Pharmacological Prevention of Ectopic Erythrophagocytosis by Cilostazol Mitigates Ferroptosis in NASH
Abstract
:1. Introduction
2. Results
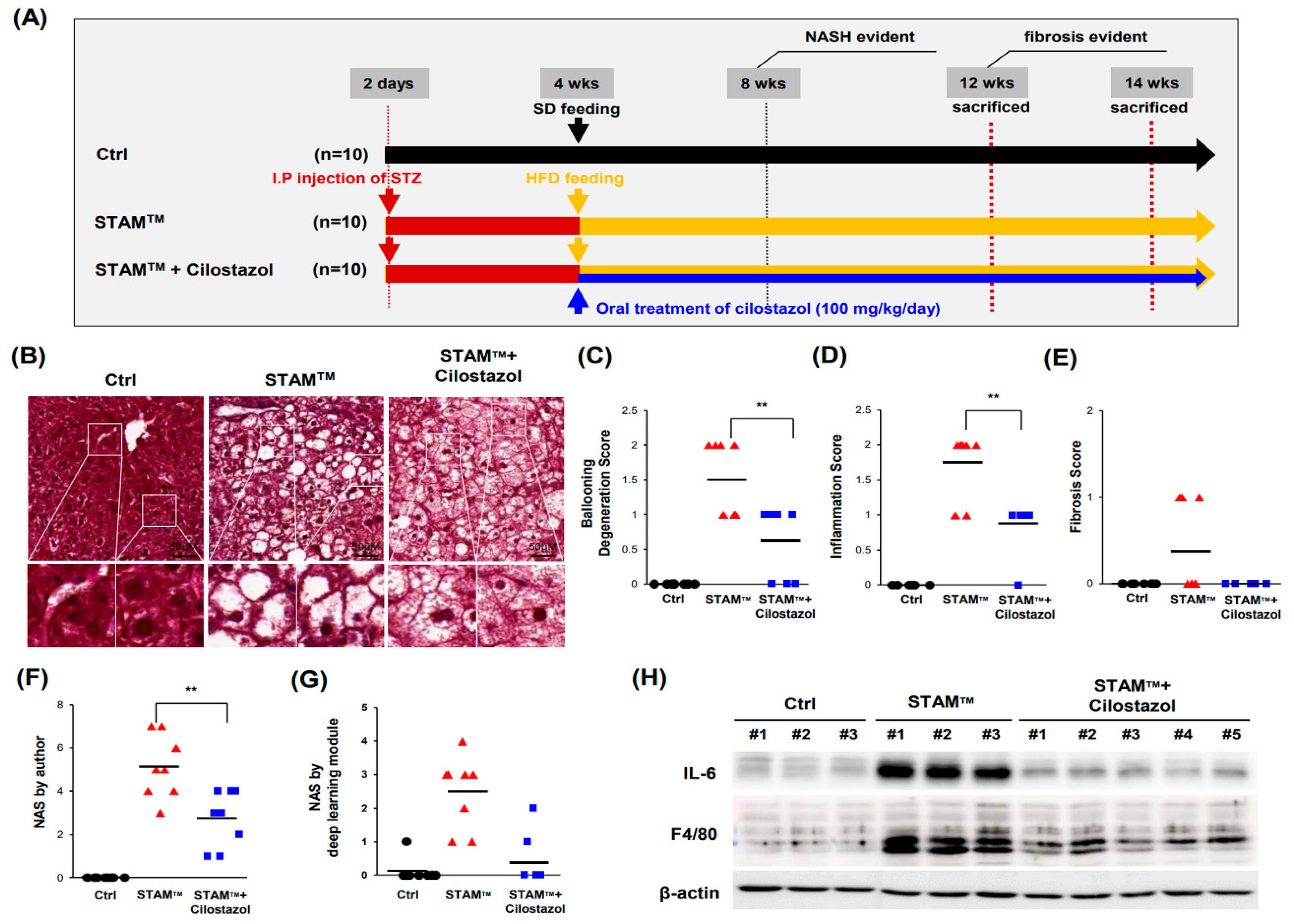
2.1. NASH Was Induced in STAMTM Mice
2.2. Cilostazol Ameliorates Ballooning Degeneration and Inflammation in the NASH Model
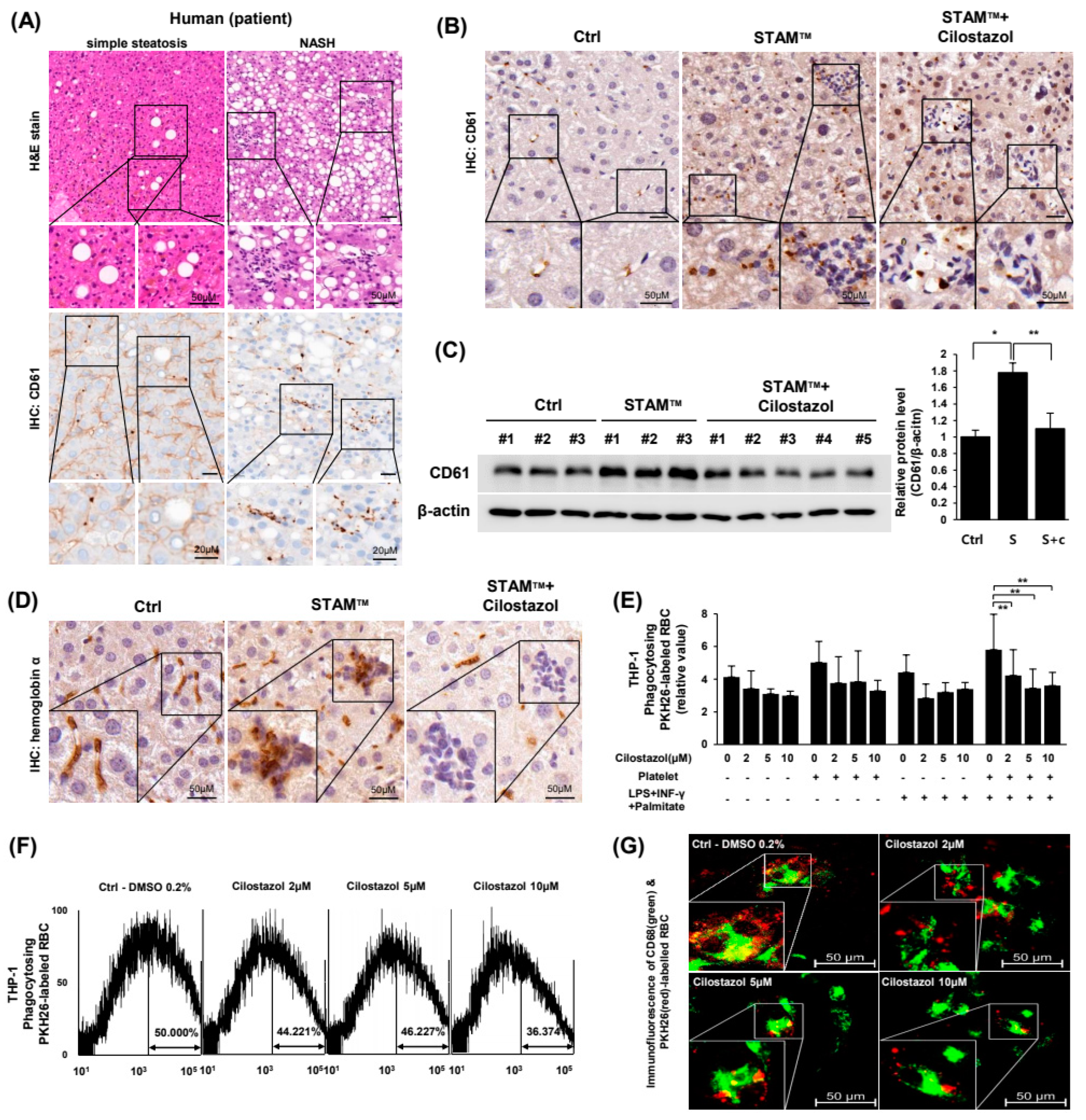
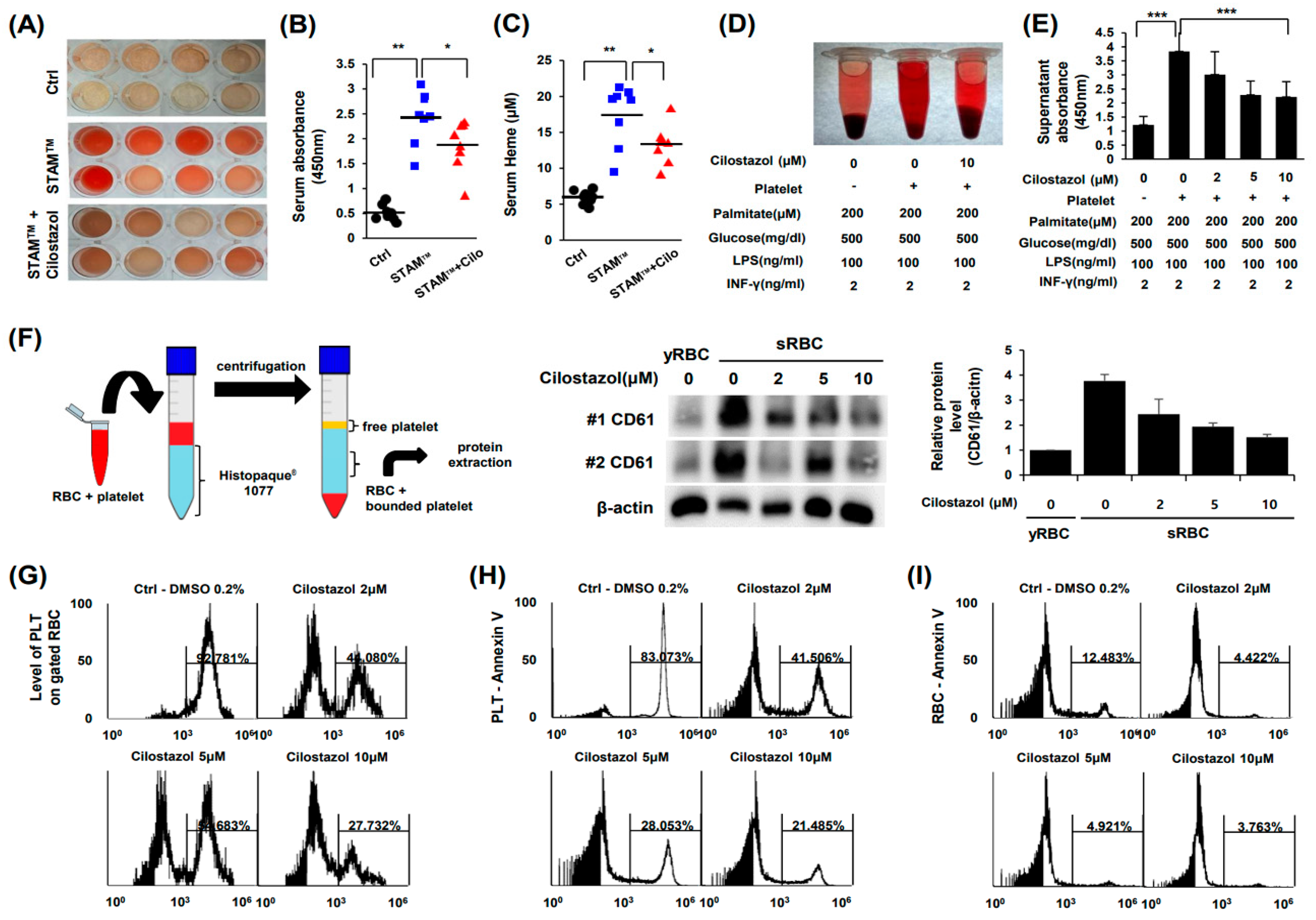
2.3. Cilostazol Resolved the Augmentation of PLT and RBC Accumulation in NASH Livers and Prevented the PLT-Induced Increase in Erythrophagocytosis In Vitro
2.4. Cilostazol Inhibited PLT-RBC Contact-Induced Phosphatidylserine Exposure and Hemolysis
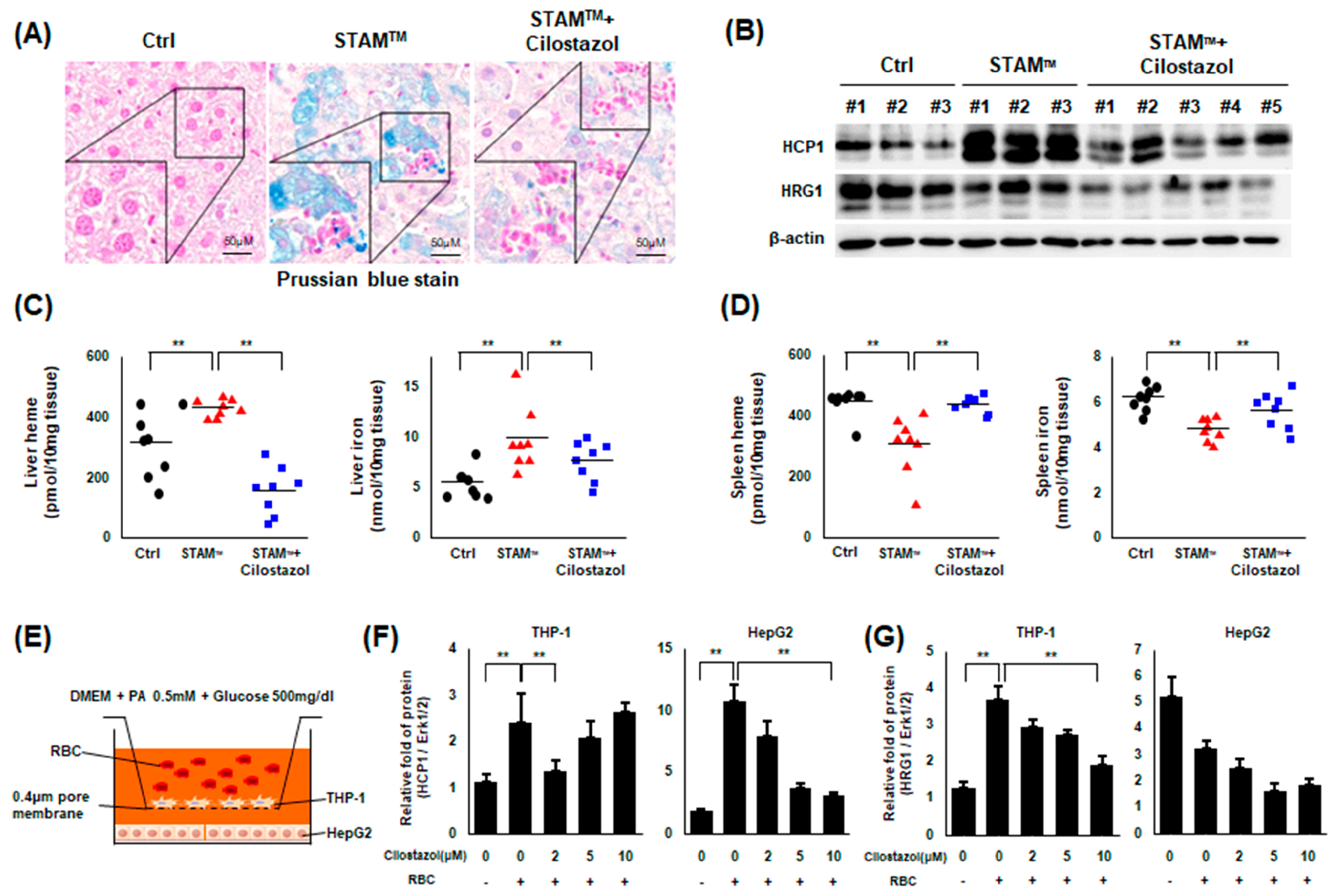
2.5. Cilostazol Improved Iron Overload in NASH Livers
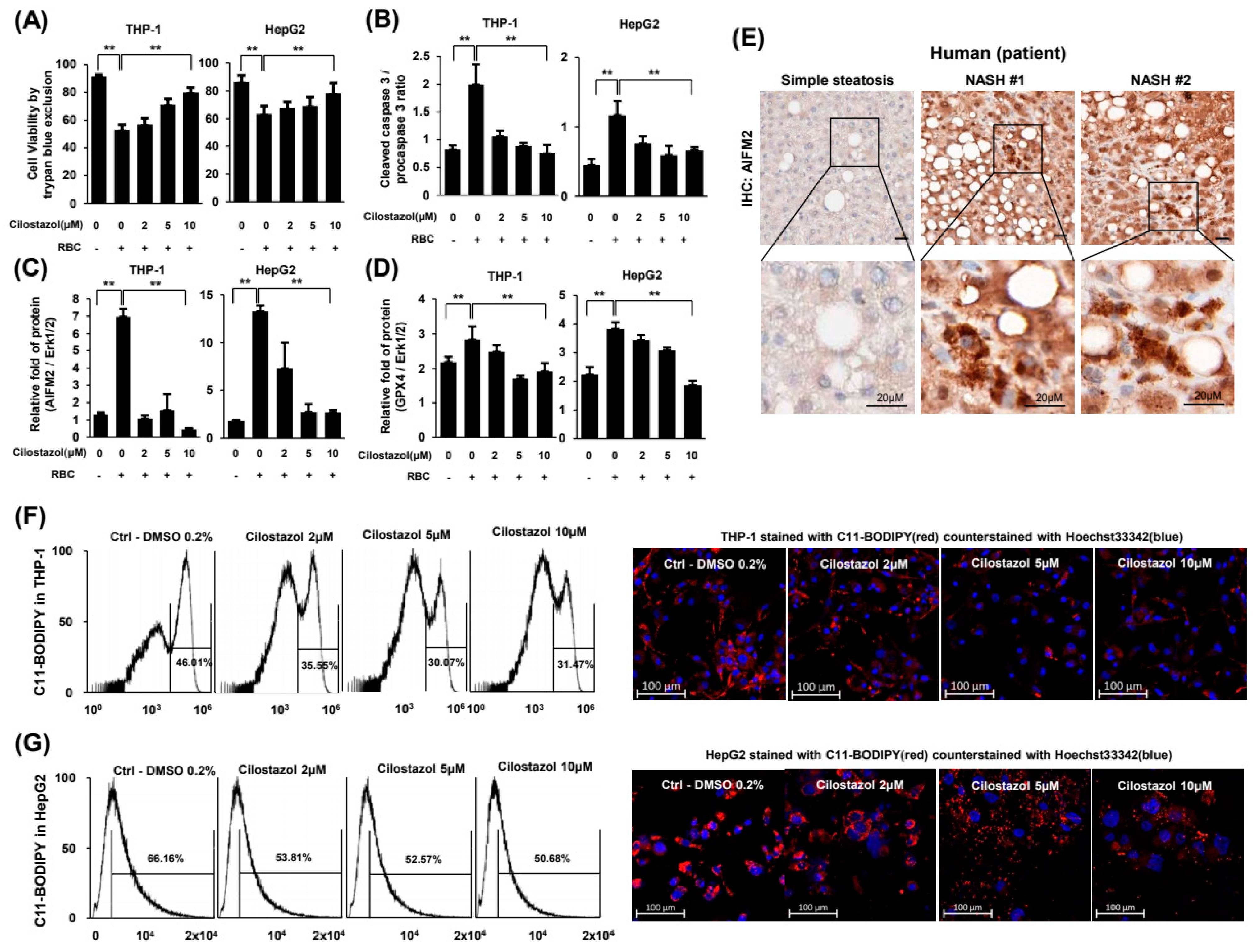
2.6. Cilostazol Attenuated RBC-Induced Ferroptosis of Hepatocytes and Phagocytes
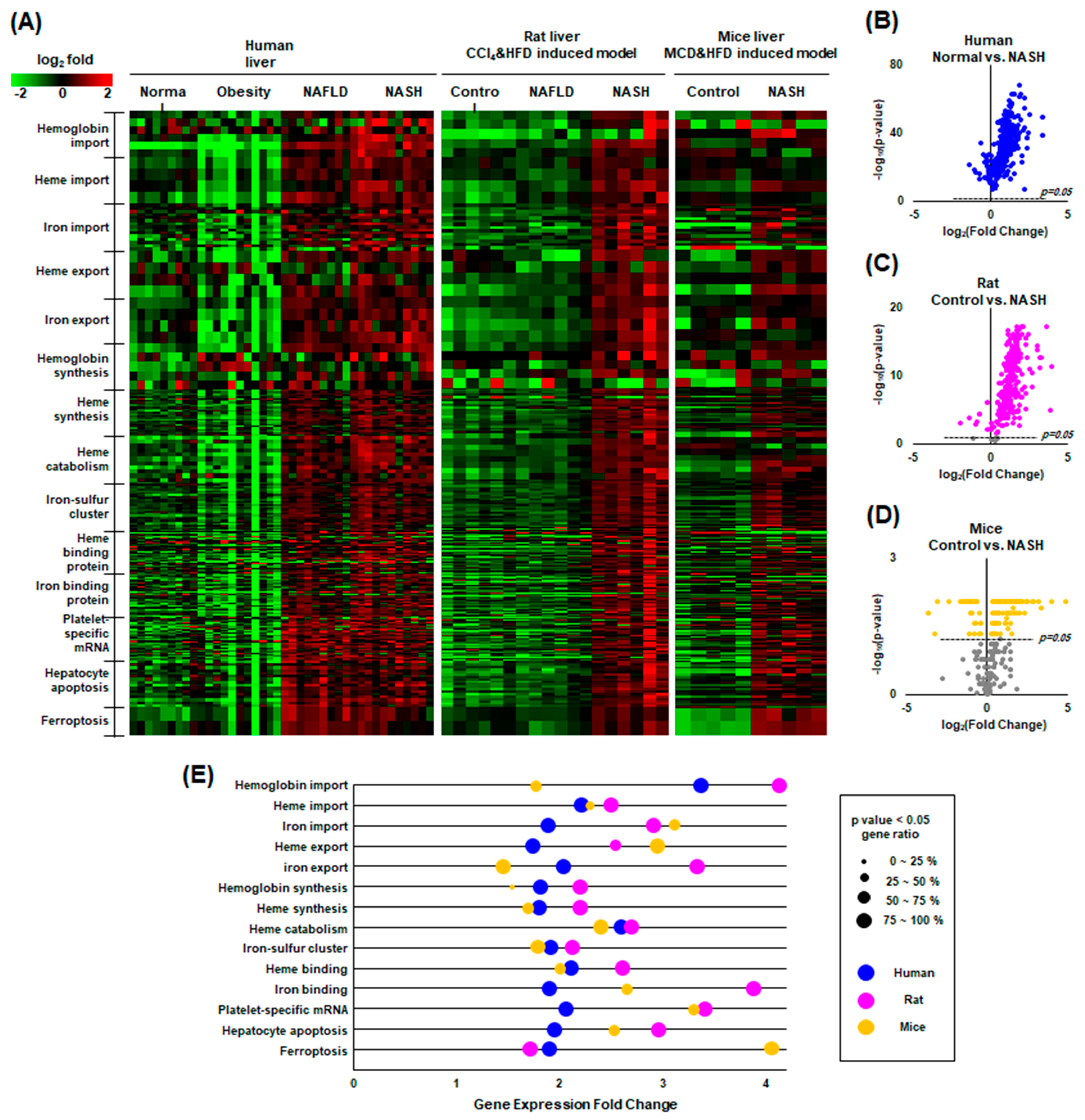
2.7. Our Findings Were Validated through Gene Expression Omnibus (GEO) Data Analysis
3. Discussion
4. Materials and Methods
4.1. Human Pathology Samples
4.2. Animal Model
4.3. Analysis of Lipid Metabolites
4.4. Intraperitoneal Glucose Tolerance Tests (GTTs) and Insulin Tolerance Tests (ITTs)
4.5. Histology
4.6. Immunohistochemistry
4.7. Preparation of RBCs and PLTs
4.8. Cell Culture
4.9. Coculture System
4.10. Hemolysis Analysis
4.11. In Vitro Simulation of NASH and RBC-PLT Interaction Assay
4.12. Erythrophagocytosis Assay
4.13. Ferroptosis Assay
4.14. Gene Expression Omnibus (GEO) Data Collection and Analysis
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Chen, H.; Wang, C.; Liang, L. Pathogenesis of non-alcoholic fatty liver disease in children and adolescence: From “two hit theory” to “multiple hit model”. World J. Gastroenterol. 2018, 24, 2974–2983. [Google Scholar] [CrossRef]
- Diggs, L.P.; Greten, T.F. The effects of platelet accumulation in fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 393–394. [Google Scholar] [CrossRef] [PubMed]
- Wong CH, Y.; Jenne, C.N.; Petri, B.; Chrobok, N.L.; Kubes, P. Nucleation of platelets with blood-borne pathogens on Kupffer cells precedes other innate immunity and contributes to bacterial clearance. Nat. Immunol. 2013, 14, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, M.; Fujii, H.; Sumida, Y.; Hyogo, H.; Itoh, Y.; Ono, M.; Eguchi, Y.; Suzuki, Y.; Aoki, N.; Kanemasa, K.; et al. Platelet count for predicting fibrosis in nonalcoholic fatty liver disease. J. Gastroenterol. 2011, 46, 1300–1306. [Google Scholar] [CrossRef]
- Alkhouri, N.; Kistangari, G.; Campbell, C.; Lopez, R.; Zein, N.N.; Feldstein, A.E. Mean platelet volume as a marker of increased cardiovascular risk in patients with nonalcoholic steatohepatitis. Hepatology 2012, 55, 331. [Google Scholar] [CrossRef]
- Fujita, K.; Nozaki, Y.; Wada, K.; Yoneda, M.; Endo, H.; Takahashi, H.; Iwasaki, T.; Inamori, M.; Abe, Y.; Kobayashi, N.; et al. Effectiveness of antiplatelet drugs against experimental non-alcoholic fatty liver disease. Gut 2008, 57, 1583–1591. [Google Scholar] [CrossRef]
- Malehmir, M.; Pfister, D.; Gallage, S.; Szydlowska, M.; Inverso, D.; Kotsiliti, E.; Leone, V.; Peiseler, M.; Surewaard, B.G.; Rath, D.; et al. Platelet GPIbalpha is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat. Med. 2019, 25, 641–655. [Google Scholar] [CrossRef]
- Kolodgie, F.D.; Gold, H.K.; Burke, A.P.; Fowler, D.R.; Kruth, H.S.; Weber, D.K.; Farb, A.; Guerrero, L.J.; Hayase, M.; Kutys, R.; et al. Intraplaque hemorrhage and progression of coronary atheroma. N. Engl. J. Med. 2003, 349, 2316–2325. [Google Scholar] [CrossRef]
- Jeney, V.; Balla, G.; Balla, J. Red blood cell, hemoglobin and heme in the progression of atherosclerosis. Front. Physiol. 2014, 5, 379. [Google Scholar] [CrossRef]
- Klatt, C.; Krüger, I.; Zey, S.; Krott, K.J.; Spelleken, M.; Gowert, N.S.; Oberhuber, A.; Pfaff, L.; Lückstädt, W.; Jurk, K.; et al. Platelet-RBC interaction mediated by FasL/FasR induces procoagulant activity important for thrombosis. J. Clin. Investig. 2018, 128, 3906–3925. [Google Scholar] [CrossRef] [PubMed]
- Theurl, I.; Hilgendorf, I.; Nairz, M.; Tymoszuk, P.; Haschka, D.; Asshoff, M.; He, S.; Gerhardt, L.M.; Holderried, T.A.; Seifert, M.; et al. On-demand erythrocyte disposal and iron recycling requires transient macrophages in the liver. Nat. Med. 2016, 22, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Otogawa, K.; Kinoshita, K.; Fujii, H.; Sakabe, M.; Shiga, R.; Nakatani, K.; Ikeda, K.; Nakajima, Y.; Ikura, Y.; Ueda, M.; et al. Erythrophagocytosis by liver macrophages (Kupffer cells) promotes oxidative stress, inflammation, and fibrosis in a rabbit model of steatohepatitis: Implications for the pathogenesis of human nonalcoholic steatohepatitis. Am. J. Pathol. 2007, 170, 967–980. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kwon, W.Y.; Park, J.B.; Lee, M.H.; Oh, Y.J.; Suh, S.; Baek, Y.H.; Jeong, J.S.; Yoo, Y.H. Hepatic STAMP2 mediates recombinant FGF21-induced improvement of hepatic iron overload in nonalcoholic fatty liver disease. FASEB J. 2020, 34, 12354–12366. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Park, C.H.; Park, J.B.; Ko, K.; Lee, M.H.; Chung, J.; Yoo, Y.H. Hepatic STAMP2 alleviates polychlorinated biphenyl-induced steatosis and hepatic iron overload in NAFLD models. Environ. Toxicol. 2022, 37, 2223–2234. [Google Scholar] [CrossRef] [PubMed]
- Wahlang, B.; McClain, C.; Barve, S.; Gobejishvili, L. Role of cAMP and phosphodiesterase signaling in liver health and disease. Cell. Signal. 2018, 49, 105–115. [Google Scholar] [CrossRef]
- Oh, Y.J.; Kim, H.Y.; Lee, M.H.; Suh, S.H.; Choi, Y.; Nam, T.G.; Kwon, W.Y.; Lee, S.Y.; Yoo, Y.H. Cilostazol Improves HFD-Induced Hepatic Steatosis by Upregulating Hepatic STAMP2 Expression through AMPK. Mol. Pharmacol. 2018, 94, 1401–1411. [Google Scholar] [CrossRef]
- Kherallah, R.Y.; Khawaja, M.; Olson, M.; Angiolillo, D.; Birnbaum, Y. Cilostazol: A Review of Basic Mechanisms and Clinical Uses. Cardiovasc. Drugs Ther. 2022, 36, 777–792. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Heinemann, F.; Birk, G.; Stierstorfer, B. Deep learning enables pathologist-like scoring of NASH models. Sci. Rep. 2019, 9, 18454. [Google Scholar] [CrossRef]
- Paquette, M.; Yan, M.; Ramírez-Reyes, J.M.; El-Houjeiri, L.; Biondini, M.; Dufour, C.R.; Jeong, H.; Pacis, A.; Giguère, V.; Estall, J.L.; et al. Loss of hepatic Flcn protects against fibrosis and inflammation by activating autophagy pathways. Sci. Rep. 2021, 11, 21268. [Google Scholar] [CrossRef] [PubMed]
- Hottz, E.D.; Medeiros-de-Moraes, I.M.; Vieira-de-Abreu, A.; de Assis, E.F.; Vals-de-Souza, R.; Castro-Faria-Neto, H.C.; Weyrich, A.S.; Zimmerman, G.A.; Bozza, F.A.; Bozza, P.T. Platelet activation and apoptosis modulate monocyte inflammatory responses in dengue. J. Immunol. 2014, 193, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, S.; Garrick, M.D.; Arredondo, M. Heme carrier protein 1 transports heme and is involved in heme-Fe metabolism. Am. J. Physiol.-Cell Physiol. 2012, 302, C1780–C1785. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, A.; Wree, A.; Feldstein, A.E. Biomarkers of liver cell death. J. Hepatol. 2014, 60, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Afonso, M.B.; Rodrigues, P.M.; Carvalho, T.; Caridade, M.; Borralho, P.; Cortez-Pinto, H.; Castro, R.E.; Rodrigues, C.M. Necroptosis is a key pathogenic event in human and experimental murine models of non-alcoholic steatohepatitis. Clin. Sci. 2015, 129, 721–739. [Google Scholar] [CrossRef]
- Wen, Q.; Liu, J.; Kang, R.; Zhou, B.; Tang, D. The release and activity of HMGB1 in ferroptosis. Biochem. Biophys. Res. Commun. 2019, 510, 278–283. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Fracanzani, A.L.; Fargion, S.; Valenti, L. Iron in fatty liver and in the metabolic syndrome: A promising therapeutic target. J. Hepatol. 2011, 55, 920–932. [Google Scholar] [CrossRef]
- Schleicher, R.I.; Reichenbach, F.; Kraft, P.; Kumar, A.; Lescan, M.; Todt, F.; Göbel, K.; Hilgendorf, I.; Geisler, T.; Bauer, A.; et al. Platelets induce apoptosis via membrane-bound FasL. Blood 2015, 126, 1483–1493. [Google Scholar] [CrossRef]
- Foller, M.; Huber, S.M.; Lang, F. Erythrocyte programmed cell death. IUBMB Life 2008, 60, 661–668. [Google Scholar] [CrossRef]
- Suppli, M.P.; Rigbolt, K.T.; Veidal, S.S.; Heebøll, S.; Eriksen, P.L.; Demant, M.; Bagger, J.I.; Nielsen, J.C.; Oró, D.; Thrane, S.W.; et al. Hepatic transcriptome signatures in patients with varying degrees of nonalcoholic fatty liver disease compared with healthy normal-weight individuals. Am. J. Physiol.-Gastrointest. Liver Physiol. 2019, 316, G462–G472. [Google Scholar] [CrossRef] [PubMed]
- Maeso-Díaz, R.; Boyer-Diaz, Z.; Lozano, J.J.; Ortega-Ribera, M.; Peralta, C.; Bosch, J.; Gracia-Sancho, J. New rat model of advanced NASH mimicking pathophysiological features and transcriptomic signature of the human disease. Cells 2019, 8, 1062. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Shibazaki, Y.; Wakamatsu, K.; Honda, Y.; Kawauchi, Y.; Suzuki, K.; Arumugam, S.; Watanabe, K.; Ichida, T.; Asakura, H.; et al. A murine model for non-alcoholic steatohepatitis showing evidence of association between diabetes and hepatocellular carcinoma. Med. Mol. Morphol. 2013, 46, 141–152. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.B.; Ko, K.; Baek, Y.H.; Kwon, W.Y.; Suh, S.; Han, S.-H.; Kim, Y.H.; Kim, H.Y.; Yoo, Y.H. Pharmacological Prevention of Ectopic Erythrophagocytosis by Cilostazol Mitigates Ferroptosis in NASH. Int. J. Mol. Sci. 2023, 24, 12862. https://doi.org/10.3390/ijms241612862
Park JB, Ko K, Baek YH, Kwon WY, Suh S, Han S-H, Kim YH, Kim HY, Yoo YH. Pharmacological Prevention of Ectopic Erythrophagocytosis by Cilostazol Mitigates Ferroptosis in NASH. International Journal of Molecular Sciences. 2023; 24(16):12862. https://doi.org/10.3390/ijms241612862
Chicago/Turabian StylePark, Joon Beom, Kangeun Ko, Yang Hyun Baek, Woo Young Kwon, Sunghwan Suh, Song-Hee Han, Yun Hak Kim, Hye Young Kim, and Young Hyun Yoo. 2023. "Pharmacological Prevention of Ectopic Erythrophagocytosis by Cilostazol Mitigates Ferroptosis in NASH" International Journal of Molecular Sciences 24, no. 16: 12862. https://doi.org/10.3390/ijms241612862
APA StylePark, J. B., Ko, K., Baek, Y. H., Kwon, W. Y., Suh, S., Han, S.-H., Kim, Y. H., Kim, H. Y., & Yoo, Y. H. (2023). Pharmacological Prevention of Ectopic Erythrophagocytosis by Cilostazol Mitigates Ferroptosis in NASH. International Journal of Molecular Sciences, 24(16), 12862. https://doi.org/10.3390/ijms241612862





