Impact of a Whey Protein Hydrolysate Treated by Electrodialysis with Ultrafiltration Membrane on the Development of Metabolic Syndrome and the Modulation of Gut Microbiota in Mice
Abstract
:1. Introduction
2. Results
2.1. Physiological Effects of Fraction Supplementation
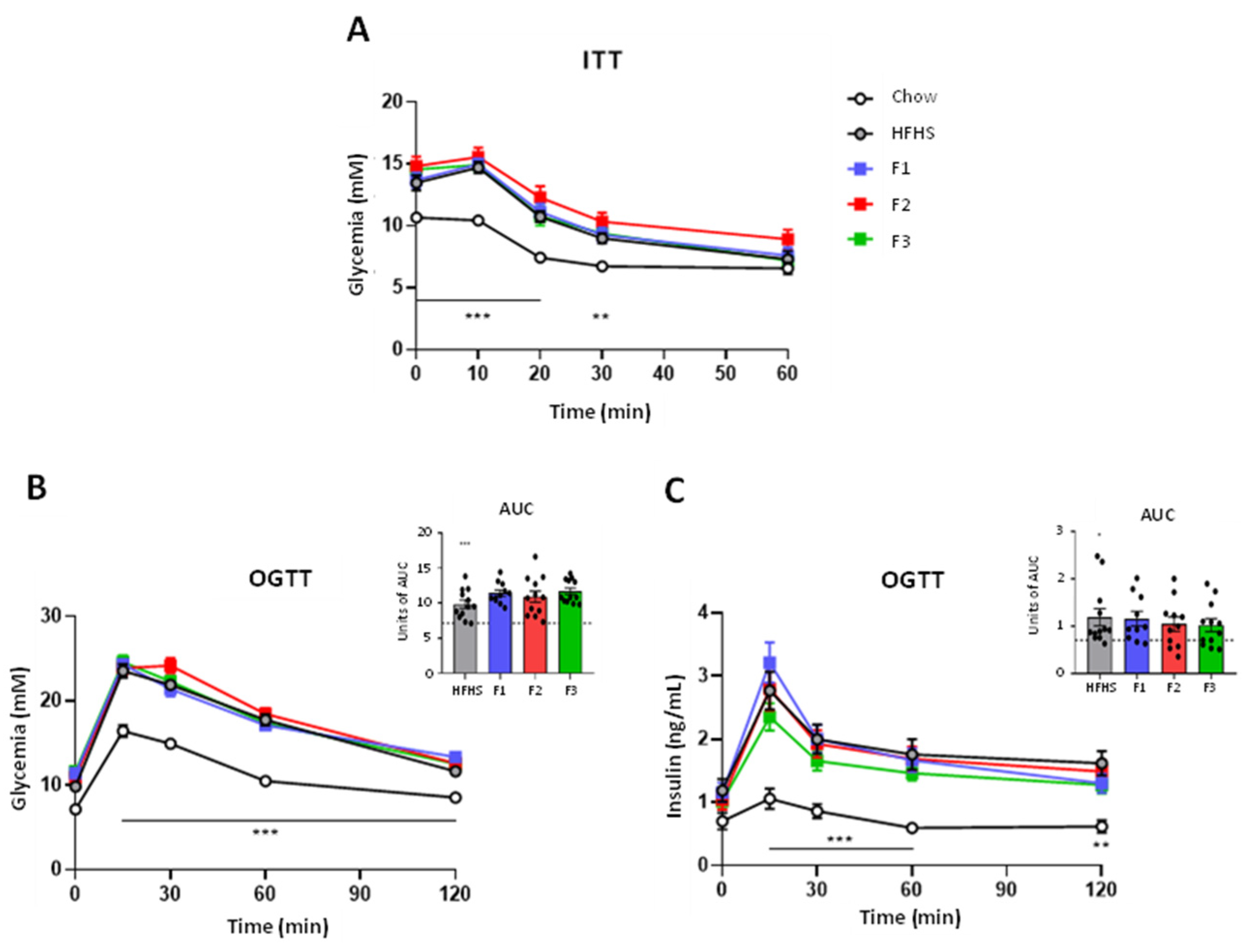
2.2. Glucose Homeostasis and Insulin Sensitivity
2.3. Gut Microbiota Analyses
3. Discussion
4. Materials and Methods
4.1. Supplementation Samples
4.2. Animals and Dietary Treatments
4.3. Insulin Tolerance Test
4.4. Oral Glucose Tolerance Test
4.5. Fecal Sample Processing and 16S rRNA Gene-Based Sequencing
4.6. Gut Microbiota Analyses
4.7. Functional Prediction of Gut Bacterial Communities
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic Syndrome—A New World-Wide Definition. A Consens. Statement Int. Diabetes Fed. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Materna, A.C.; Friedman, J.; Campos-Baptista, M.I.; Blackburn, M.C.; Perrotta, A.; Erdman, S.E.; Alm, E.J. Host Lifestyle Affects Human Microbiota on Daily Timescales. Genome Biol. 2014, 15, R89. [Google Scholar] [CrossRef] [PubMed]
- Vernocchi, P.; Del Chierico, F.; Putignani, L. Gut Microbiota Metabolism and Interaction with Food Components. Int. J. Mol. Sci. 2020, 21, 3688. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between Microbiota and Immunity in Health and Disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Zhou, A.; Yuan, Y.; Yang, M.; Huang, Y.; Li, X.; Li, S.; Yang, S.; Tang, B. Crosstalk Between the Gut Microbiota and Epithelial Cells Under Physiological and Infectious Conditions. Front. Cell. Infect. Microbiol. 2022, 12, 832672. [Google Scholar] [CrossRef]
- Do, M.H.; Lee, E.; Oh, M.-J.; Kim, Y.; Park, H.-Y. High-Glucose or -Fructose Diet Cause Changes of the Gut Microbiota and Metabolic Disorders in Mice without Body Weight Change. Nutrients 2018, 10, 761. [Google Scholar] [CrossRef]
- Thingholm, L.B.; Rühlemann, M.C.; Koch, M.; Fuqua, B.; Laucke, G.; Boehm, R.; Bang, C.; Franzosa, E.A.; Hübenthal, M.; Rahnavard, A.; et al. Obese Individuals with and without Type 2 Diabetes Show Different Gut Microbial Functional Capacity and Composition. Cell Host Microbe 2019, 26, 252–264.e10. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Marcone, S.; Belton, O.; Fitzgerald, D.J. Milk-derived Bioactive Peptides and Their Health Promoting Effects: A Potential Role in Atherosclerosis. Br. J. Clin. Pharmacol. 2017, 83, 152–162. [Google Scholar] [CrossRef]
- Patel, S. Functional Food Relevance of Whey Protein: A Review of Recent Findings and Scopes Ahead. J. Funct. Foods 2015, 19, 308–319. [Google Scholar] [CrossRef]
- Faucher, M.; Geoffroy, T.; Thibodeau, J.; Gaaloul, S.; Bazinet, L. Semi-Industrial Production of a DPP-IV and ACE Inhibitory Peptide Fraction from Whey Protein Concentrate Hydrolysate by Electrodialysis with Ultrafiltration Membrane. Membranes 2022, 12, 409. [Google Scholar] [CrossRef] [PubMed]
- Drouin, N.; Kubáň, P.; Rudaz, S.; Pedersen-Bjergaard, S.; Schappler, J. Electromembrane Extraction: Overview of the Last Decade. TrAC Trends Anal. Chem. 2019, 113, 357–363. [Google Scholar] [CrossRef]
- Huang, C.; Chen, Z.; Gjelstad, A.; Pedersen-Bjergaard, S.; Shen, X. Electromembrane Extraction. TrAC Trends Anal. Chem. 2017, 95, 47–56. [Google Scholar] [CrossRef]
- Margolis, J. Electrophoretic Method for Preparative Separation of Charged Molecules in Liquids. U.S. Patent No. 5,039,386, 13 August 1991. [Google Scholar]
- Faupel, M.D.; van Oostrum, J. Means and Devices for Electro-Filtration of Molecules. U.S. Patent Application No. 12/441,948, 2009. [Google Scholar]
- Bazinet, L.; Firdaous, L. Separation of Bioactive Peptides by Membrane Processes: Technologies and Devices. Recent Pat. Biotechnol. 2013, 7, 9–27. [Google Scholar] [CrossRef]
- Bazinet, L.; Geoffroy, T.R. Electrodialytic Processes: Market Overview, Membrane Phenomena, Recent Developments and Sustainable Strategies. Membranes 2020, 10, 221. [Google Scholar] [CrossRef]
- Sun, L.; Chen, Q.; Lu, H.; Wang, J.; Zhao, J.; Li, P. Electrodialysis with Porous Membrane for Bioproduct Separation: Technology, Features, and Progress. Food Res. Int. 2020, 137, 109343. [Google Scholar] [CrossRef]
- Cournoyer, A.; Bazinet, L. Electrodialysis Processes an Answer to Industrial Sustainability: Toward the Concept of Eco-Circular Economy?—A Review. Membranes 2023, 13, 205. [Google Scholar] [CrossRef]
- Dlask, O.; Václavíková, N. Electrodialysis with Ultrafiltration Membranes for Peptide Separation. Chem. Pap. 2018, 72, 261–271. [Google Scholar] [CrossRef]
- Lambeir, A.-M.; Durinx, C.; Scharpé, S.; Meester, I. Dipeptidyl-Peptidase IV from Bench to Bedside: An Update on Structural Properties, Functions, and Clinical Aspects of the Enzyme DPP IV. Crit. Rev. Clin. Lab. Sci. 2003, 40, 209–294. [Google Scholar] [CrossRef]
- Chaudhari, D.D.; Singh, R.; Mallappa, R.H.; Rokana, N.; Kaushik, J.K.; Bajaj, R.; Batish, V.K.; Grover, S. Evaluation of Casein & Whey Protein Hydrolysates as Well as Milk Fermentates from Lactobacillus Helveticus for Expression of Gut Hormones. Indian J. Med. Res. 2017, 146, 409–419. [Google Scholar] [CrossRef]
- Foltz, M.; Ansems, P.; Schwarz, J.; Tasker, M.C.; Lourbakos, A.; Gerhardt, C.C. Protein Hydrolysates Induce CCK Release from Enteroendocrine Cells and Act as Partial Agonists of the CCK1 Receptor. J. Agric. Food Chem. 2008, 56, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Froy, O. Biochemical and Metabolic Mechanisms by Which Dietary Whey Protein May Combat Obesity and Type 2 Diabetes. J. Nutr. Biochem. 2013, 24, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Elovaris, R.A.; Hutchison, A.T.; Lange, K.; Horowitz, M.; Feinle-Bisset, C.; Luscombe-Marsh, N.D. Plasma Free Amino Acid Responses to Whey Protein and Their Relationships with Gastric Emptying, Blood Glucose- and Appetite-Regulatory Hormones and Energy Intake in Lean Healthy Men. Nutrients 2019, 11, 2465. [Google Scholar] [CrossRef]
- Rigamonti, A.E.; Leoncini, R.; De Col, A.; Tamini, S.; Cicolini, S.; Abbruzzese, L.; Cella, S.G.; Sartorio, A. The Appetite−Suppressant and GLP-1-Stimulating Effects of Whey Proteins in Obese Subjects Are Associated with Increased Circulating Levels of Specific Amino Acids. Nutrients 2020, 12, 775. [Google Scholar] [CrossRef] [PubMed]
- Hooton, D.; Lentle, R.; Monro, J.; Wickham, M.; Simpson, R. The Secretion and Action of Brush Border Enzymes in the Mammalian Small Intestine. In Reviews of Physiology, Biochemistry and Pharmacology; Nilius, B., Gudermann, T., Jahn, R., Lill, R., Petersen, O.H., de Tombe, P.P., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 59–118. ISBN 978-3-319-22503-6. [Google Scholar]
- Shen, W.; Matsui, T. Intestinal Absorption of Small Peptides: A Review. Int. J. Food Sci. Technol. 2019, 54, 1942–1948. [Google Scholar] [CrossRef]
- Lacroix, I.M.E.; Chen, X.-M.; Kitts, D.D.; Li-Chan, E.C.Y. Investigation into the Bioavailability of Milk Protein-Derived Peptides with Dipeptidyl-Peptidase IV Inhibitory Activity Using Caco-2 Cell Monolayers. Food Funct. 2017, 8, 701–709. [Google Scholar] [CrossRef]
- Geerlings, A.; Villar, I.C.; Zarco, F.H.; Sánchez, M.; Vera, R.; Gomez, A.Z.; Boza, J.; Duarte, J. Identification and Characterization of Novel Angiotensin-Converting Enzyme Inhibitors Obtained from Goat Milk. J. Dairy Sci. 2006, 89, 3326–3335. [Google Scholar] [CrossRef]
- Mirzapour-Kouhdasht, A.; Garcia-Vaquero, M. Cardioprotective Peptides from Milk Processing and Dairy Products: From Bioactivity to Final Products Including Commercialization and Legislation. Foods 2022, 11, 1270. [Google Scholar] [CrossRef]
- Chatterjee, A.; Kanawjia, S.K.; Khetra, Y.; Saini, P. Discordance between in Silico & in Vitro Analyses of ACE Inhibitory & Antioxidative Peptides from Mixed Milk Tryptic Whey Protein Hydrolysate. J. Food Sci. Technol. 2015, 52, 5621–5630. [Google Scholar] [CrossRef]
- Bernard, J.R.; Liao, Y.-H.; Doerner, P.G.; Ding, Z.; Hsieh, M.; Wang, W.; Nelson, J.L.; Ivy, J.L. An Amino Acid Mixture Is Essential to Optimize Insulin-Stimulated Glucose Uptake and GLUT4 Translocation in Perfused Rodent Hindlimb Muscle. J. Appl. Physiol. 2012, 113, 97–104. [Google Scholar] [CrossRef]
- Binder, E.; Bermúdez-Silva, F.J.; André, C.; Elie, M.; Romero-Zerbo, S.Y.; Leste-Lasserre, T.; Belluomo, L.; Duchampt, A.; Clark, S.; Aubert, A.; et al. Leucine Supplementation Protects from Insulin Resistance by Regulating Adiposity Levels. PLoS ONE 2013, 8, e74705. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, H.; Fan, W.; Jin, Q.; Chao, T.; Wu, Y.; Huang, J.; Hao, L.; Yang, X. Leucine Supplementation Differently Modulates Branched-Chain Amino Acid Catabolism, Mitochondrial Function and Metabolic Profiles at the Different Stage of Insulin Resistance in Rats on High-Fat Diet. Nutrients 2017, 9, 565. [Google Scholar] [CrossRef]
- Nishitani, S.; Takehana, K.; Fujitani, S.; Sonaka, I. Branched-Chain Amino Acids Improve Glucose Metabolism in Rats with Liver Cirrhosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G1292–G1300. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, K.; LeBlanc, R.E.; Loh, D.; Schwartz, G.J.; Yu, Y.-H. Increasing Dietary Leucine Intake Reduces Diet-Induced Obesity and Improves Glucose and Cholesterol Metabolism in Mice via Multimechanisms. Diabetes 2007, 56, 1647–1654. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; Nilaweera, K.; Ross, P.R.; Shanahan, F.; O’Toole, P.W.; Cotter, P.D. The Gut Microbiota and Its Relationship to Diet and Obesity. Gut Microbes 2012, 3, 186–202. [Google Scholar] [CrossRef]
- Daniel, D.S.; Lee, S.M.; Dykes, G.A.; Rahman, S. Public Health Risks of Multiple-Drug-Resistant Enterococcus spp. in Southeast Asia. Appl. Environ. Microbiol. 2015, 81, 6090–6097. [Google Scholar] [CrossRef]
- Hanchi, H.; Mottawea, W.; Sebei, K.; Hammami, R. The Genus Enterococcus: Between Probiotic Potential and Safety Concerns—An Update. Front. Microbiol. 2018, 9, 1791. [Google Scholar] [CrossRef]
- Dziarski, R.; Park, S.Y.; Kashyap, D.R.; Dowd, S.E.; Gupta, D. Pglyrp-Regulated Gut Microflora Prevotella falsenii, Parabacteroides distasonis and Bacteroides eggerthii Enhance and Alistipes finegoldii Attenuates Colitis in Mice. PLoS ONE 2016, 11, e0146162. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Goel, R.; Kumar, A.; Qi, Y.; Lobaton, G.; Hosaka, K.; Mohammed, M.; Handberg, E.M.; Richards, E.M.; Pepine, C.J.; et al. Imbalance of Gut Microbiome and Intestinal Epithelial Barrier Dysfunction in Patients with High Blood Pressure. Clin. Sci. Lond. Engl. 1979 2018, 132, 701–718. [Google Scholar] [CrossRef]
- den Besten, G.; Lange, K.; Havinga, R.; van Dijk, T.H.; Gerding, A.; van Eunen, K.; Müller, M.; Groen, A.K.; Hooiveld, G.J.; Bakker, B.M.; et al. Gut-Derived Short-Chain Fatty Acids Are Vividly Assimilated into Host Carbohydrates and Lipids. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G900–G910. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Lordan, C.; Ross, R.P.; Cotter, P.D. Gut Microbes from the Phylogenetically Diverse Genus Eubacterium and Their Various Contributions to Gut Health. Gut Microbes 2020, 12, 1802866. [Google Scholar] [CrossRef]
- Kadel, S.; Persico, M.; Thibodeau, J.; Lainé, C.; Bazinet, L. Use of redundancy analysis and multivariate regression models to select the significant membrane properties affecting peptide migration during electrodialysis with filtration membranes. Sep. Purif. Technol. 2019, 221, 114–125. [Google Scholar] [CrossRef]
- Geoffroy, T.R.; Bernier, M.E.; Thibodeau, J.; Francezon, N.; Beaulieu, L.; Mikhaylin, S.; Langevin, M.E.; Lutin, F.; Bazinet, L. Semi-industrial scale-up of EDUF technology for the electroseparation of bioactive cationic peptides: Impact of process parameters and cell configurations on eco-efficiency. J. Membr. Sci. 2022, 641, 119856. [Google Scholar] [CrossRef]
- Geoffroy, T.R.; Thibodeau, J.; Faucher, M.; Langevin, M.E.; Lutin, F.; Bazinet, L. Relationship between Feed Concentration and Biactive Cationic Peptide Recovery: Impact on Ecoefficiency of EDUF at Semi-Industrial Scale. Sep. Purif. Technol. 2022, 286, 120403. [Google Scholar] [CrossRef]
- Kadel, S.; Pellerin, G.; Thibodeau, J.; Perreault, V.; Lainé, C.; Bazinet, L. How molecular weight cut-offs and physicochemical properties of polyether sulfone membrane affect peptides migration and selectivity during electrodialysis with filtration membranes. Membranes 2019, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Persico, M.; Dhulster, P.; Bazinet, L. Redundancy analysis for determination of the main physicochemical characteristics of filtration membranes explaining their fouling by peptides. J. Membr. Sci. 2018, 563, 708–717. [Google Scholar] [CrossRef]
- Persico, M.; Daigle, G.; Kadel, S.; Perreault, V.; Pellerin, G.; Thibodeau, J.; Bazinet, L. Predictive models for determination of peptide fouling based on the physicochemical characteristics of filtration membranes. Sep. Purif. Technol. 2020, 240, 116602. [Google Scholar] [CrossRef]
- Marie, G.C.U.; Perreault, V.; Henaux, L.; Carnovale, V.; Aluko, R.; Marette, A.; Doyen, A.; Bazinet, L. Impact of a high hydrostatic pressure pretreatment on the separation of bioactive peptides from flaxseed protein hydrolysates by electrodialysis with ultrafiltration membranes. Sep. Purif. Technol. 2019, 211, 242–251. [Google Scholar] [CrossRef]
- González-Muñoz, A.; Valle, M.; Marette, A.; Aluko, R.; Bazinet, L.; Enrione, J. Production of antihypertensive and antidiabetic peptide fractions from quinoa (Chenopodium quinoa Willd.) by electrodialysis with ultrafiltration membranes. Food Sci. Hum. Wellness 2022, 11, 1650–1659. [Google Scholar] [CrossRef]
- Anhe, F.F.; Roy, D.; Pilon, G.; Dudonne, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, e872–e883. [Google Scholar] [CrossRef]
- Durand, R.; Ouellette, A.; Houde, V.P.; Guénard, F.; Varin, T.V.; Marcotte, B.; Pilon, G.; Fraboulet, E.; Vohl, M.C.; Marette, A.; et al. Animal and cellular studies demonstrate some of the beneficial impacts of herring milt hydrolysates on obesity-induced glucose intolerance and inflammation. Nutrients 2020, 12, 3235. [Google Scholar] [CrossRef] [PubMed]
- Benoit, N.; Dubois, M.J.; Pilon, G.; Varin, T.V.; Marette, A.; Bazinet, L. Effects of herring milt hydrolysates and fractions in a diet-induced obesity model. Foods 2021, 10, 2046. [Google Scholar] [CrossRef] [PubMed]
- Renaud, V.; Houde, V.P.; Pilon, G.; Varin, T.V.; Roblet, C.; Marette, A.; Boutin, Y.; Bazinet, L. The Concentration of Organic Acids in Cranberry Juice Modulates the Gut Microbiota in Mice. Int. J. Mol. Sci. 2021, 22, 11537. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, S.; Yu, F.; Zhou, L.; Wang, K. Noncoding RNAs as Molecular Targets of Resveratrol Underlying Its Anticancer Effects. J. Agric. Food Chem. 2019, 67, 4709–4719. [Google Scholar] [CrossRef] [PubMed]
- Caspi, R.; Billington, R.; Ferrer, L.; Foerster, H.; Fulcher, C.A.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Latendresse, M.; Mueller, L.A.; et al. The MetaCyc Database of Metabolic Pathways and Enzymes and the BioCyc Collection of Pathway/Genome Databases. Nucleic Acids Res. 2016, 44, D471–D480. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for Prediction of Metagenome Functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Barati, M.; Javanmardi, F.; Mousavi Jazayeri, S.M.H.; Jabbari, M.; Rahmani, J.; Barati, F.; Nickho, H.; Davoodi, S.H.; Roshanravan, N.; Mousavi Khaneghah, A. Techniques, Perspectives, and Challenges of Bioactive Peptide Generation: A Comprehensive Systematic Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1488–1520. [Google Scholar] [CrossRef] [PubMed]
- Sypka, M.; Jodłowska, I.; Białkowska, A.M. Keratinases as Versatile Enzymatic Tools for Sustainable Development. Biomolecules 2021, 11, 1900. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Feng, C.; Hong, H.; Zhang, Y.; Luo, Z.; Wang, Q.; Luo, Y.; Tan, Y. Novel ACE Inhibitory Peptides Derived from Whey Protein Hydrolysates: Identification and Molecular Docking Analysis. Food Biosci. 2022, 48, 101737. [Google Scholar] [CrossRef]





| Chow | HFHS | F1 | F2 | F3 | |
|---|---|---|---|---|---|
| Total weight gain (g) | 4.24 ± 0.40 *** | 9.98 ± 0.92 | 10.56 ± 1.05 | 11.61 ± 0.96 | 10.22 ± 0.96 |
| Total energy intake (kcal) | 602.59 ± 14.41 ** | 693.25 ± 20.28 | 669.05 ± 16.71 | 708.97 ± 15.00 | 665.93 ± 11.24 |
| Visceral fat pad (g) | 1.16 ± 0.11 *** | 3.40 ± 0.32 | 3.38 ± 0.39 | 3.83 ± 0.37 | 3.45 ± 0.28 |
| Subcutaneous fat pad (g) | 0.34 ± 0.03 *** | 0.80 ± 0.07 | 0.90 ± 0.08 | 0.99 ± 0.14 | 0.89 ± 0.09 |
| Brown adipose tissue (g) | 0.08 ± 0.01 *** | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.13 ± 0.01 | 0.12 ± 0.01 |
| Total lean mass (g) | 19.71 ± 0.49 | 20.3 ± 0.33 | 20.18 ± 0.33 | 20.58 ± 0.35 | 19.75 ± 0.33 |
| Total fat mass (g) | 2.73 ± 0.31 *** | 7.28 ± 0.64 | 7.77 ± 0.76 | 8.29 ± 0.83 | 7.71 ± 0.56 |
| Gastrocnemius (g) | 0.26 ± 0.01 | 0.26 ± 0.00 | 0.26 ± 0.00 | 0.27 ± 0.01 | 0.25 ± 0.00 |
| Soleus (g) | 0.02 ± 0.00 * | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 |
| Brain (g) | 0.43 ± 0.01 | 0.43 ± 0.00 | 0.43 ± 0.00 | 0.43 ± 0.01 | 0.42 ± 0.00 |
| Heart (g) | 0.14 ± 0.00 | 0.13 ± 0.00 | 0.13 ± 0.00 | 0.13 ± 0.00 | 0.14 ± 0.00 |
| Kidneys (g) | 0.33 ± 0.01 | 0.35 ± 0.01 | 0.34 ± 0.00 | 0.35 ± 0.01 | 0.33 ± 0.01 |
| Liver (g) | 1.06 ± 0.04 | 0.99 ± 0.06 | 1.09 ± 0.04 | 1.12 ± 0.06 | 1.06 ± 0.03 |
| Pancreas (g) | 0.29 ± 0.01 | 0.32 ± 0.03 | 0.26 ± 0.01 # | 0.31 ± 0.02 | 0.27 ± 0.01 & |
| F1 | F2 | F3 | |
|---|---|---|---|
| Proteins (g/100 g on a dry basis) | 38.40 ± 0.21 | 40.54 ± 0.19 | 19.56 ± 0.03 |
| Ash content (g/100 g on a dry basis) | 0.89 ± 0.03 | 0.87 ± 0.03 | 1.02 ± 0.03 |
| Calcium | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.08 ± 0.00 |
| Potassium | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.02 ± 0.01 |
| Magnesium | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.02 ± 0.00 |
| Phosphorus | 0.06 ± 0.01 | 0.09 ± 0.01 | 0.22 ± 0.02 |
| Sodium | 0.21 ± 0.01 | 0.22 ± 0.01 | 0.11 ± 0.00 |
| Lactose (g/100 g on a dry basis) | 44.94 ± 0.92 | 39.52 ± 0.43 | 68.76 ± 1.96 |
| Moisture content (g/100 g on a dry basis) | 1.07 ± 0.00 | 5.42 ± 0.11 | 2.51 ± 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renaud, V.; Faucher, M.; Dubois, M.-J.; Pilon, G.; Varin, T.; Marette, A.; Bazinet, L. Impact of a Whey Protein Hydrolysate Treated by Electrodialysis with Ultrafiltration Membrane on the Development of Metabolic Syndrome and the Modulation of Gut Microbiota in Mice. Int. J. Mol. Sci. 2023, 24, 12968. https://doi.org/10.3390/ijms241612968
Renaud V, Faucher M, Dubois M-J, Pilon G, Varin T, Marette A, Bazinet L. Impact of a Whey Protein Hydrolysate Treated by Electrodialysis with Ultrafiltration Membrane on the Development of Metabolic Syndrome and the Modulation of Gut Microbiota in Mice. International Journal of Molecular Sciences. 2023; 24(16):12968. https://doi.org/10.3390/ijms241612968
Chicago/Turabian StyleRenaud, Valentine, Mélanie Faucher, Marie-Julie Dubois, Geneviève Pilon, Thibault Varin, André Marette, and Laurent Bazinet. 2023. "Impact of a Whey Protein Hydrolysate Treated by Electrodialysis with Ultrafiltration Membrane on the Development of Metabolic Syndrome and the Modulation of Gut Microbiota in Mice" International Journal of Molecular Sciences 24, no. 16: 12968. https://doi.org/10.3390/ijms241612968
APA StyleRenaud, V., Faucher, M., Dubois, M.-J., Pilon, G., Varin, T., Marette, A., & Bazinet, L. (2023). Impact of a Whey Protein Hydrolysate Treated by Electrodialysis with Ultrafiltration Membrane on the Development of Metabolic Syndrome and the Modulation of Gut Microbiota in Mice. International Journal of Molecular Sciences, 24(16), 12968. https://doi.org/10.3390/ijms241612968







