Comparative Thrombin Generation in Animal Plasma: Sensitivity to Human Factor XIa and Tissue Factor
Abstract
:1. Introduction
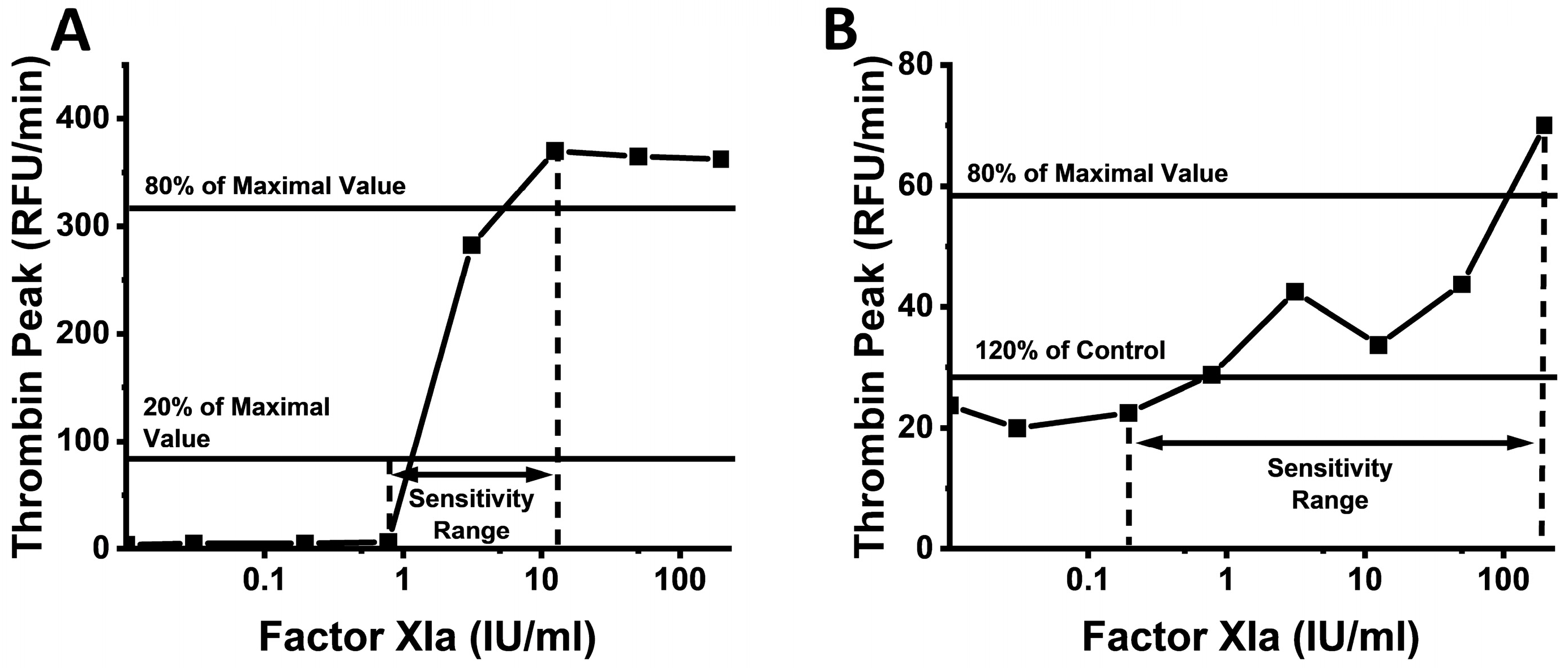
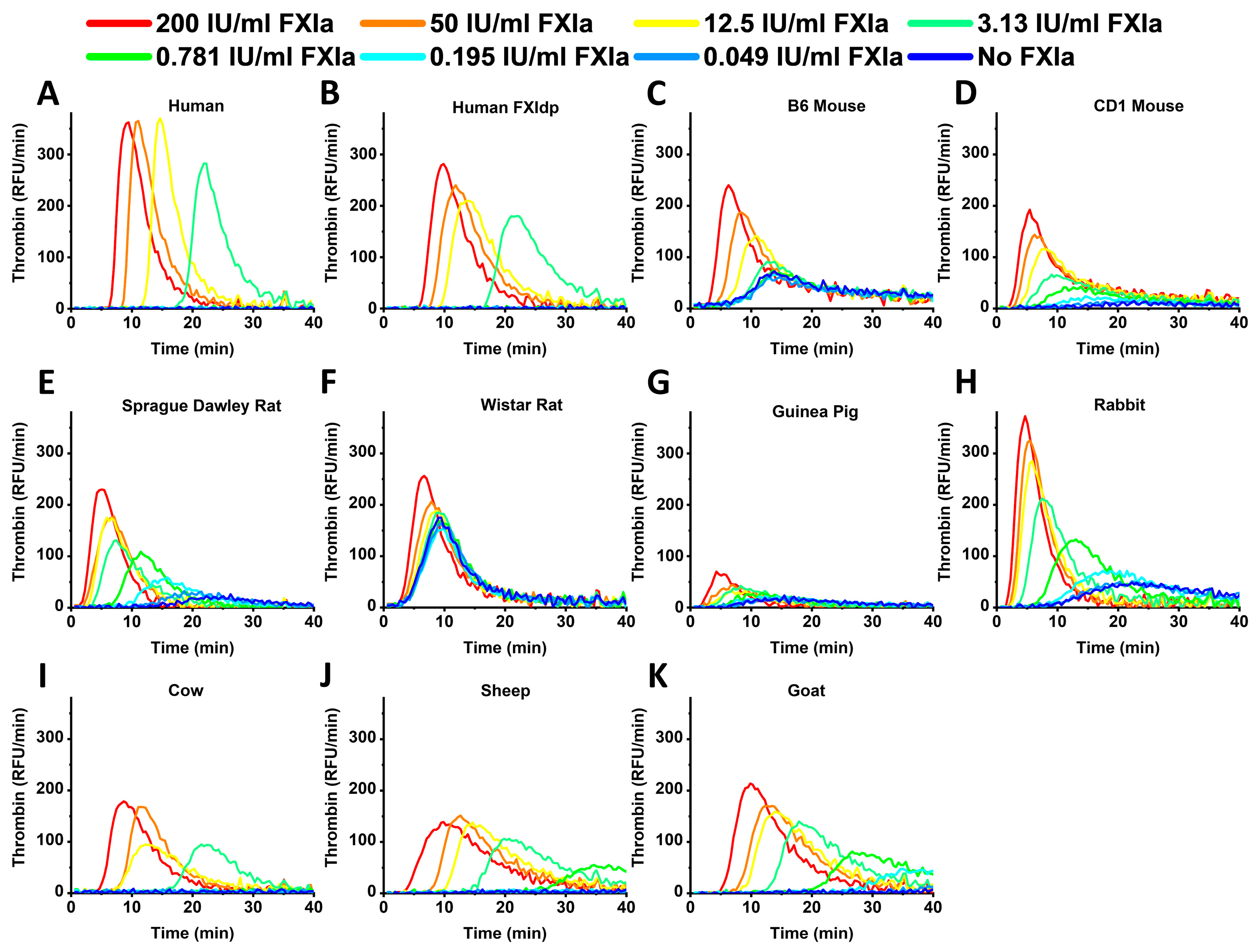
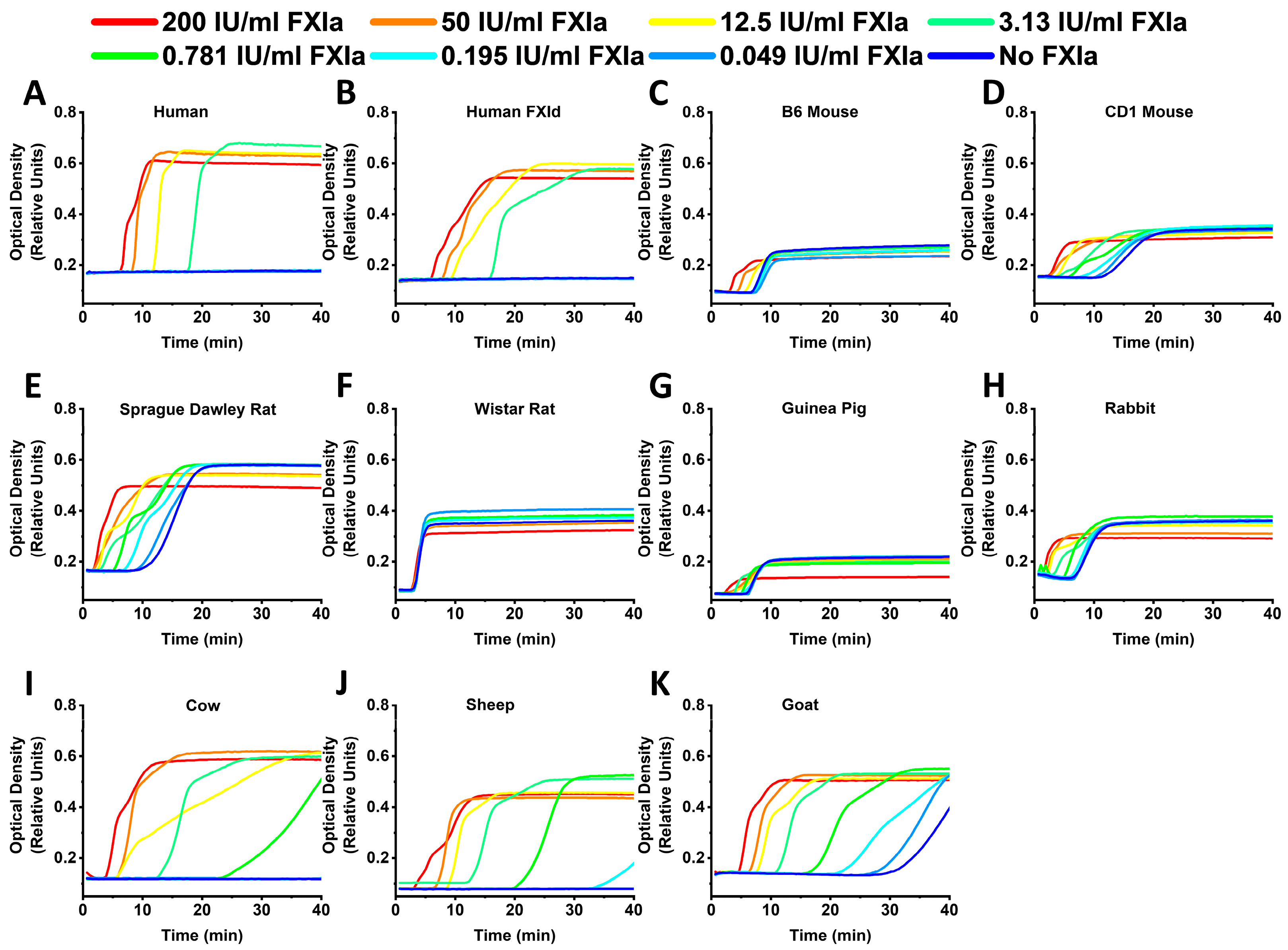
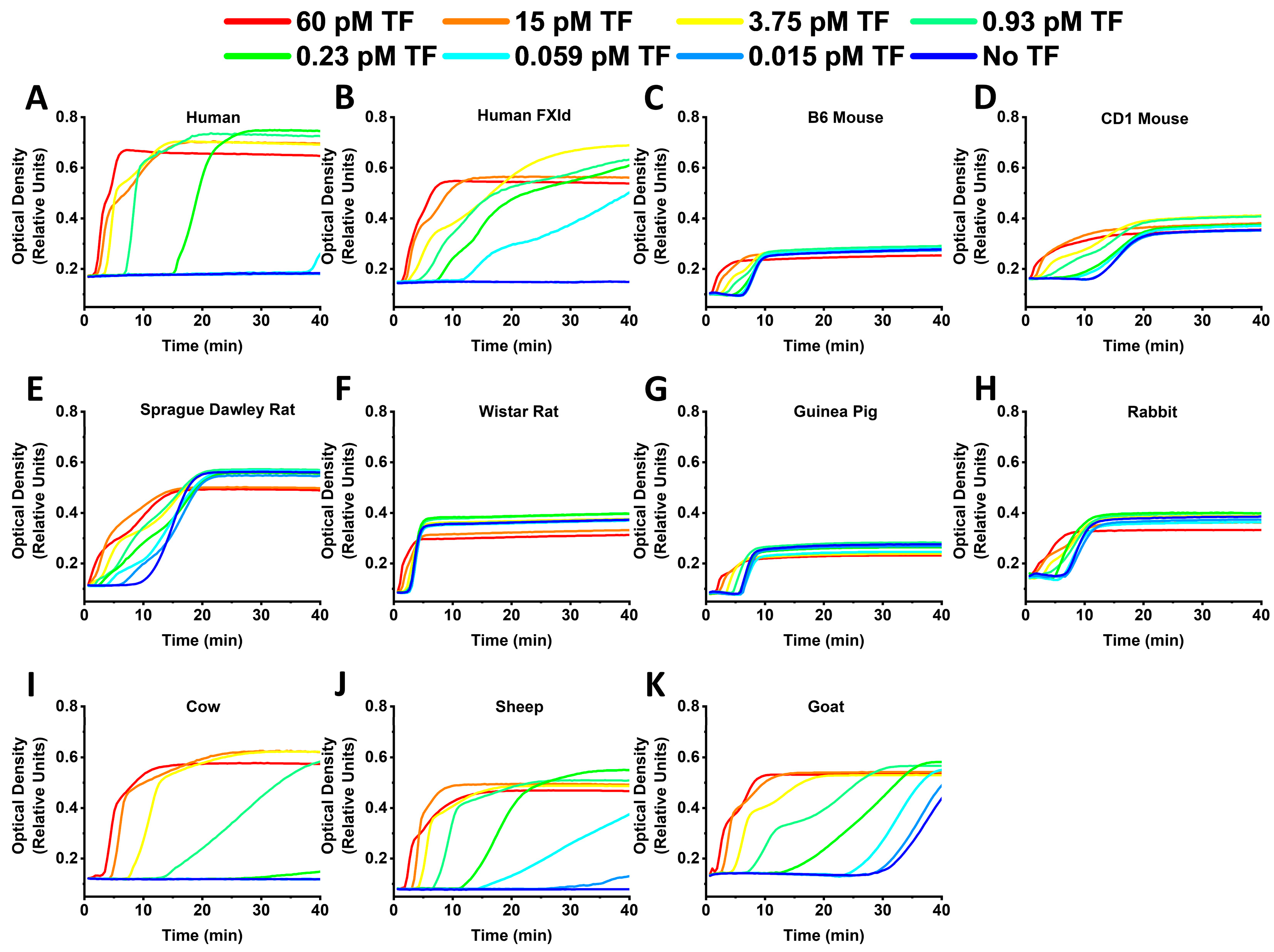
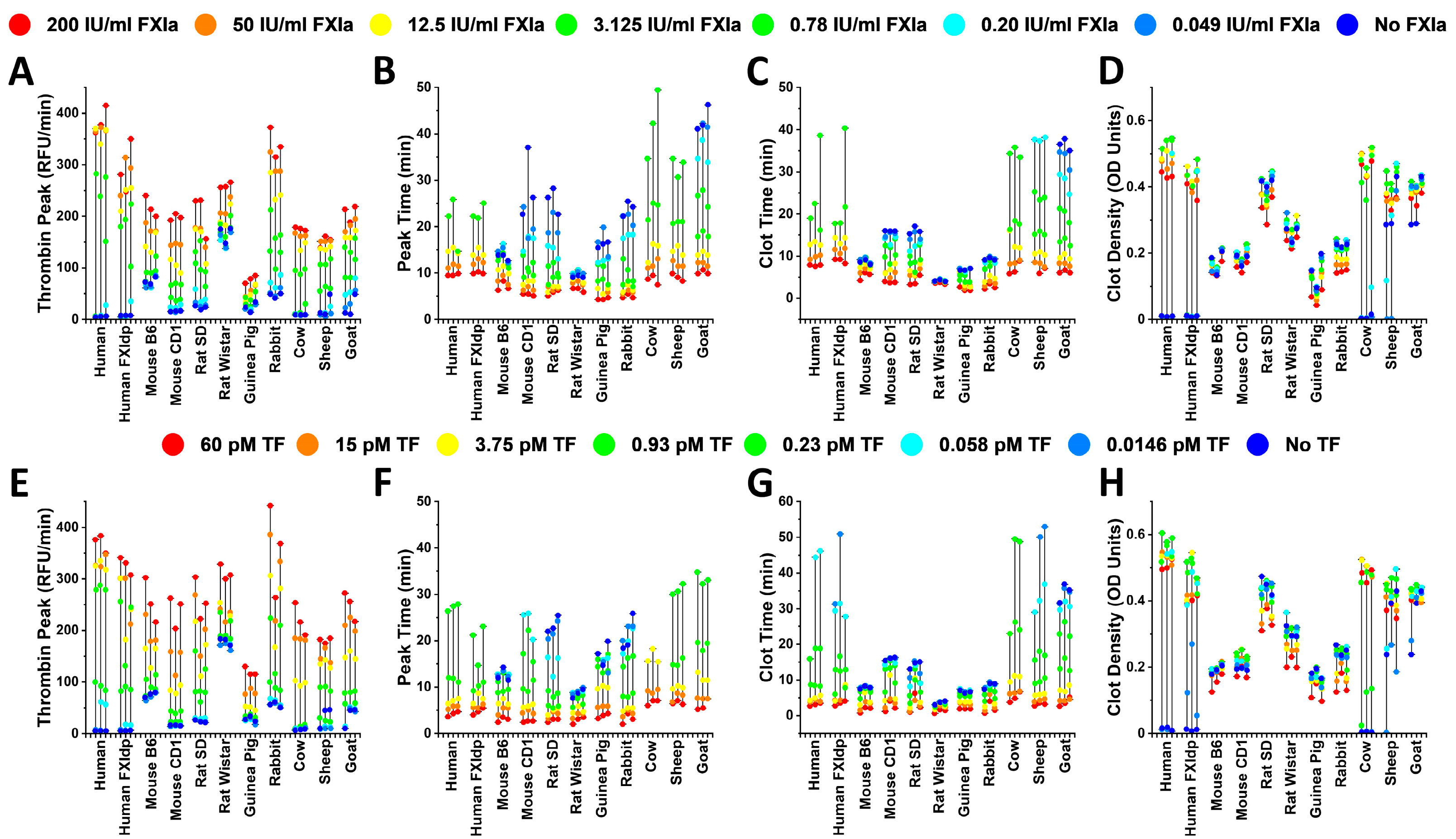
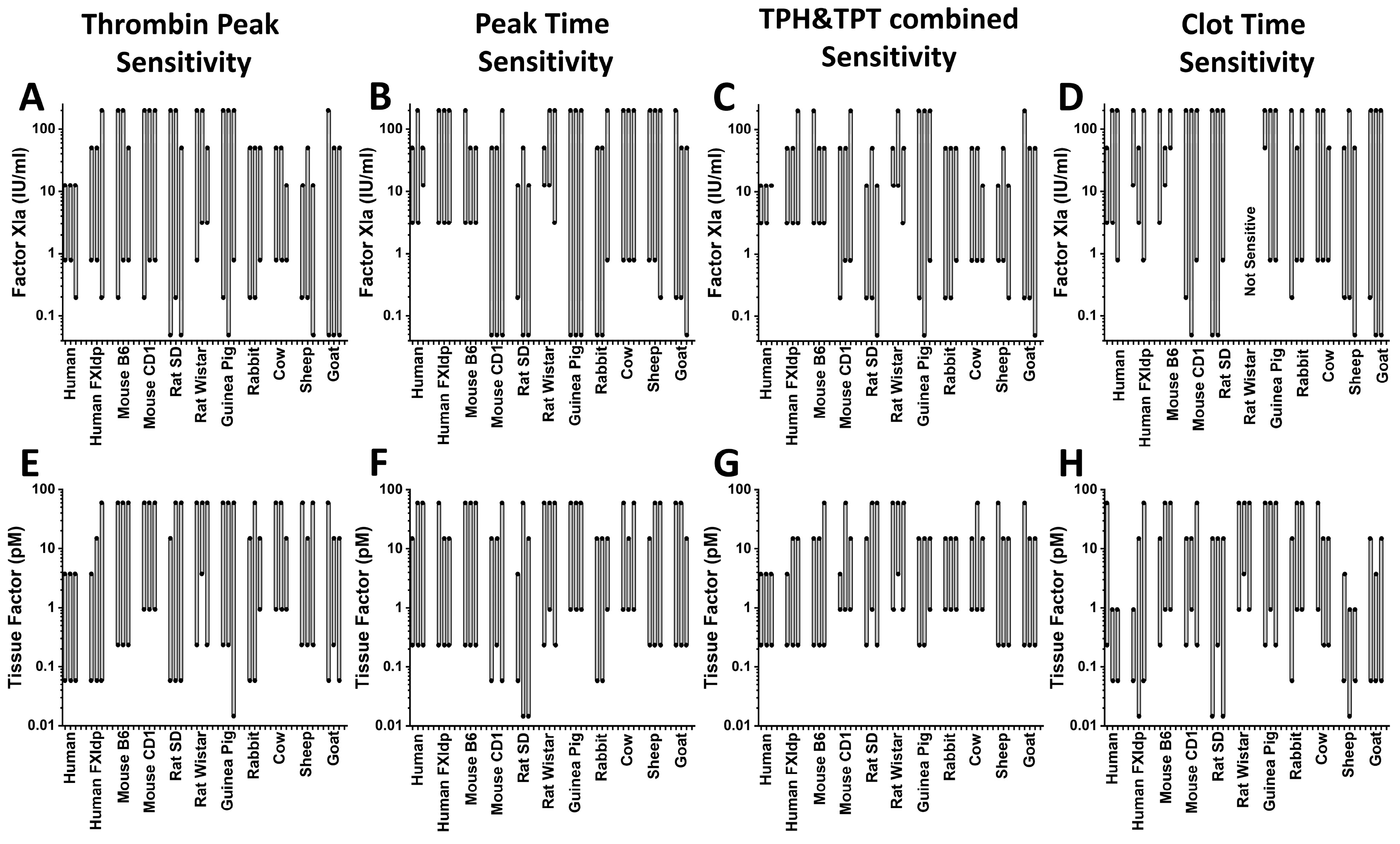
2. Results
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hemker, H.C.; Giesen, P.; Al Dieri, R.; Regnault, V.; de Smedt, E.; Wagenvoord, R.; Lecompte, T.; Beguin, S. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol. Haemost. Thromb. 2003, 33, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, A. Thrombin Generation Assay and Its Application in the Clinical Laboratory. Clin. Chem. 2016, 62, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Hemker, H.C.; Giesen, P.; AlDieri, R.; Regnault, V.; de Smed, E.; Wagenvoord, R.; Lecompte, T.; Beguin, S. The calibrated automated thrombogram (CAT): A universal routine test for hyper- and hypocoagulability. Pathophysiol. Haemost. Thromb. 2002, 32, 249–253. [Google Scholar] [CrossRef] [PubMed]
- van de Berg, T.W.; Beckers, E.A.M.; Heubel-Moenen, F.; Henskens, Y.M.C.; Thomassen, S.; Hackeng, T.M. Sensitive measurement of clinically relevant factor VIII levels in thrombin generation assays requires presence of factor XIa. Thromb. Haemost. 2023. [Google Scholar] [CrossRef] [PubMed]
- Parunov, L.A.; Shea, M.E.; Liang, Y.; Surov, S.S.; Chattopadhyay, M.; Lee, T.K.; Scott, D.E.; Ovanesov, M.V. Thrombin generation test based on a 96-channel pipettor for evaluation of FXIa procoagulant activity in pharmaceuticals. Nat. Protoc. 2021, 16, 3981–4003. [Google Scholar] [CrossRef] [PubMed]
- Lund, J.; Jensen, K.; Burnier, L.; Ezban, M. In vitro effects of combining Mim8 with factor VIII, FVIIa, and activated prothrombin complex concentrates in thrombin generation assays. J. Thromb. Haemost. 2023, 21, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Brooks, M.B.; Stokol, T.; Catalfamo, J.L. Comparative hemostasis: Animal models and new hemostasis tests. Clin. Lab. Med. 2011, 31, 139–159. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sonkar, V.K.; Swamy, J.; Ahmed, A.; Sharathkumar, A.A.; Pierce, G.L.; Dayal, S. DNase 1 Protects From Increased Thrombin Generation and Venous Thrombosis During Aging: Cross-Sectional Study in Mice and Humans. J. Am. Heart Assoc. 2022, 11, e021188. [Google Scholar] [CrossRef]
- Verhoef, D.; Tjalma, A.V.R.; Cheung, K.L.; Reitsma, P.H.; Bos, M.H.A. Elevated anti-human factor Xa activity in rabbit and rodent plasma: Implications for preclinical assessment of human factor X in animal models of hemostasis. Thromb. Res. 2021, 198, 154–162. [Google Scholar] [CrossRef]
- Wang, B.; Yan, X.; Chen, F.; Yang, A.; Lu, Y.; Wu, Y. Plasma kallikrein contributes to ambient particulate matter-induced lung injury. Biochem. Biophys. Res. Commun. 2019, 518, 409–415. [Google Scholar] [CrossRef]
- Karges, H.E.; Funk, K.A.; Ronneberger, H. Activity of coagulation and fibrinolysis parameters in animals. Arzneimittelforschung 1994, 44, 793–797. [Google Scholar]
- Duchemin, J.; Pan-Petesch, B.; Arnaud, B.; Blouch, M.T.; Abgrall, J.F. Influence of coagulation factors and tissue factor concentration on the thrombin generation test in plasma. Thromb. Haemost. 2008, 99, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, T.; Olsen, O.H.; Petersen, L.C. Tissue factor and factor VIIa cross-species compatibility. Front. Biosci. (Landmark Ed.) 2011, 16, 3196–3215. [Google Scholar] [CrossRef] [PubMed]
- Brooks, M.B.; Stablein, A.P.; Johnson, L.; Schultze, A.E. Preanalytic processing of rat plasma influences thrombin generation and fibrinolysis assays. Vet. Clin. Pathol. 2017, 46, 496–507. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, E.; Scaf, B.; van Oerle, R.; van Nieuwenhoven, F.A.; van Hunnik, A.; Verheule, S.; Schotten, U.; Ten Cate, H.; Spronk, H.M.H. Thrombin generation by calibrated automated thrombography in goat plasma: Optimization of an assay. Res. Pract. Thromb. Haemost. 2021, 5, e12620. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liang, Y.; Parunov, L.; Despres, D.; Eckhaus, M.; Scott, D.; Ovanesov, M.; Struble, E.B. Combined thrombogenic effects of vessel injury, pregnancy and procoagulant immune globulin administration in mice. Thromb. J. 2020, 18, 32. [Google Scholar] [CrossRef]
- Siller-Matula, J.M.; Plasenzotti, R.; Spiel, A.; Quehenberger, P.; Jilma, B. Interspecies differences in coagulation profile. Thromb Haemost 2008, 100, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Tarandovskiy, I.D.; Shin, H.K.H.; Baek, J.H.; Karnaukhova, E.; Buehler, P.W. Interspecies comparison of simultaneous thrombin and plasmin generation. Sci. Rep. 2020, 10, 3885. [Google Scholar] [CrossRef]
- Poitout-Belissent, F.; Culang, D.; Poulin, D.; Samadfan, R.; Cotton, S.; Bedard, C. Monitoring Compound-Related Effects on Coagulability in Rats and Cynomolgus and Rhesus Monkeys by Thrombin Generation Kinetic Measurement. Int. J. Toxicol. 2020, 39, 207–217. [Google Scholar] [CrossRef] [PubMed]
- McGonigle, P.; Ruggeri, B. Animal models of human disease: Challenges in enabling translation. Biochem. Pharmacol. 2014, 87, 162–171. [Google Scholar] [CrossRef]
- Kearney, K.J.; Butler, J.; Posada, O.M.; Wilson, C.; Heal, S.; Ali, M.; Hardy, L.; Ahnstrom, J.; Gailani, D.; Foster, R.; et al. Kallikrein directly interacts with and activates Factor IX, resulting in thrombin generation and fibrin formation independent of Factor XI. Proc. Natl. Acad. Sci. USA 2021, 118, e2014810118. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; van Oerle, R.; Ten Cate, H.; Laux, V.; Mackman, N.; Heitmeier, S.; Spronk, H.M.H. Plasma Kallikrein Contributes to Coagulation in the Absence of Factor XI by Activating Factor IX. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Tanratana, P.; Roest, M.; Gruber, A.; Pawlinski, R.; Wolberg, A.S.; Mackman, N.; Grover, S.P. A novel mouse whole blood thrombin generation assay sensitive to FXI- and FIX-mediated amplification of coagulation. Blood Adv. 2023, 7, 1915–1925. [Google Scholar] [CrossRef]
- Konrath, S.; Mailer, R.K.; Beerens, M.; Englert, H.; Frye, M.; Kuta, P.; Preston, R.J.S.; Maas, C.; Butler, L.M.; Roest, M.; et al. Intrinsic coagulation pathway-mediated thrombin generation in mouse whole blood. Front. Cardiovasc. Med. 2022, 9, 1008410. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.P.; Mackman, N. Intrinsic Pathway of Coagulation and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Renne, T.; Pozgajova, M.; Gruner, S.; Schuh, K.; Pauer, H.U.; Burfeind, P.; Gailani, D.; Nieswandt, B. Defective thrombus formation in mice lacking coagulation factor XII. J. Exp. Med. 2005, 202, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Merkulov, S.; Zhang, W.M.; Komar, A.A.; Schmaier, A.H.; Barnes, E.; Zhou, Y.; Lu, X.; Iwaki, T.; Castellino, F.J.; Luo, G.; et al. Deletion of murine kininogen gene 1 (mKng1) causes loss of plasma kininogen and delays thrombosis. Blood 2008, 111, 1274–1281. [Google Scholar] [CrossRef]
- Stavrou, E.X.; Fang, C.; Merkulova, A.; Alhalabi, O.; Grobe, N.; Antoniak, S.; Mackman, N.; Schmaier, A.H. Reduced thrombosis in Klkb1-/- mice is mediated by increased Mas receptor, prostacyclin, Sirt1, and KLF4 and decreased tissue factor. Blood 2015, 125, 710–719. [Google Scholar] [CrossRef]
- Cichon, S.; Martin, L.; Hennies, H.C.; Muller, F.; Van Driessche, K.; Karpushova, A.; Stevens, W.; Colombo, R.; Renne, T.; Drouet, C.; et al. Increased activity of coagulation factor XII (Hageman factor) causes hereditary angioedema type III. Am. J. Hum. Genet. 2006, 79, 1098–1104. [Google Scholar] [CrossRef]
- von Bruhl, M.L.; Stark, K.; Steinhart, A.; Chandraratne, S.; Konrad, I.; Lorenz, M.; Khandoga, A.; Tirniceriu, A.; Coletti, R.; Kollnberger, M.; et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012, 209, 819–835. [Google Scholar] [CrossRef]
- Paszek, E.; Pociask, E.; Zabczyk, M.; Piorkowski, A.; Butenas, S.; Legutko, J.; Undas, A. Active factor XI is associated with the risk of cardiovascular events in stable coronary artery disease patients. Atherosclerosis 2022, 346, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, G.; Ekezue, B.F.; Izurieta, H.S.; Selvam, N.; Ovanesov, M.V.; Divan, H.A.; Liang, Y.; Golding, B.; Forshee, R.A.; Anderson, S.A.; et al. Immune globulins and same-day thrombotic events as recorded in a large health care database during 2008 to 2012. Transfusion 2014, 54, 2553–2565. [Google Scholar] [CrossRef] [PubMed]
- Dilger, A.K.; Pabbisetty, K.B.; Corte, J.R.; De Lucca, I.; Fang, T.; Yang, W.; Pinto, D.J.P.; Wang, Y.; Zhu, Y.; Mathur, A.; et al. Discovery of Milvexian, a High-Affinity, Orally Bioavailable Inhibitor of Factor XIa in Clinical Studies for Antithrombotic Therapy. J. Med. Chem. 2022, 65, 1770–1785. [Google Scholar] [CrossRef] [PubMed]
- Heitmeier, S.; Visser, M.; Tersteegen, A.; Dietze-Torres, J.; Glunz, J.; Gerdes, C.; Laux, V.; Stampfuss, J.; Roehrig, S. Pharmacological profile of asundexian, a novel, orally bioavailable inhibitor of factor XIa. J. Thromb. Haemost. 2022, 20, 1400–1411. [Google Scholar] [CrossRef]
- Ferriere, S.; Peyron, I.; Christophe, O.D.; Kawecki, C.; Casari, C.; Muczynski, V.; Nathwani, A.; Kauskot, A.; Lenting, P.J.; Denis, C.V. A hemophilia A mouse model for the in vivo assessment of emicizumab function. Blood 2020, 136, 740–748. [Google Scholar] [CrossRef]
- Ellery, P.E.; Maroney, S.A.; Cooley, B.C.; Luyendyk, J.P.; Zogg, M.; Weiler, H.; Mast, A.E. A balance between TFPI and thrombin-mediated platelet activation is required for murine embryonic development. Blood 2015, 125, 4078–4084. [Google Scholar] [CrossRef]
- Xin, K.Z.; Chang, W.C.; Ovanesov, M.V. Interconnectedness of global hemostasis assay parameters in simultaneously evaluated thrombin generation, fibrin generation and clot lysis in normal plasma. Thromb. Res. 2016, 140, 132–139. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Tarandovskiy, I.; Surov, S.S.; Ovanesov, M.V. Comparative Thrombin Generation in Animal Plasma: Sensitivity to Human Factor XIa and Tissue Factor. Int. J. Mol. Sci. 2023, 24, 12920. https://doi.org/10.3390/ijms241612920
Liang Y, Tarandovskiy I, Surov SS, Ovanesov MV. Comparative Thrombin Generation in Animal Plasma: Sensitivity to Human Factor XIa and Tissue Factor. International Journal of Molecular Sciences. 2023; 24(16):12920. https://doi.org/10.3390/ijms241612920
Chicago/Turabian StyleLiang, Yideng, Ivan Tarandovskiy, Stepan S. Surov, and Mikhail V. Ovanesov. 2023. "Comparative Thrombin Generation in Animal Plasma: Sensitivity to Human Factor XIa and Tissue Factor" International Journal of Molecular Sciences 24, no. 16: 12920. https://doi.org/10.3390/ijms241612920
APA StyleLiang, Y., Tarandovskiy, I., Surov, S. S., & Ovanesov, M. V. (2023). Comparative Thrombin Generation in Animal Plasma: Sensitivity to Human Factor XIa and Tissue Factor. International Journal of Molecular Sciences, 24(16), 12920. https://doi.org/10.3390/ijms241612920



