Factor VII Activating Protease (FSAP) and Its Importance in Hemostasis—Part I: FSAP Structure, Synthesis and Activity Regulation: A Narrative Review
Abstract
1. Prima Facie of Factor VII Activating Protease (FSAP)
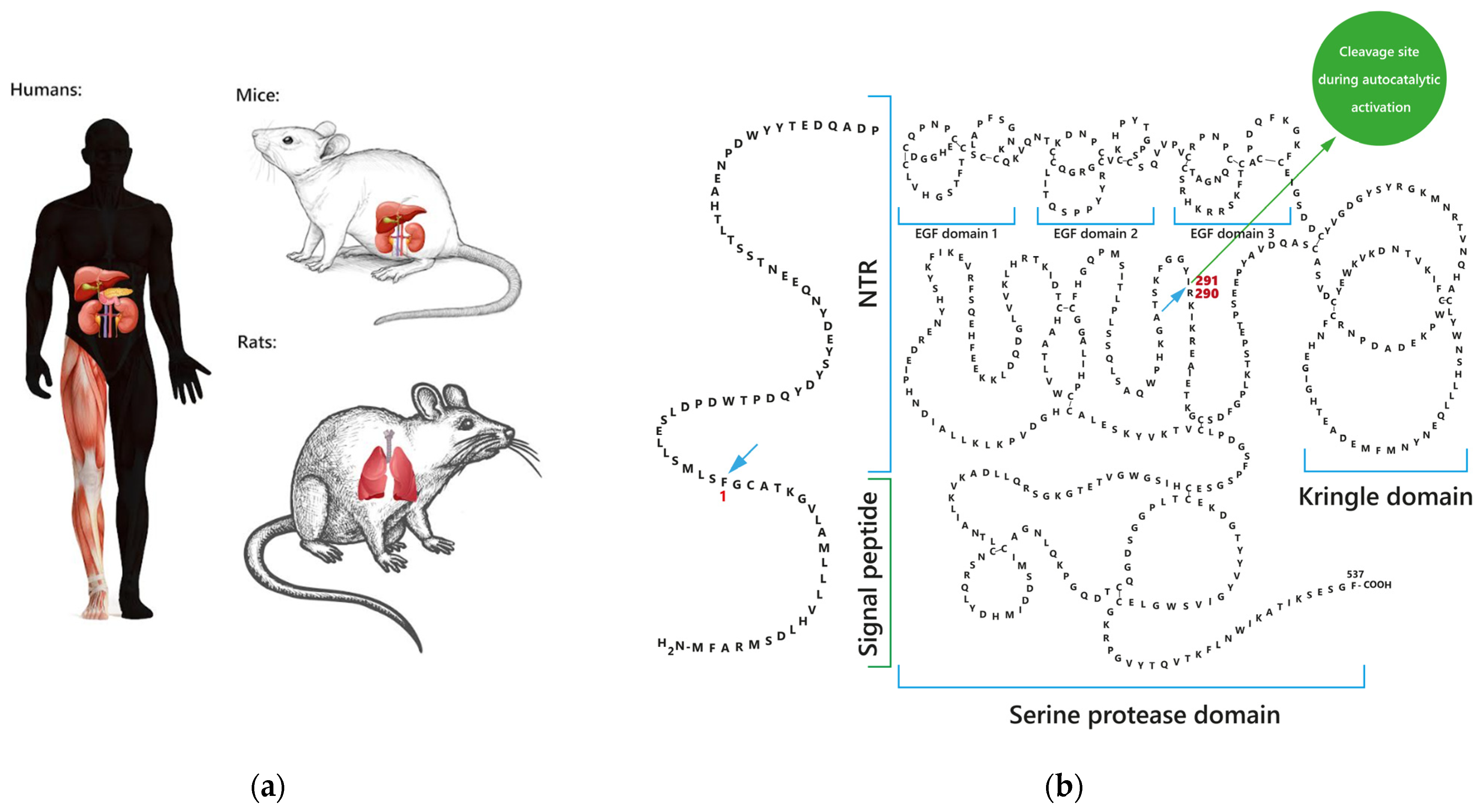
1.1. Sites of FSAP Synthesis
1.2. FSAP Forms and Structure
1.3. FSAP Levels in Human Biological Samples
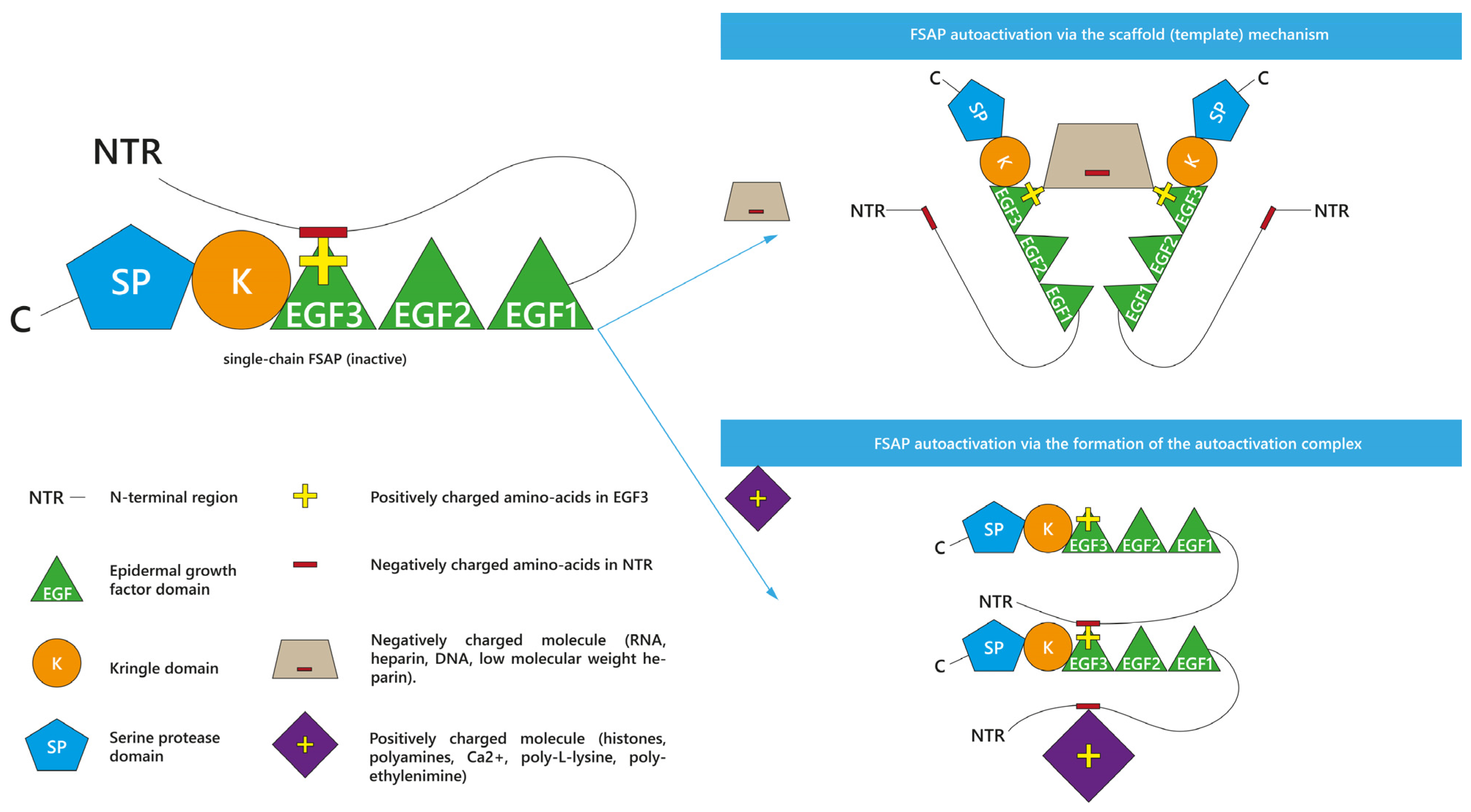
2. (Auto)activation and Activity Profile of FSAP
2.1. Enhancers and Inhibitors of FSAP Activation
2.1.1. Enhancers of scFSAP Activation
2.1.2. Inhibitors of scFSAP and Its Activation
2.2. Enhancers and Inhibitors of FSAP Activity
2.2.1. Enhancers of tcFSAP Activity
2.2.2. Inhibitors of tcFSAP and Its Activity
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi-Miura, N.H.; Tobe, T.; Sumiya, J.; Nakano, Y.; Sano, Y.; Mazda, T.; Tomita, M. Purification and characterization of a novel hyaluronan-binding protein (PHBP) from human plasma: It has three EGF, a kringle and a serine protease domain, similar to hepatocyte growth factor activator. J. Biochem. 1996, 119, 1157–1165. [Google Scholar] [CrossRef]
- Hunfeld, A.; Etscheid, M.; König, H.; Seitz, R.; Dodt, J. Detection of a novel plasma serine protease during purification of vitamin K-dependent coagulation factors. FEBS Lett. 1999, 456, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Römisch, J.; Feussner, A.; Vermöhlen, S.; Stöhr, H.A. A protease isolated from human plasma activating factor VII independent of tissue factor. Blood Coagul. Fibrinolysis 1999, 10, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Sumiya, J.; Asakawa, S.; Tobe, T.; Hashimoto, K.; Saguchi, K.; Choi-Miura, N.H.; Shimizu, Y.; Minoshima, S.; Shimizu, N.; Tomita, M. Isolation and characterization of the plasma hyaluronan-binding protein (PHBP) gene (HABP2). J. Biochem. 1997, 122, 983–990. [Google Scholar] [CrossRef]
- Choi-Miura, N.H.; Yoda, M.; Saito, K.; Takahashi, K.; Tomita, M. Identification of the substrates for plasma hyaluronan binding protein. Biol. Pharm. Bull 2001, 24, 140–143. [Google Scholar] [CrossRef][Green Version]
- Choi-Miura, N.H.; Saito, K.; Takahashi, K.; Yoda, M.; Tomita, M. Regulation mechanism of the serine protease activity of plasma hyaluronan binding protein. Biol. Pharm. Bull 2001, 24, 221–225. [Google Scholar] [CrossRef]
- Choi-Miura, N.H.; Otsuyama, K.; Sano, Y.; Saito, K.; Takahashi, K.; Tomita, M. Hepatic injury-specific conversion of mouse plasma hyaluronan binding protein to the active hetero-dimer form. Biol. Pharm. Bull 2001, 24, 892–896. [Google Scholar] [CrossRef][Green Version]
- Jeon, J.W.; Song, H.S.; Moon, E.J.; Park, S.Y.; Son, M.J.; Jung, S.Y.; Kim, J.T.; Nam, D.H.; Choi-Miura, N.H.; Kim, K.W.; et al. Anti-angiogenic action of plasma hyaluronan binding protein in human umbilical vein endothelial cells. Int. J. Oncol. 2006, 29, 209–215. [Google Scholar] [CrossRef][Green Version]
- Yamamichi, S.; Nishitani, M.; Nishimura, N.; Matsushita, Y.; Hasumi, K. Polyamine-promoted autoactivation of plasma hyaluronan-binding protein. J. Thromb. Haemost. 2010, 8, 559–566. [Google Scholar] [CrossRef]
- Yamamichi, S.; Fujiwara, Y.; Kikuchi, T.; Nishitani, M.; Matsushita, Y.; Hasumi, K. Extracellular histone induces plasma hyaluronan-binding protein (factor VII activating protease) activation in vivo. Biochem. Biophys. Res. Commun. 2011, 409, 483–488. [Google Scholar] [CrossRef]
- Etscheid, M.; Hunfeld, A.; König, H.; Seitz, R.; Dodt, J. Activation of proPHBSP, the zymogen of a plasma hyaluronan binding serine protease, by an intermolecular autocatalytic mechanism. Biol. Chem. 2000, 381, 1223–1231. [Google Scholar] [CrossRef]
- Mambetsariev, N.; Mirzapoiazova, T.; Mambetsariev, B.; Sammani, S.; Lennon, F.E.; Garcia, J.G.; Singleton, P.A. Hyaluronic Acid binding protein 2 is a novel regulator of vascular integrity. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Mirzapoiazova, T.; Mambetsariev, N.; Lennon, F.E.; Mambetsariev, B.; Berlind, J.E.; Salgia, R.; Singleton, P.A. HABP2 is a Novel Regulator of Hyaluronan-Mediated Human Lung Cancer Progression. Front. Oncol. 2015, 5, 164. [Google Scholar] [CrossRef] [PubMed]
- Stavenuiter, F.; Ebberink, E.H.T.M.; Mertens, K.; Meijer, A.B. Role of glycine 221 in catalytic activity of hyaluronan-binding protein 2. J. Biol. Chem. 2017, 292, 6381–6388. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.M.; Stass, S.A.; Kagan, J. The human urokinase-plasminogen activator gene (PLAU) is located on chromosome 10q24 centromeric to the HOX11 gene. Genomics 1993, 16, 301–302. [Google Scholar] [CrossRef]
- Leiting, S.; Seidl, S.; Martinez-Palacian, A.; Muhl, L.; Kanse, S.M. Transforming Growth Factor-β (TGF-β) Inhibits the Expression of Factor VII-activating Protease (FSAP) in Hepatocytes. J. Biol. Chem. 2016, 291, 21020–21028. [Google Scholar] [CrossRef]
- Sedding, D.; Daniel, J.M.; Muhl, L.; Hersemeyer, K.; Brunsch, H.; Kemkes-Matthes, B.; Braun-Dullaeus, R.C.; Tillmanns, H.; Weimer, T.; Preissner, K.T.; et al. The G534E polymorphism of the gene encoding the factor VII-activating protease is associated with cardiovascular risk due to increased neointima formation. J. Exp. Med. 2006, 203, 2801–2807. [Google Scholar] [CrossRef]
- Roderfeld, M.; Weiskirchen, R.; Atanasova, S.; Gressner, A.M.; Preissner, K.T.; Roeb, E.; Kanse, S.M. Altered factor VII activating protease expression in murine hepatic fibrosis and its influence on hepatic stellate cells. Liver Int. 2009, 29, 686–691. [Google Scholar] [CrossRef]
- Parahuleva, M.S.; Kanse, S.M.; Parviz, B.; Barth, A.; Tillmanns, H.; Bohle, R.M.; Sedding, D.G.; Hölschermann, H. Factor Seven Activating Protease (FSAP) expression in human monocytes and accumulation in unstable coronary atherosclerotic plaques. Atherosclerosis 2008, 196, 164–171. [Google Scholar] [CrossRef]
- Joshi, A.U.; Orset, C.; Engelhardt, B.; Baumgart-Vogt, E.; Gerriets, T.; Vivien, D.; Kanse, S.M. Deficiency of Factor VII activating protease alters the outcome of ischemic stroke in mice. Eur. J. Neurosci. 2015, 41, 965–975. [Google Scholar] [CrossRef]
- Wygrecka, M.; Markart, P.; Fink, L.; Guenther, A.; Preissner, K.T. Raised protein levels and altered cellular expression of factor VII activating protease (FSAP) in the lungs of patients with acute respiratory distress syndrome (ARDS). Thorax 2007, 62, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Knoblauch, B.; Kellert, J.; Battmann, A.; Preissner, K.; Roemisch, J. A histological study of FVII-activating protease (FSAP) in human tissue. Ann. Haematol. 2002, 81, A42. [Google Scholar]
- Römisch, J. Factor VII activating protease (FSAP): A novel protease in hemostasis. Biol. Chem. 2002, 383, 1119–1124. [Google Scholar] [CrossRef]
- Parahuleva, M.S.; Ball, N.; Parviz, B.; Zandt, D.; Abdallah, Y.; Tillmanns, H.; Hoelschermann, H.; Kanse, S.M. Factor seven activating protease (FSAP) expression in human placenta and its role in trophoblast migration. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 167, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Mu, E.; Liu, X.; Chen, S.; Zhi, L.; Li, X.; Xu, X.; Ma, X. Changes in factor VII-activating protease in a bleomycin-induced lung injury rat model and its influence on human pulmonary fibroblasts in vitro. Int. J. Mol. Med. 2010, 26, 549–555. [Google Scholar] [CrossRef]
- Nakazawa, F.; Kannemeier, C.; Shibamiya, A.; Song, Y.; Tzima, E.; Schubert, U.; Koyama, T.; Niepmann, M.; Trusheim, H.; Engelmann, B.; et al. Extracellular RNA is a natural cofactor for the (auto-)activation of Factor VII-activating protease (FSAP). Biochem. J 2005, 385, 831–838. [Google Scholar] [CrossRef]
- Daniel, J.M.; Reichel, C.A.; Schmidt-Woell, T.; Dutzmann, J.; Zuchtriegel, G.; Krombach, F.; Herold, J.; Bauersachs, J.; Sedding, D.G.; Kanse, S.M. Factor VII-activating protease deficiency promotes neointima formation by enhancing leukocyte accumulation. J. Thromb. Haemost. 2016, 14, 2058–2067. [Google Scholar] [CrossRef]
- Kannemeier, C.; Al-Fakhri, N.; Preissner, K.T.; Kanse, S.M. Factor VII-activating protease (FSAP) inhibits growth factor-mediated cell proliferation and migration of vascular smooth muscle cells. FASEB J. 2004, 18, 728–730. [Google Scholar] [CrossRef]
- Parahuleva, M.S.; Worsch, M.; Euler, G.; Choukeir, M.; Mardini, A.; Parviz, B.; Kanse, S.M.; Portig, I.; Khayrutdinov, E.; Schieffer, B.; et al. Factor VII Activating Protease Expression in Human Platelets and Accumulation in Symptomatic Carotid Plaque. J. Am. Heart Assoc. 2020, 9, e016445. [Google Scholar] [CrossRef]
- Parahuleva, M.S.; Langanke, E.; Hölschermann, H.; Parviz, B.; Abdallah, Y.; Stracke, S.; Tillmanns, H.; Kanse, S.M. Nicotine modulation of factor VII activating protease (FSAP) expression in human monocytes. J. Atheroscler. Thromb. 2012, 19, 962–969. [Google Scholar] [CrossRef][Green Version]
- Parahuleva, M.S.; Hölschermann, H.; Erdogan, A.; Langanke, E.; Prickartz, I.; Parviz, B.; Weiskirchen, R.; Tillmanns, H.; Kanse, S.M. Factor seven ativating potease (FSAP) levels during normal pregnancy and in women using oral contraceptives. Thromb. Res. 2010, 126, e36–e40. [Google Scholar] [CrossRef] [PubMed]
- Parahuleva, M.S.; Hölschermann, H.; Zandt, D.; Pons-Kühnemann, J.; Parviz, B.; Weiskirchen, R.; Staubitz, A.; Tillmanns, H.; Erdogan, A.; Kanse, S.M. Circulating factor VII activating protease (FSAP) is associated with clinical outcome in acute coronary syndrome. Circ. J. 2012, 76, 2653–2661. [Google Scholar] [CrossRef]
- Römisch, J.; Feussner, A.; Stöhr, H.A. Quantitation of the factor VII- and single-chain plasminogen activator-activating protease in plasmas of healthy subjects. Blood Coagul. Fibrinolysis 2001, 12, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, R.; Gram, J.B.; Sand, N.P.R.; Nørgaard, B.L.; Diederichsen, A.C.P.; Vitzthum, F.; Schwarz, H.; Sidelmann, J.J. Factor VII-activating protease: Sex-related association with coronary artery calcification. Blood Coagul. Fibrinolysis 2017, 28, 558–563. [Google Scholar] [CrossRef]
- Tian, D.S.; Qin, C.; Zhou, L.Q.; Yang, S.; Chen, M.; Xiao, J.; Shang, K.; Bosco, D.B.; Wu, L.J.; Wang, W. FSAP aggravated endothelial dysfunction and neurological deficits in acute ischemic stroke due to large vessel occlusion. Signal Transduct. Target Ther. 2022, 7, 6. [Google Scholar] [CrossRef]
- Olsson, M.; Stanne, T.M.; Pedersen, A.; Lorentzen, E.; Kara, E.; Martinez-Palacian, A.; Rønnow Sand, N.P.; Jacobsen, A.F.; Sandset, P.M.; Sidelmann, J.J.; et al. Genome-wide analysis of genetic determinants of circulating factor VII-activating protease (FSAP) activity. J. Thromb. Haemost 2018, 16, 2024–2034. [Google Scholar] [CrossRef]
- Sidelmann, J.J.; Skouby, S.O.; Kluft, C.; Winkler, U.; Vitzthum, F.; Schwarz, H.; Gram, J.; Jespersen, J. Plasma factor VII-activating protease is increased by oral contraceptives and induces factor VII activation in-vivo. Thromb. Res. 2011, 128, e67–e72. [Google Scholar] [CrossRef]
- Kannemeier, C.; Feussner, A.; Stöhr, H.A.; Weisse, J.; Preissner, K.T.; Römisch, J. Factor VII and single-chain plasminogen activator-activating protease: Activation and autoactivation of the proenzyme. Eur. J. Biochem. 2001, 268, 3789–3796. [Google Scholar] [CrossRef] [PubMed]
- Wygrecka, M.; Morty, R.E.; Markart, P.; Kanse, S.M.; Andreasen, P.A.; Wind, T.; Guenther, A.; Preissner, K.T. Plasminogen activator inhibitor-1 is an inhibitor of factor VII-activating protease in patients with acute respiratory distress syndrome. J. Biol. Chem. 2007, 282, 21671–21682. [Google Scholar] [CrossRef] [PubMed]
- Zeerleder, S.; Zwart, B.; te Velthuis, H.; Stephan, F.; Manoe, R.; Rensink, I.; Aarden, L.A. Nucleosome-releasing factor: A new role for factor VII-activating protease (FSAP). FASEB J. 2008, 22, 4077–4084. [Google Scholar] [CrossRef]
- Kanse, S.M.; Declerck, P.J.; Ruf, W.; Broze, G.; Etscheid, M. Factor VII-activating protease promotes the proteolysis and inhibition of tissue factor pathway inhibitor. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 427–433. [Google Scholar] [CrossRef]
- Stavenuiter, F.; Dienava-Verdoold, I.; Boon-Spijker, M.G.; Brinkman, H.J.; Meijer, A.B.; Mertens, K. Factor seven activating protease (FSAP): Does it activate factor VII. J. Thromb. Haemost. 2012, 10, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Stephan, F.; Hazelzet, J.A.; Bulder, I.; Boermeester, M.A.; van Till, J.O.; van der Poll, T.; Wuillemin, W.A.; Aarden, L.A.; Zeerleder, S. Activation of factor VII-activating protease in human inflammation: A sensor for cell death. Crit. Care 2011, 15, R110. [Google Scholar] [CrossRef] [PubMed]
- Sidelmann, J.J.; Vitzthum, F.; Funding, E.; Münster, A.M.; Gram, J.; Jespersen, J. Factor VII-activating protease in patients with acute deep venous thrombosis. Thromb. Res. 2008, 122, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Etscheid, M.; Subramaniam, S.; Lochnit, G.; Zabczyk, M.; Undas, A.; Lang, I.M.; Hanschmann, K.M.; Kanse, S.M. Altered structure and function of fibrinogen after cleavage by Factor VII Activating Protease (FSAP). Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3397–3406. [Google Scholar] [CrossRef]
- Römisch, J.; Vermöhlen, S.; Feussner, A.; Stöhr, H. The FVII activating protease cleaves single-chain plasminogen activators. Haemostasis 1999, 29, 292–299. [Google Scholar] [CrossRef]
- Nielsen, N.V.; Roedel, E.; Manna, D.; Etscheid, M.; Morth, J.P.; Kanse, S.M. Characterization of the enzymatic activity of the serine protease domain of Factor VII activating protease (FSAP). Sci. Rep. 2019, 9, 18990. [Google Scholar] [CrossRef]
- Muhl, L.; Galuska, S.P.; Oörni, K.; Hernández-Ruiz, L.; Andrei-Selmer, L.C.; Geyer, R.; Preissner, K.T.; Ruiz, F.A.; Kovanen, P.T.; Kanse, S.M. High negative charge-to-size ratio in polyphosphates and heparin regulates factor VII-activating protease. FEBS J. 2009, 276, 4828–4839. [Google Scholar] [CrossRef]
- Kanse, S.M.; Parahuleva, M.; Muhl, L.; Kemkes-Matthes, B.; Sedding, D.; Preissner, K.T. Factor VII-activating protease (FSAP): Vascular functions and role in atherosclerosis. Thromb. Haemost. 2008, 99, 286–289. [Google Scholar] [CrossRef]
- Sperling, C.; Maitz, M.F.; Grasso, S.; Werner, C.; Kanse, S.M. A Positively Charged Surface Triggers Coagulation Activation Through Factor VII Activating Protease (FSAP). ACS Appl. Mater. Interfaces 2017, 9, 40107–40116. [Google Scholar] [CrossRef]
- Altincicek, B.; Shibamiya, A.; Trusheim, H.; Tzima, E.; Niepmann, M.; Linder, D.; Preissner, K.T.; Kanse, S.M. A positively charged cluster in the epidermal growth factor-like domain of Factor VII-activating protease (FSAP) is essential for polyanion binding. Biochem. J. 2006, 394, 687–692. [Google Scholar] [CrossRef]
- Muhl, L.; Hersemeyer, K.; Preissner, K.T.; Weimer, T.; Kanse, S.M. Structure-function analysis of factor VII activating protease (FSAP): Sequence determinants for heparin binding and cellular functions. FEBS Lett. 2009, 583, 1994–1998. [Google Scholar] [CrossRef]
- Etscheid, M.; Muhl, L.; Pons, D.; Jukema, J.W.; König, H.; Kanse, S.M. The Marburg I polymorphism of factor VII activating protease is associated with low proteolytic and low pro-coagulant activity. Thromb. Res. 2012, 130, 935–941. [Google Scholar] [CrossRef]
- Roedel, E.K.; Schwarz, E.; Kanse, S.M. The factor VII-activating protease (FSAP) enhances the activity of bone morphogenetic protein-2 (BMP-2). J. Biol. Chem. 2013, 288, 7193–7203. [Google Scholar] [CrossRef] [PubMed]
- Sidelmann, J.J.; Skouby, S.O.; Vitzthum, F.; Schwarz, H.; Jespersen, J. Hormone therapy affects plasma measures of factor VII-activating protease in younger postmenopausal women. Climacteric 2010, 13, 340–346. [Google Scholar] [CrossRef]
- Parahuleva, M.S.; Kanse, S.; Hölschermann, H.; Zheleva, K.; Zandt, D.; Worsch, M.; Parviz, B.; Güttler, N.; Tillmanns, H.; Böning, A.; et al. Association of circulating factor seven activating protease (FSAP) and of oral Omega-3 fatty acids supplements with clinical outcome in patients with atrial fibrillation: The OMEGA-AF study. J. Thromb. Thrombolysis 2014, 37, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Hanson, E.; Kanse, S.M.; Joshi, A.; Jood, K.; Nilsson, S.; Blomstrand, C.; Jern, C. Plasma factor VII-activating protease antigen levels and activity are increased in ischemic stroke. J. Thromb. Haemost. 2012, 10, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Artunc, F.; Bohnert, B.N.; Schneider, J.C.; Staudner, T.; Sure, F.; Ilyaskin, A.V.; Wörn, M.; Essigke, D.; Janessa, A.; Nielsen, N.V.; et al. Proteolytic activation of the epithelial sodium channel (ENaC) by factor VII activating protease (FSAP) and its relevance for sodium retention in nephrotic mice. Pflugers. Arch. 2022, 474, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Kanse, S.M.; Gallenmueller, A.; Zeerleder, S.; Stephan, F.; Rannou, O.; Denk, S.; Etscheid, M.; Lochnit, G.; Krueger, M.; Huber-Lang, M. Factor VII-activating protease is activated in multiple trauma patients and generates anaphylatoxin C5a. J. Immunol. 2012, 188, 2858–2865. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Thielmann, I.; Morowski, M.; Pragst, I.; Sandset, P.M.; Nieswandt, B.; Etscheid, M.; Kanse, S.M. Defective thrombus formation in mice lacking endogenous factor VII activating protease (FSAP). Thromb. Haemost. 2015, 113, 870–880. [Google Scholar] [CrossRef] [PubMed]
- de Jong, H.K.; Koh, G.C.; Bulder, I.; Stephan, F.; Wiersinga, W.J.; Zeerleder, S.S. Diabetes-independent increase of factor VII-activating protease activation in patients with Gram-negative sepsis (melioidosis). J. Thromb. Haemost. 2015, 13, 41–46. [Google Scholar] [CrossRef]
- Marsman, G.; Stephan, F.; de Leeuw, K.; Bulder, I.; Ruinard, J.T.; de Jong, J.; Westra, J.; Bultink, I.E.; Voskuyl, A.E.; Aarden, L.A.; et al. FSAP-mediated nucleosome release from late apoptotic cells is inhibited by autoantibodies present in SLE. Eur. J. Immunol. 2016, 46, 762–771. [Google Scholar] [CrossRef]
- Marsman, G.; von Richthofen, H.; Bulder, I.; Lupu, F.; Hazelzet, J.; Luken, B.M.; Zeerleder, S. DNA and factor VII-activating protease protect against the cytotoxicity of histones. Blood Adv. 2017, 1, 2491–2502. [Google Scholar] [CrossRef] [PubMed]
- Wasmuth, H.E.; Tag, C.G.; Van de Leur, E.; Hellerbrand, C.; Mueller, T.; Berg, T.; Puhl, G.; Neuhaus, P.; Samuel, D.; Trautwein, C.; et al. The Marburg I variant (G534E) of the factor VII-activating protease determines liver fibrosis in hepatitis C infection by reduced proteolysis of platelet-derived growth factor BB. Hepatology 2009, 49, 775–780. [Google Scholar] [CrossRef]
- Roemisch, J.; Feussner, A.; Nerlich, C.; Stoehr, H.A.; Weimer, T. The frequent Marburg I polymorphism impairs the pro-urokinase activating potency of the factor VII activating protease (FSAP). Blood Coagul. Fibrinolysis 2002, 13, 433–441. [Google Scholar] [CrossRef]
- Semeraro, F.; Ammollo, C.T.; Semeraro, N.; Colucci, M. Extracellular histones promote fibrinolysis by single-chain urokinase-type plasminogen activator in a factor seven activating protease-dependent way. Thromb. Res. 2020, 196, 193–199. [Google Scholar] [CrossRef]
- Stephan, F.; Dienava-Verdoold, I.; Bulder, I.; Wouters, D.; Mast, A.E.; Te Velthuis, H.; Aarden, L.A.; Zeerleder, S. Tissue factor pathway inhibitor is an inhibitor of factor VII-activating protease. J. Thromb. Haemost. 2012, 10, 1165–1171. [Google Scholar] [CrossRef]
- Stephan, F.; Marsman, G.; Bakker, L.M.; Bulder, I.; Stavenuiter, F.; Aarden, L.A.; Zeerleder, S. Cooperation of factor VII-activating protease and serum DNase I in the release of nucleosomes from necrotic cells. Arthritis. Rheumatol. 2014, 66, 686–693. [Google Scholar] [CrossRef]
- Bustamante, A.; Díaz-Fernández, B.; Giralt, D.; Boned, S.; Pagola, J.; Molina, C.A.; García-Berrocoso, T.; Kanse, S.M.; Montaner, J. Factor seven activating protease (FSAP) predicts response to intravenous thrombolysis in acute ischemic stroke. Int. J. Stroke 2016, 11, 646–655. [Google Scholar] [CrossRef]
- Gramstad, O.R.; Kandanur, S.P.S.; Etscheid, M.; Nielsen, E.W.; Kanse, S.M. Factor VII activating protease (FSAP) is not essential in the pathophysiology of angioedema in patients with C1 inhibitor deficiency. Mol. Immunol. 2022, 142, 95–104. [Google Scholar] [CrossRef]
- Grasso, S.; Neumann, A.; Lang, I.M.; Etscheid, M.; von Köckritz-Blickwede, M.; Kanse, S.M. Interaction of factor VII activating protease (FSAP) with neutrophil extracellular traps (NETs). Thromb. Res. 2018, 161, 36–42. [Google Scholar] [CrossRef]
- Muhl, L.; Nykjaer, A.; Wygrecka, M.; Monard, D.; Preissner, K.T.; Kanse, S.M. Inhibition of PDGF-BB by Factor VII-activating protease (FSAP) is neutralized by protease nexin-1, and the FSAP-inhibitor complexes are internalized via LRP. Biochem. J. 2007, 404, 191–196. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shibamiya, A.; Muhl, L.; Tannert-Otto, S.; Preissner, K.T.; Kanse, S.M. Nucleic acids potentiate Factor VII-activating protease (FSAP)-mediated cleavage of platelet-derived growth factor-BB and inhibition of vascular smooth muscle cell proliferation. Biochem. J 2007, 404, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Uslu, Ö.; Herold, J.; Kanse, S.M. VEGF-A-Cleavage by FSAP and Inhibition of Neo-Vascularization. Cells 2019, 8, 1396. [Google Scholar] [CrossRef] [PubMed]
- Byskov, K.; Le Gall, S.M.; Thiede, B.; Camerer, E.; Kanse, S.M. Protease activated receptors (PAR)-1 and -2 mediate cellular effects of factor VII activating protease (FSAP). FASEB J. 2020, 34, 1079–1090. [Google Scholar] [CrossRef]
| Measurement | In Women | In Men | p-Value (Women vs. Men) * | References |
|---|---|---|---|---|
| FSAP levels | 11.15 µg/mL (3.60–16.43 µg/mL) | 10.51 µg/mL (4.36–16.15 µg/mL) | 0.07 | [33] |
| 97.4% | 87.5% | <0.001 | [34] | |
| FSAP activity | 955 mPEU/mL (50–1453 mPEU/mL) | 841 mPEU/mL (290–1326 mPEU/mL) | 0.0005 | [33] |
| 81.1% | 68.7% | <0.001 | [34] | |
| The ratio of percentage FSAP activity to percentage FSAP levels | 0.79 | 0.84 | <0.001 | [34] |
| Name | Studies In Vitro Describing the Influence |
|---|---|
| Single-chain urokinase plasminogen activator (scuPA) | [38] |
| Two-chain urokinase plasminogen activator (tcuPA) | [38] |
| High molecular mass urokinase plasminogen activator (HMMuPA) | [38] |
| Histones | [10] 1, [59,63,66,69,70,71] |
| Nucleosomes (predigested) | [63] |
| Chromatin | [59] |
| Polyamines (spermidine, spermine, putrescine) | [9] |
| Neutrophil extracellular traps (NETs) (disintegrated) | [71] |
| Apoptotic and necrotic cells | [40,43,67,68] |
| Poly-L-lysine (PLL) | [11,50] |
| Polyethylenimine (PEI) | [50] |
| Name | Studies In Vitro Describing the Influence | Contradictory Data |
|---|---|---|
| RNA | [9,10,26,51] | RNA was excluded as the cell-derived structure promoting FSAP activation [40,43]. RNA failed to promote FSAP activation in human plasma [10]. |
| DNA | Effect at high levels of DNA [51] | Neither DNA homologue [26] nor DNA [66,71] was able to affect FSAP activation in vitro. |
| Heparin | [9,10,11,17,18,38,48,51,52,53,64] | Heparin failed to promote FSAP activation in human plasma [10,59,65]. |
| Low molecular weight heparin (LMWH) | Weak effect [48]. | LMWH inhibited spermidine-induced FSAP autoactivation in vitro [9]. |
| Name | Studies In Vitro Describing the Influence | Contradictory Data |
|---|---|---|
| α-2-antiplasmin (AP) | [11] | FSAP-AP complexes are considered as the marker of completed FSAP activation [10,43,50,59,61,62,63,67,68,69,70,71]. |
| C1-esteraze inhibitor (C1-inh) | [11] | FSAP-C1-inh complexes are considered as the marker of completed FSAP activation [43,67,70]. |
| Plasminogen activator inhibitor type 1 (PAI-1) | Limited scFSAP-PAI-1 binding [39]. | Another experiment of the same authors indicated that scFSAP did not bind with PAI-1. |
| Low molecular weight heparin (LMWH) | LMWH inhibited spermidine-induced FSAP autoactivation [9]. | LMWH weakly promotes FSAP activation [48]. |
| Name | Studies In Vitro Describing the Influence | Contradictory Data |
|---|---|---|
| RNA | [73] | RNA increased inhibitor-driven reduction of tcFSAP activity [39]. |
| DNA | Weak effect [73]. | DNA did not alter FSAP activity effectively [73]. |
| Polyamines | Minimal effect [9]. | nd 1 |
| Heparin | [3,18,25,28,42,46,48,52,73,74,75] | Heparin did not alter significantly FSAP activity [9,28,72,75] or FSAP activity was reduced in heparin presence [54]. Heparin increased inhibitor-driven reduction of tcFSAP activity [11,46,48,52]. |
| Low molecular weight heparin (LMWH) | [48] | LMWH did not alter significantly FSAP activity [28]. LMWH increased inhibitor-driven reduction of tcFSAP activity [48]. |
| Name | Studies In Vitro Describing the Influence |
|---|---|
| α-2-antiplasmin (AP) | [2,10] 1, [11,39], [43] 1, [46,50], [59] 1, [61,62,63] 1, [67,68,69,70,71] |
| Plasminogen activator inhibitor-1 (PAI-1) | [39] 1, [48,52,72] |
| Protease nexin-1 (PN-1) | [72,73] |
| Antithrombin (AT) + heparin | [11,46,48] |
| Name | Studies In Vitro Describing the Influence | Contradictory Data |
|---|---|---|
| Heparin | [11,46,48,52] | Heparin promoted [3,18,25,28,42,46,48,52,73,74,75] or did not alter FSAP activity [9,28,72,75]. Heparin did not promote PAI-1-driven inhibition of FSAP [39]. |
| Low molecular weight heparin (LMWH) | [48] | LMWH did not alter significantly [28] or increased tcFSAP activity [48]. |
| C1-esterase inhibitor (C1-inh) | [2,6,39,43] 1, [46,67,70] | Murine plasma levels of tcFSAP-C1-inh complexes were insignificant after histone injection [10]. |
| Tissue factor pathway inhibitor (TFPI) | [67] | TFPI failed to inhibit tcFSAP activity [46]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiatkowska, I.; Żekanowska, E.; Lattanzi, S.; Alexandre, A.M.; Kister-Kowalska, A.; Słomka, A. Factor VII Activating Protease (FSAP) and Its Importance in Hemostasis—Part I: FSAP Structure, Synthesis and Activity Regulation: A Narrative Review. Int. J. Mol. Sci. 2023, 24, 5473. https://doi.org/10.3390/ijms24065473
Kwiatkowska I, Żekanowska E, Lattanzi S, Alexandre AM, Kister-Kowalska A, Słomka A. Factor VII Activating Protease (FSAP) and Its Importance in Hemostasis—Part I: FSAP Structure, Synthesis and Activity Regulation: A Narrative Review. International Journal of Molecular Sciences. 2023; 24(6):5473. https://doi.org/10.3390/ijms24065473
Chicago/Turabian StyleKwiatkowska, Iga, Ewa Żekanowska, Simona Lattanzi, Andrea M. Alexandre, Agata Kister-Kowalska, and Artur Słomka. 2023. "Factor VII Activating Protease (FSAP) and Its Importance in Hemostasis—Part I: FSAP Structure, Synthesis and Activity Regulation: A Narrative Review" International Journal of Molecular Sciences 24, no. 6: 5473. https://doi.org/10.3390/ijms24065473
APA StyleKwiatkowska, I., Żekanowska, E., Lattanzi, S., Alexandre, A. M., Kister-Kowalska, A., & Słomka, A. (2023). Factor VII Activating Protease (FSAP) and Its Importance in Hemostasis—Part I: FSAP Structure, Synthesis and Activity Regulation: A Narrative Review. International Journal of Molecular Sciences, 24(6), 5473. https://doi.org/10.3390/ijms24065473






