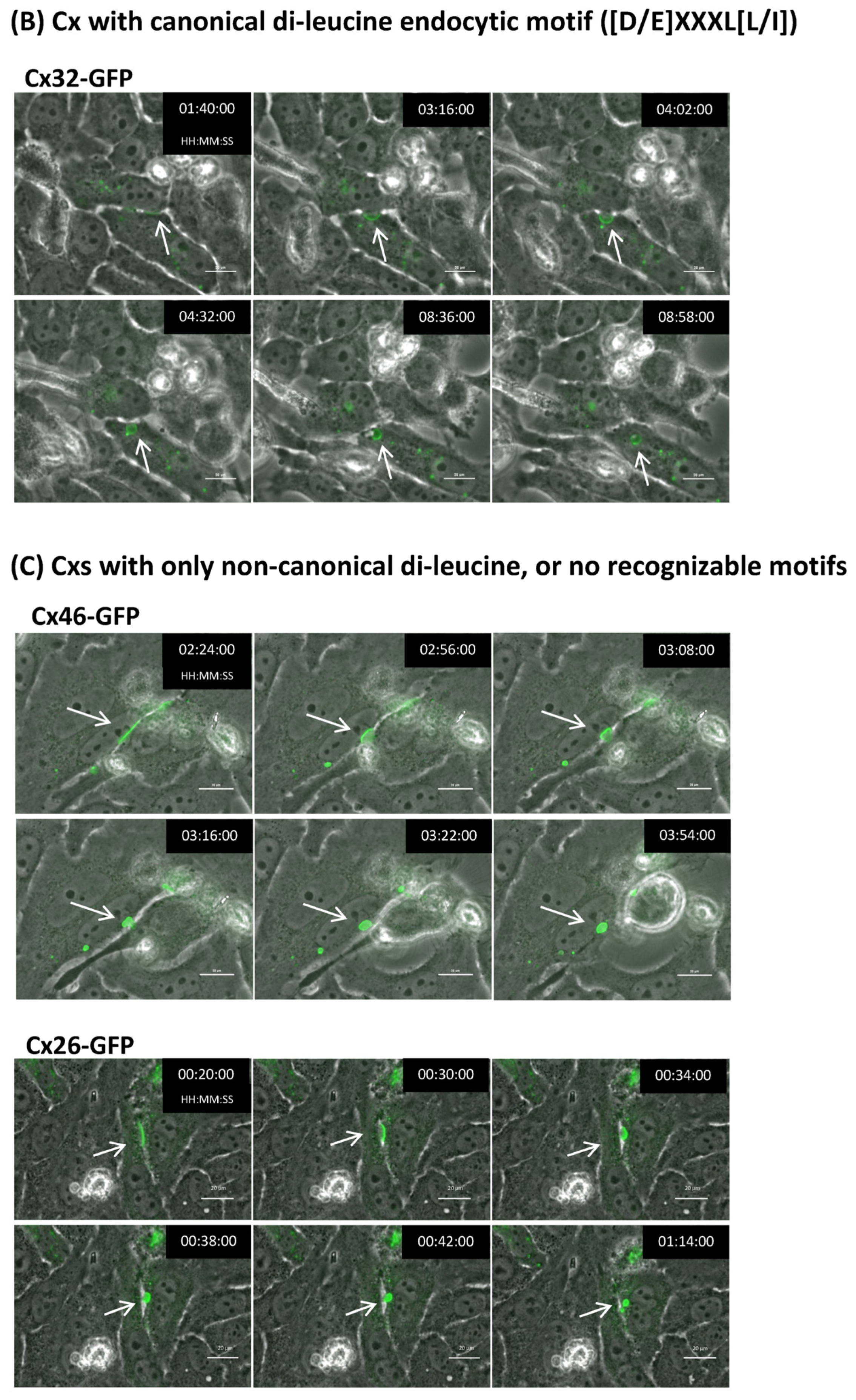
2.7. Connexins with and without Identifiable Endocytic Motifs Endocytose to Form Typical Cytoplasmic Annular Gap Junctions
3. Discussion
4. Materials and Methods
4.1. Endocytic Motif Search
4.2. Live Cell Imaging
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Herve, J.C.; Derangeon, M. Gap-junction-mediated cell-to-cell communication. Cell Tissue Res. 2013, 352, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.M.; Gilula, N.B. The gap junction communication channel. Cell 1996, 84, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.S.; Axelsen, L.N.; Sorgen, P.L.; Verma, V.; Delmar, M.; Holstein-Rathlou, N.H. Gap junctions. Compr. Physiol. 2012, 2, 1981–2035. [Google Scholar] [CrossRef] [PubMed]
- Falk, M.M.; Bell, C.L.; Kells Andrews, R.K.; Murray, S.A. Formation, trafficking and processing of annular gap junctions. BMC Cell Biol. 2016, 17 (Suppl. 1), 22. [Google Scholar] [CrossRef] [PubMed]
- Thévenin, A.F.; Kowal, T.J.; Fong, J.T.; Kells, R.M.; Fisher, C.G.; Falk, M.M. Proteins and mechanisms regulating gap junction assembly, internalization and degradation. Physiology 2013, 28, 93–116. [Google Scholar] [CrossRef]
- Berthoud, V.M.; Minogue, P.J.; Laing, J.G.; Beyer, E.C. Pathways for degradation of connexins and gap junctions. Cardiovasc. Res. 2004, 62, 256–267. [Google Scholar] [CrossRef]
- Fallon, R.F.; Goodenough, D.A. Five-hour half-life of mouse liver gap-junction protein. J. Cell Biol. 1981, 90, 521–526. [Google Scholar] [CrossRef]
- Laird, D.W.; Puranam, K.L.; Revel, J.P. Turnover and phosphorylation dynamics of connexin43 gap junction protein in cultured cardiac myocytes. Biochem. J. 1991, 273 Pt 1, 67–72. [Google Scholar] [CrossRef]
- Bitsikas, V.; Correa, I.R., Jr.; Nichols, B.J. Clathrin-independent pathways do not contribute significantly to endocytic flux. Elife 2014, 3, e03970. [Google Scholar] [CrossRef]
- Gumpert, A.M.; Varco, J.S.; Baker, S.M.; Piehl, M.; Falk, M.M. Double-membrane gap junction internalization requires the clathrin-mediated endocytic machinery. FEBS Lett. 2008, 582, 2887–2892. [Google Scholar] [CrossRef]
- Nickel, B.M.; DeFranco, B.H.; Gay, V.L.; Murray, S.A. Clathrin and Cx43 gap junction plaque endoexocytosis. Biochem. Biophys. Res. Commun. 2008, 374, 679–682. [Google Scholar] [CrossRef]
- Piehl, M.; Lehmann, C.; Gumpert, A.; Denizot, J.P.; Segretain, D.; Falk, M.M. Internalization of large double-membrane intercellular vesicles by a clathrin-dependent endocytic process. Mol. Biol. Cell 2007, 18, 337–347. [Google Scholar] [CrossRef]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Kirchhausen, T.; Owen, D.; Harrison, S.C. Molecular structure, function, and dynamics of clathrin-mediated membrane traffic. Cold Spring Harb. Perspect. Biol. 2014, 6, a016725. [Google Scholar] [CrossRef]
- Bonifacino, J.S.; Traub, L.M. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003, 72, 395–447. [Google Scholar] [CrossRef] [PubMed]
- Traub, L.M. Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J. Cell Biol. 2003, 163, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Traub, L.M.; Bonifacino, J.S. Cargo recognition in clathrin-mediated endocytosis. Cold Spring Harb. Perspect. Biol. 2013, 5, a016790. [Google Scholar] [CrossRef]
- Gephart, J.D.; Singh, B.; Higginbotham, J.N.; Franklin, J.L.; Gonzalez, A.; Folsch, H.; Coffey, R.J. Identification of a novel mono-leucine basolateral sorting motif within the cytoplasmic domain of amphiregulin. Traffic 2011, 12, 1793–1804. [Google Scholar] [CrossRef] [PubMed]
- Kozik, P.; Francis, R.W.; Seaman, M.N.; Robinson, M.S. A screen for endocytic motifs. Traffic 2010, 11, 843–855. [Google Scholar] [CrossRef]
- Pandey, K.N. Endocytosis and trafficking of natriuretic peptide receptor-A: Potential role of short sequence motifs. Membranes 2015, 5, 253–287. [Google Scholar] [CrossRef]
- Wang, C.C.; Sato, K.; Otsuka, Y.; Otsu, W.; Inaba, M. Clathrin-mediated endocytosis of mammalian erythroid AE1 anion exchanger facilitated by a YXXPhi or a noncanonical YXXXPhi motif in the N-terminal stretch. J. Vet. Med. Sci. 2012, 74, 17–25. [Google Scholar] [CrossRef]
- Abascal, F.; Zardoya, R. Evolutionary analyses of gap junction protein families. Biochim. Biophys. Acta 2013, 1828, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Beyer, E.C.; Berthoud, V.M. Gap junction gene and protein families: Connexins, innexins, and pannexins. Biochim. Biophys. Acta Biomembr. 2018, 1860, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Sohl, G.; Willecke, K. Gap junctions and the connexin protein family. Cardiovasc. Res. 2004, 62, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Eastman, S.D.; Chen, T.H.; Falk, M.M.; Mendelson, T.C.; Iovine, M.K. Phylogenetic analysis of three complete gap junction gene families reveals lineage-specific duplications and highly supported gene classes. Genomics 2006, 87, 265–274. [Google Scholar] [CrossRef]
- Watanabe, M. Gap Junction in the teleost fish lineage: Duplicated connexins may contribute to skin pattern formation and body shape determination. Front. Cell Dev. Biol. 2017, 5, 13. [Google Scholar] [CrossRef]
- Fong, J.T.; Kells, R.M.; Falk, M.M. Two tyrosine-based sorting signals in the Cx43 C-terminus cooperate to mediate gap junction endocytosis. Mol. Biol. Cell 2013, 24, 2834–2848. [Google Scholar] [CrossRef]
- Collins, B.M.; McCoy, A.J.; Kent, H.M.; Evans, P.R.; Owen, D.J. Molecular architecture and functional model of the endocytic AP2 complex. Cell 2002, 109, 523–535. [Google Scholar] [CrossRef]
- Ray, A.; Katoch, P.; Jain, N.; Mehta, P.P. Dileucine-like motifs in the C-terminal tail of connexin32 control its endocytosis and assembly into gap junctions. J. Cell Sci. 2018, 131, jcs207340. [Google Scholar] [CrossRef]
- Elfgang, C.; Eckert, R.; Lichtenberg-Frate, H.; Butterweck, A.; Traub, O.; Klein, R.A.; Hulser, D.F.; Willecke, K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J. Cell Biol. 1995, 129, 805–817. [Google Scholar] [CrossRef]
- Thomas, M.A.; Zosso, N.; Scerri, I.; Demaurex, N.; Chanson, M.; Staub, O. A tyrosine-based sorting signal is involved in connexin43 stability and gap junction turnover. J. Cell Sci. 2003, 116, 2213–2222. [Google Scholar] [CrossRef] [PubMed]
- Defourny, J.; Audouard, C.; Davy, A.; Thiry, M. Efnb2 haploinsufficiency induces early gap junction plaque disassembly and endocytosis in the cochlea. Brain Res. Bull. 2021, 174, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ruan, Z.; Yang, X.; Chu, K.; Wu, H.; Li, Y.; Huang, Y. Connexin 31.1 degradation requires the Clathrin-mediated autophagy in NSCLC cell H1299. J. Cell. Mol. Med. 2015, 19, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Chen, S.; Shao, Q.; Chen, J.; Bijian, K.; Laird, D.W.; Alaoui-Jamali, M.A. Dynamin 2 interacts with connexin 26 to regulate its degradation and function in gap junction formation. Int. J. Biochem. Cell Biol. 2014, 55, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Schlingmann, B.; Schadzek, P.; Hemmerling, F.; Schaarschmidt, F.; Heisterkamp, A.; Ngezahayo, A. The role of the C-terminus in functional expression and internalization of rat connexin46 (rCx46). J. Bioenerg. Biomembr. 2013, 45, 59–70. [Google Scholar] [CrossRef]
- Catarino, S.; Ramalho, J.S.; Marques, C.; Pereira, P.; Girao, H. Ubiquitin-mediated internalization of connexin43 is independent of the canonical endocytic tyrosine-sorting signal. Biochem. J. 2011, 437, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Girao, H.; Catarino, S.; Pereira, P. Eps15 interacts with ubiquitinated Cx43 and mediates its internalization. Exp. Cell Res. 2009, 315, 3587–3597. [Google Scholar] [CrossRef]
- Leithe, E.; Kjenseth, A.; Sirnes, S.; Stenmark, H.; Brech, A.; Rivedal, E. Ubiquitylation of the gap junction protein connexin-43 signals its trafficking from early endosomes to lysosomes in a process mediated by Hrs and Tsg101. J. Cell Sci. 2009, 122, 3883–3893. [Google Scholar] [CrossRef]
- Ribeiro-Rodrigues, T.M.; Catarino, S.; Marques, C.; Ferreira, J.V.; Martins-Marques, T.; Pereira, P.; Girao, H. AMSH-mediated deubiquitination of Cx43 regulates internalization and degradation of gap junctions. FASEB J. 2014, 28, 4629–4641. [Google Scholar] [CrossRef]
- Kells-Andrews, R.M.; Margraf, R.M.; Fisher, C.G.; Falk, M.M. Connexin 43 K63-polyubiquitylation on lysines 264 and 303 regulates gap junction internalization. J. Cell Sci. 2018, 131, jcs204321. [Google Scholar] [CrossRef]
- Komander, D. The emerging complexity of protein ubiquitination. Biochem. Soc. Trans. 2009, 37, 937–953. [Google Scholar] [CrossRef] [PubMed]
- Komander, D.; Rape, M. The ubiquitin code. Annu. Rev. Biochem. 2012, 81, 203–229. [Google Scholar] [CrossRef] [PubMed]
- Basheer, W.A.; Harris, B.S.; Mentrup, H.L.; Abreha, M.; Thames, E.L.; Lea, J.B.; Swing, D.A.; Copeland, N.G.; Jenkins, N.A.; Price, R.L.; et al. Cardiomyocyte-specific overexpression of the ubiquitin ligase Wwp1 contributes to reduction in Connexin 43 and arrhythmogenesis. J. Mol. Cell Cardiol. 2015, 88, 1–13. [Google Scholar] [CrossRef]
- Chen, V.C.; Kristensen, A.R.; Foster, L.J.; Naus, C. Association of connexin43 with E3 ubiquitin ligase TRIM21 reveals a mechanism for gap junction phosphodegron control. J. Proteome Res. 2012, 11, 6134–6146. [Google Scholar] [CrossRef]
- Fykerud, T.A.; Kjenseth, A.; Schink, K.O.; Sirnes, S.; Bruun, J.; Omori, Y.; Brech, A.; Rivedal, E.; Leithe, E. Smad ubiquitination regulatory factor-2 controls gap junction intercellular communication by modulating endocytosis and degradation of connexin43. J. Cell Sci. 2012, 125, 3966–3976. [Google Scholar] [CrossRef] [PubMed]
- Leykauf, K.; Salek, M.; Bomke, J.; Frech, M.; Lehmann, W.D.; Durst, M.; Alonso, A. Ubiquitin protein ligase Nedd4 binds to connexin43 by a phosphorylation-modulated process. J. Cell Sci. 2006, 119, 3634–3642. [Google Scholar] [CrossRef]
- Kim, H.C.; Huibregtse, J.M. Polyubiquitination by HECT E3s and the determinants of chain type specificity. Mol. Cell Biol. 2009, 29, 3307–3318. [Google Scholar] [CrossRef]
- Spagnol, G.; Kieken, F.; Kopanic, J.L.; Li, H.; Zach, S.; Stauch, K.L.; Grosely, R.; Sorgen, P.L. Structural studies of the Nedd4 WW domains and their selectivity for the connexin43 (Cx43) carboxyl terminus. J. Biol. Chem. 2016, 291, 7637–7650. [Google Scholar] [CrossRef] [PubMed]
- Lynn, B.D.; Li, X.; Hormuzdi, S.G.; Griffiths, E.K.; McGlade, C.J.; Nagy, J.I. E3 ubiquitin ligases LNX1 and LNX2 localize at neuronal gap junctions formed by connexin36 in rodent brain and molecularly interact with connexin36. Eur. J. Neurosci. 2018, 48, 3062–3081. [Google Scholar] [CrossRef]
- Kopanic, J.L.; Al-mugotir, M.H.; Kieken, F.; Zach, S.; Trease, A.J.; Sorgen, P.L. Characterization of the connexin45 carboxyl-terminal domain structure and interactions with molecular partners. Biophys. J. 2014, 106, 2184–2195. [Google Scholar] [CrossRef]
- Keyel, P.A.; Mishra, S.K.; Roth, R.; Heuser, J.E.; Watkins, S.C.; Traub, L.M. A single common portal for clathrin-mediated endocytosis of distinct cargo governed by cargo-selective adaptors. Mol. Biol. Cell. 2006, 17, 4300–4317. [Google Scholar] [CrossRef] [PubMed]
- Traub, L.M.; Lukacs, G.L. Decoding ubiquitin sorting signals for clathrin-dependent endocytosis by CLASPs. J. Cell Sci. 2007, 120, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Benmerah, A.; Lamaze, C.; Begue, B.; Schmid, S.L.; Dautry-Varsat, A.; Cerf-Bensussan, N. AP-2/Eps15 interaction is required for receptor-mediated endocytosis. J. Cell Biol. 1998, 140, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, R.C.; Wendland, B. Endocytosis of membrane receptors: Two pathways are better than one. Proc. Natl. Acad. Sci. USA 2005, 102, 2679–2680. [Google Scholar] [CrossRef]
- Kotova, A.; Timonina, K.; Zoidl, G.R. Endocytosis of Connexin 36 is Mediated by Interaction with Caveolin-1. Int. J. Mol. Sci. 2020, 21, 5401. [Google Scholar] [CrossRef]


| Cx Gene Name | Cx Protein Name | Type of Endocytic Signal | Location of Endocytic Signal | |
|---|---|---|---|---|
| Intracellular-Loop | C-Terminal Domain | |||
| A1 | CX43 * | Tyrosine based (YXXF) | --- | Y230VFF; Y265AYF; Y286KLV |
| A4 | CX37 * | --- | Y266LPV; Y281NGL | |
| A8 | CX50 | --- | Y266QLL; Y278FPL; (Y316QETL); Y323AQV | |
| A9 | CX59 | --- | (D283YNLL); Y284NLL; Y297PSL; (L500TNNLI) | |
| A10 | CX62 | --- | (L433SRLL); Y513VCV | |
| B4 | CX30.3 | Y117DNL | --- | |
| C1 | CX45 * | Y141PEM | Y301TEL | |
| C2 | CX47 | --- | Y330SLV | |
| D2 | CX36 * | Y109STV | --- | |
| E1 | CX23 | --- | Y200FPF | |
| B1 | CX32 * | Di-leucine based | (E103KKMLR) | E247INKLL; (L260KDILR) |
| B6 | CX30 | --- | E243MNELI | |
| C3 | CX30.2 (CX31.3) | E111EETLI | --- | |
| D3 | CX31.9 | --- | E233AQKLL | |
| A3 | CX46 * | Non-canonical or Not identifiable | --- | (D296FKLL); (E367AGAAPLL) |
| A5 | CX40 | (E105KRKLR) | (L145QGTLL) | |
| B2 | CX26 * | --- | --- | |
| B3 | CX31 | --- | --- | |
| B5 | CX31.1 | --- | (L245SGDLI); (D255SHPPLL) | |
| B7 | CX25 | --- | --- | |
| D4 | CX40.1 | --- | --- | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fisher, C.G.; Falk, M.M. Endocytosis and Endocytic Motifs across the Connexin Gene Family. Int. J. Mol. Sci. 2023, 24, 12851. https://doi.org/10.3390/ijms241612851
Fisher CG, Falk MM. Endocytosis and Endocytic Motifs across the Connexin Gene Family. International Journal of Molecular Sciences. 2023; 24(16):12851. https://doi.org/10.3390/ijms241612851
Chicago/Turabian StyleFisher, Charles G., and Matthias M. Falk. 2023. "Endocytosis and Endocytic Motifs across the Connexin Gene Family" International Journal of Molecular Sciences 24, no. 16: 12851. https://doi.org/10.3390/ijms241612851






