Investigational Drugs for the Treatment of Postherpetic Neuralgia: Systematic Review of Randomized Controlled Trials
Abstract
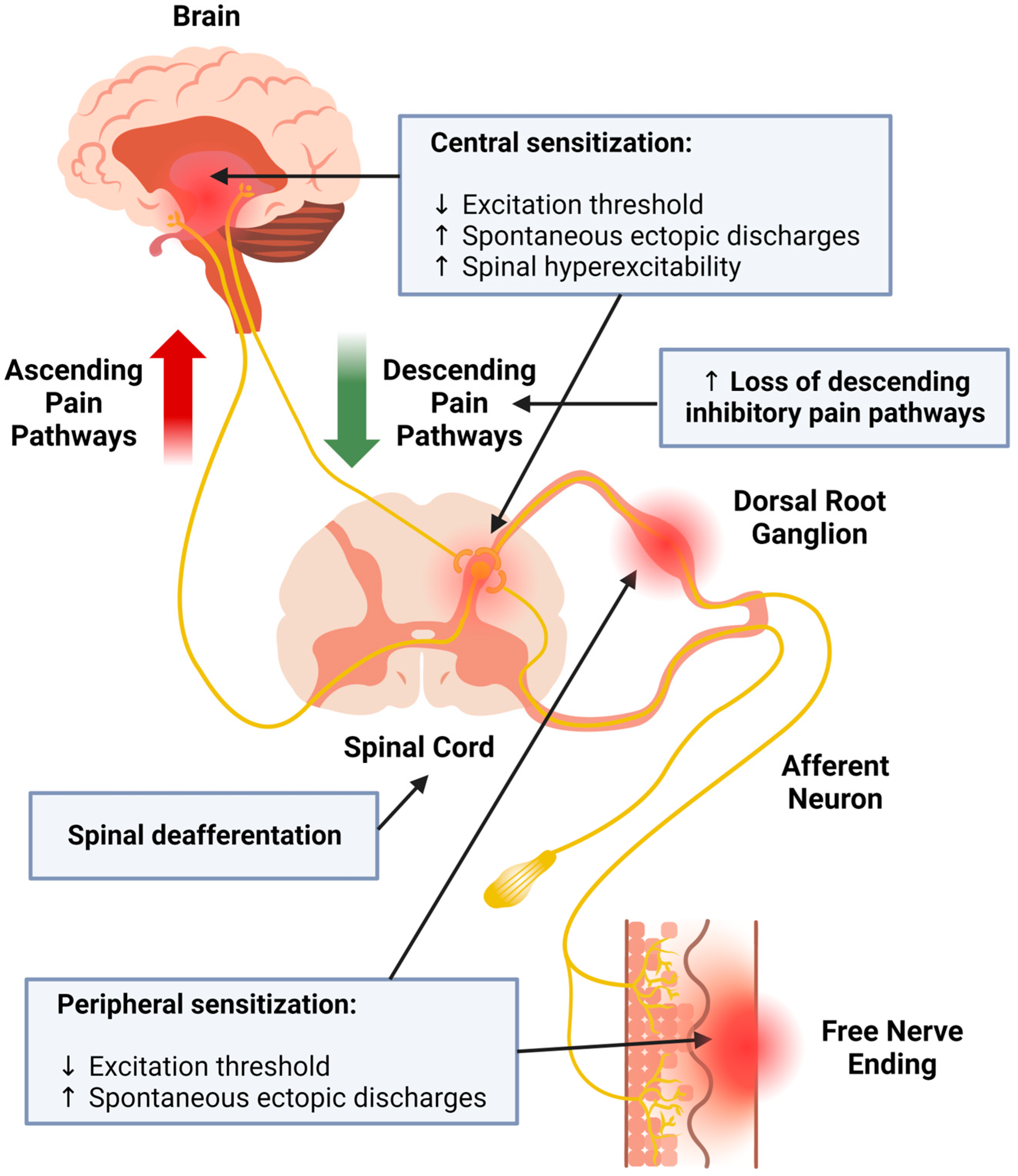
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Review Question Statement and PICOS Elements
2.3. Information Sources and Search Strategy
2.4. Inclusion and Exclusion Criteria
2.5. Article Selection
2.6. Data Extraction
3. Results and Discussion
3.1. Article Selection
3.2. Study Characteristics
3.3. Future Insights According to Pharmacological Target
3.3.1. Angiotensin II Type 2 Receptor Antagonism
3.3.2. Voltage-Gated Calcium Channel (VGCC) α2δ Subunit Inhibition
3.3.3. Voltage-Gated Sodium Channel (VGSC) Blockade
3.3.4. Cyclooxygenase-1 (COX-1) Inhibition
3.3.5. Adaptor-Associated Kinase 1 (AAK1) Inhibition
3.3.6. Lanthionine Synthetase C-like Protein (LANCL) Activation
3.3.7. N-Methyl-D-aspartate (NMDA) Receptor Antagonism
3.3.8. Mu Opioid Agonism
3.3.9. Nerve Growth Factor (NGF) Inhibition
3.3.10. Other Targets
3.3.11. Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, R.W.; Rice, A.S.C. Clinical Practice. Postherpetic Neuralgia. N. Engl. J. Med. 2014, 371, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.R.; Kong, C.L.; Porco, T.C.; Kim, E.; Ebert, C.D.; Acharya, N.R. Herpes Zoster and Postherpetic Neuralgia: Changing Incidence Rates from 1994 to 2018 in the United States. Clin. Infect. Dis. 2021, 73, e3210. [Google Scholar] [CrossRef] [PubMed]
- Hadley, G.R.; Gayle, J.A.; Ripoll, J.; Jones, M.R.; Argoff, C.E.; Kaye, R.J.; Kaye, A.D. Post-Herpetic Neuralgia: A Review. Curr. Pain Headache Rep. 2016, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Mallick-Searle, T.; Snodgrass, B.; Brant, J.M. Postherpetic Neuralgia: Epidemiology, Pathophysiology, and Pain Management Pharmacology. J. Multidiscip. Healthc. 2016, 9, 447. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; Portenoy, R.K. Pain and Its Persistence in Herpes Zoster. Pain 1996, 67, 241–251. [Google Scholar] [CrossRef]
- Sampathkumar, P.; Drage, L.A.; Martin, D.P. Herpes Zoster (Shingles) and Postherpetic Neuralgia. Mayo Clin. Proc. 2009, 84, 274–280. [Google Scholar] [CrossRef]
- Johnson, R.W.; Wasner, G.; Saddier, P.; Baron, R. Postherpetic Neuralgia: Epidemiology, Pathophysiology and Management. Expert Rev. Neurother. 2007, 7, 1581–1595. [Google Scholar] [CrossRef]
- Gruver, C.; Guthmiller, K.B. Postherpetic Neuralgia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Harden, R.N.; Kaye, A.D.; Kintanar, T.; Argoff, C.E. Evidence-Based Guidance for the Management of Postherpetic Neuralgia in Primary Care. Postgrad. Med. 2013, 125, 191–202. [Google Scholar] [CrossRef]
- Nalamachu, S.; Morley-Forster, P. Diagnosing and Managing Postherpetic Neuralgia. Drugs Aging 2012, 29, 863–869. [Google Scholar] [CrossRef]
- Schutzer-Weissmann, J.; Farquhar-Smith, P. Post-Herpetic Neuralgia—A Review of Current Management and Future Directions. Expert Opin. Pharmacother. 2017, 18, 1739–1750. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- To Evaluate the Efficacy and Safety of HSK16149 Capsule in Chinese Patients with Postherpetic Neuralgia—Full Text View—ClinicalTrials.Gov. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05140863 (accessed on 24 June 2023).
- Kato, J.; Matsui, N.; Kakehi, Y.; Murayama, E.; Ohwada, S.; Sugihara, M. Mirogabalin for the Management of Postherpetic Neuralgia: A Randomized, Double-Blind, Placebo-Controlled Phase 3 Study in Asian Patients. Pain 2019, 160, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Kato, J.; Matsui, N.; Kakehi, Y.; Murayama, E.; Ohwada, S. Long-Term Safety and Efficacy of Mirogabalin in Asian Patients with Postherpetic Neuralgia: Results from an Open-Label Extension of a Multicenter Randomized, Double-Blind, Placebo-Controlled Trial. Medicine 2020, 99, e21976. [Google Scholar] [CrossRef]
- Huffman, C.L.; Goldenberg, J.N.; Weintraub, J.; Sanin, L.; Driscoll, J.; Yang, R.; Chew, M.L.; Scavone, J.M. Efficacy and Safety of Once-Daily Controlled-Release Pregabalin for the Treatment of Patients with Postherpetic Neuralgia: A Double-Blind, Enriched Enrollment Randomized Withdrawal, Placebo-Controlled Trial. Clin. J. Pain 2017, 33, 569–578. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, H.; Xi, L.; Hong, Z.; He, L.; Fu, Y.; Fang, H.; Shang, N.; Yan, P.; Fan, D. A Randomized, Double-Blind, Placebo-Controlled Trial to Evaluate the Efficacy and Safety of Pregabalin for Postherpetic Neuralgia in a Population of Chinese Patients. Pain Pr. 2017, 17, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Han, K.A.; Lee, Y.H.; Son, H.S.; Song, K.H.; Kim, S.Y.; Chung, C.H.; Jang, H.C.; Lee, K.W.; Cha, B.Y.; Song, K.H.; et al. Efficacy and Safety of a New Sustained-Release Pregabalin Formulation Compared with Immediate-Release Pregabalin in Patients With Peripheral Neuropathic Pain: A Randomized Noninferiority Phase 3 Trial. Clin. J. Pain 2022, 38, 343–350. [Google Scholar] [CrossRef]
- Price, N.; Namdari, R.; Neville, J.; Proctor, K.J.W.; Kaber, S.; Vest, J.; Fetell, M.; Malamut, R.; Sherrington, R.P.; Pimstone, S.N.; et al. Safety and Efficacy of a Topical Sodium Channel Inhibitor (TV-45070) in Patients with Postherpetic Neuralgia (PHN): A Randomized, Controlled, Proof-of-Concept, Crossover Study, With a Subgroup Analysis of the Nav1.7 R1150W Genotype. Clin. J. Pain 2017, 33, 310–318. [Google Scholar] [CrossRef]
- Tan, X.; Ma, L.; Yuan, J.; Zhang, D.; Wang, J.; Zhou, W.; Cao, S. Intravenous Infusion of Lidocaine Enhances the Efficacy of Conventional Treatment of Postherpetic Neuralgia. J. Pain Res. 2019, 12, 2537–2545. [Google Scholar] [CrossRef]
- Kawai, S.; Hasegawa, J.; Ito, H.; Fukuuchi, Y.; Nakano, H.; Ohtani, H.; Sasaki, K.; Adachi, T. Efficacy and Safety of Twice-Daily Tramadol Hydrochloride Bilayer Sustained-Release Tablets with an Immediate Release Component for Postherpetic Neuralgia: Results of a Phase III, Randomized, Double-Blind, Placebo-Controlled, Treatment-Withdrawal Study. Pain Pract. 2023, 23, 277–289. [Google Scholar] [CrossRef]
- Gavin, P.D.; Tremper, L.; Smith, A.; Williams, G.; Brooker, C. Transdermal Oxycodone Patch for the Treatment of Postherpetic Neuralgia: A Randomized, Double-Blind, Controlled Trial. Pain Manag. 2017, 7, 255–267. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, C.; Zeng, T.; Li, Z.; Xia, Y.; Tao, G.; Zhu, T.; Lu, L.; Li, J.; Huang, T.; et al. Intravenous Patient-Controlled Analgesia Hydromorphone Combined with Pregabalin for the Treatment of Postherpetic Neuralgia: A Multicenter, Randomized Controlled Study. Korean J. Pain 2021, 34, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Rice, A.S.C.; Dworkin, R.H.; Finnerup, N.B.; Attal, N.; Anand, P.; Freeman, R.; Piaia, A.; Callegari, F.; Doerr, C.; Mondal, S.; et al. Efficacy and Safety of EMA401 in Peripheral Neuropathic Pain: Results of 2 Randomised, Double-Blind, Phase 2 Studies in Patients with Postherpetic Neuralgia and Painful Diabetic Neuropathy. Pain 2021, 162, 2578–2589. [Google Scholar] [CrossRef] [PubMed]
- Efficacy and Safety of TRK-700 in Patient with Post-Herpetic Neuralgia—Full Text View—ClinicalTrials.Gov. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT02701374 (accessed on 24 June 2023).
- Efficacy and Safety of LX9211 in Patients with Postherpetic Neuralgia—Full Text View—ClinicalTrials.Gov. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04662281 (accessed on 24 June 2023).
- Oral LAT8881 in Neuropathic Pain—Full Text View—ClinicalTrials.Gov. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03865953 (accessed on 25 June 2023).
- To Evaluate the Efficacy and Safety of SR419 in Patients with Postherpetic Neuralgia (PHN)—Full Text View—ClinicalTrials.Gov. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05357677 (accessed on 25 June 2023).
- The Study on the Esketamine in the Treatment of Postherpetic Neuralgia—Full Text View—ClinicalTrials.Gov. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04664530 (accessed on 25 June 2023).
- Wang, H.; Romano, G.; Fedgchin, M.; Russell, L.; Sanga, P.; Kelly, K.M.; Frustaci, M.E.; Thipphawong, J. Fulranumab in Patients With Pain Associated With Postherpetic Neuralgia and Postraumatic Neuropathy: Efficacy, Safety, and Tolerability Results From a Randomized, Double-Blind, Placebo-Controlled, Phase-2 Study. Clin. J. Pain 2017, 33, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Riediger, C.; Haschke, M.; Bitter, C.; Fabbro, T.; Schaeren, S.; Urwyler, A.; Ruppen, W. The Analgesic Effect of Combined Treatment with Intranasal S-Ketamine and Intranasal Midazolam Compared with Morphine Patient-Controlled Analgesia in Spinal Surgery Patients: A Pilot Study. J. Pain Res. 2015, 8, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, J.M.K.; Schatman, M.E. EMA401: An Old Antagonist of the AT2R for a New Indication in Neuropathic Pain. J. Pain Res. 2017, 10, 439. [Google Scholar] [CrossRef][Green Version]
- Balogh, M.; Aguilar, C.; Nguyen, N.T.; Shepherd, A.J. Angiotensin Receptors and Neuropathic Pain. Pain Rep. 2021, 6, e869. [Google Scholar] [CrossRef]
- Pulakat, L.; Sumners, C. Angiotensin Type 2 Receptors: Painful, or Not? Front. Pharmacol. 2020, 11, 571994. [Google Scholar] [CrossRef]
- Smith, M.T.; Wyse, B.D.; Edwards, S.R. Small Molecule Angiotensin II Type 2 Receptor (AT₂R) Antagonists as Novel Analgesics for Neuropathic Pain: Comparative Pharmacokinetics, Radioligand Binding, and Efficacy in Rats. Pain Med. 2013, 14, 692–705. [Google Scholar] [CrossRef]
- Rice, A.S.C.; Dworkin, R.H.; McCarthy, T.D.; Anand, P.; Bountra, C.; McCloud, P.I.; Hill, J.; Cutter, G.; Kitson, G.; Desem, N.; et al. EMA401, an Orally Administered Highly Selective Angiotensin II Type 2 Receptor Antagonist, as a Novel Treatment for Postherpetic Neuralgia: A Randomised, Double-Blind, Placebo-Controlled Phase 2 Clinical Trial. Lancet 2014, 383, 1637–1647. [Google Scholar] [CrossRef]
- Safety and Tolerability of CFTX-1554 in Healthy Subjects—Full Text View—ClinicalTrials.Gov. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05260658 (accessed on 25 June 2023).
- Confo Therapeutics Announces Global Licensing Agreement with Lilly for Peripheral Pain Candidate, CFTX-1554—Confo Therapeutics. Available online: https://www.confotherapeutics.com/2023/03/02/confo-therapeutics-announces-global-licensing-agreement-with-lilly-for-peripheral-pain-candidate-cftx-1554/ (accessed on 25 June 2023).
- Boroujerdi, A.; Zeng, J.; Sharp, K.; Kim, D.; Steward, O.; Luo, Z.D. Calcium Channel Alpha-2-Delta-1 Protein Upregulation in Dorsal Spinal Cord Mediates Spinal Cord Injury-Induced Neuropathic Pain States. Pain 2011, 152, 649–655. [Google Scholar] [CrossRef]
- Meaadi, J.; Obara, I.; Eldabe, S.; Nazar, H. The Safety and Efficacy of Gabapentinoids in the Management of Neuropathic Pain: A Systematic Review with Meta-Analysis of Randomised Controlled Trials. Int. J. Clin. Pharm. 2023, 45, 556–565. [Google Scholar] [CrossRef]
- Coderre, T.J.; Kumar, N.; Lefebvre, C.D.; Yu, J.S.C. Evidence That Gabapentin Reduces Neuropathic Pain by Inhibiting the Spinal Release of Glutamate. J. Neurochem. 2005, 94, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Kavoussi, R. Pregabalin: From Molecule to Medicine. Eur. Neuropsychopharmacol. 2006, 16, S128–S133. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Shen, Z.; Wang, X.; Zhao, J.; Liu, W.; Jiang, G. A Meta-Analysis of Randomized Controlled Trials Comparing the Efficacy and Safety of Pregabalin and Gabapentin in the Treatment of Postherpetic Neuralgia. Pain Ther. 2023, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.A.; Roy, A.; Burrell, A.; Fairchild, C.J.; Fuldeore, M.J.; Ollendorf, D.A.; Wong, P.K. Medication Compliance and Persistence: Terminology and Definitions. Value Health 2008, 11, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Coleman, C.I.; Limone, B.; Sobieraj, D.M.; Lee, S.; Roberts, M.S.; Kaur, R.; Alam, T. Dosing Frequency and Medication Adherence in Chronic Disease. J. Manag. Care Pharm. 2012, 18, 527–539. [Google Scholar] [CrossRef]
- Srivastava, K.; Arora, A.; Kataria, A.; Cappelleri, J.C.; Sadosky, A.; Peterson, A.M. Impact of Reducing Dosing Frequency on Adherence to Oral Therapies: A Literature Review and Meta-Analysis. Patient Prefer. Adherence 2013, 7, 419. [Google Scholar] [CrossRef]
- Sills, G.J. The Mechanisms of Action of Gabapentin and Pregabalin. Curr. Opin. Pharmacol. 2006, 6, 108–113. [Google Scholar] [CrossRef]
- Kim, S.; Hwang, K.M.; Park, Y.S.; Nguyen, T.T.; Park, E.S. Preparation and Evaluation of Non-Effervescent Gastroretentive Tablets Containing Pregabalin for Once-Daily Administration and Dose Proportional Pharmacokinetics. Int. J. Pharm. 2018, 550, 160–169. [Google Scholar] [CrossRef]
- Vinik, A.; Rosenstock, J.; Sharma, U.; Feins, K.; Hsu, C.; Merante, D. Efficacy and Safety of Mirogabalin (DS-5565) for the Treatment of Diabetic Peripheral Neuropathic Pain: A Randomized, Double-Blind, Placebo- and Active Comparator-Controlled, Adaptive Proof-of-Concept Phase 2 Study. Diabetes Care 2014, 37, 3253–3261. [Google Scholar] [CrossRef]
- Zajączkowska, R.; Mika, J.; Leppert, W.; Kocot-Kępska, M.; Malec-Milewska, M.; Wordliczek, J. Mirogabalin-A Novel Selective Ligand for the A2δ Calcium Channel Subunit. Pharmaceuticals 2021, 14, 112. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Arakawa, N.; Domon, Y.; Matsuda, F.; Inoue, T.; Kitano, Y.; Takahashi, M.; Yamamura, N.; Kai, K. Pharmacological, Pharmacokinetics and Safety Profiles of DS-5565, a Novel A2δ Ligand. J. Neurol. Sci. 2013, 333, e535. [Google Scholar] [CrossRef]
- Kato, J.; Baba, M.; Kuroha, M.; Kakehi, Y.; Murayama, E.; Wasaki, Y.; Ohwada, S. Safety and Efficacy of Mirogabalin for Peripheral Neuropathic Pain: Pooled Analysis of Two Pivotal Phase III Studies. Clin. Ther. 2021, 43, 822–835.e16. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Mirogabalin: First Global Approval. Drugs 2019, 79, 463–468. [Google Scholar] [CrossRef]
- To Evaluate the Efficacy and Safety of HSK16149 Capsule in Chinese Patients with Herpetic Neuralgia—Full Text View—ClinicalTrials.Gov. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05763550 (accessed on 27 June 2023).
- Guo, X.; Zhang, T.; Yuan, G.; Yukun, L.; Hua Ma, J.; Hong-Mei, L. The Efficacy and Safety of HSK 16149 in Chinese with Diabetic Peripheral Neuropathic Pain—A Randomized, Double-Blinded, Placebo and Pregabalin-Controlled Phase II/III Study. Diabetes 2023, 72, 224. [Google Scholar] [CrossRef]
- Gou, X.; Yu, X.; Bai, D.; Tan, B.; Cao, P.; Qian, M.; Zheng, X.; Chen, L.; Shi, Z.; Li, Y.; et al. Pharmacology and Mechanism of Action of HSK16149, a Selective Ligand of α 2 δ Subunit of Voltage-Gated Calcium Channel with Analgesic Activity in Animal Models of Chronic Pain. J. Pharmacol. Exp. Ther. 2021, 376, 330–337. [Google Scholar] [CrossRef]
- Deng, L.; Dourado, M.; Reese, R.M.; Huang, K.; Shields, S.D.; Stark, K.L.; Maksymetz, J.; Lin, H.; Kaminker, J.S.; Jung, M.; et al. Nav1.7 Is Essential for Nociceptor Action Potentials in the Mouse in a Manner Independent of Endogenous Opioids. Neuron 2023, 111, 1–18. [Google Scholar] [CrossRef]
- Yu, F.H.; Catterall, W.A. Overview of the Voltage-Gated Sodium Channel Family. Genome Biol. 2003, 4, 207. [Google Scholar] [CrossRef]
- Khaliq, W.; Alam, S.; Puri, N.K.; Hobson, A. Topical Lidocaine for the Treatment of Postherpetic Neuralgia. Cochrane Database Syst. Rev. 2013, 2013, CD004846. [Google Scholar] [CrossRef]
- Liu, H.; Lu, F.; Zhou, D.; Yin, Y.; Li, J.; Yang, B.; Song, L.; Ye, L.; Xiao, H. The Analgesic and Emotional Response to Intravenous Lidocaine Infusion in the Treatment of Postherpetic Neuralgia. Clin. J. Pain 2018, 34, 1025–1031. [Google Scholar] [CrossRef]
- Moulin, D.E.; Morley-Forster, P.K.; Pirani, Z.; Rohfritsch, C.; Stitt, L. Intravenous Lidocaine in the Management of Chronic Peripheral Neuropathic Pain: A Randomized-Controlled Trial. Can. J. Anaesth. 2019, 66, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Eslicarbazepine Acetate as Therapy in Post-Herpetic Neuralgia—Full Text View—ClinicalTrials.Gov. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT01124097 (accessed on 27 June 2023).
- Eslicarbazepine Acetate as Therapy in Diabetic Neuropathic Pain—Full Text View—ClinicalTrials.Gov. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT01129960 (accessed on 27 June 2023).
- Kocot-Kępska, M.; Zajaczkowska, R.; Mika, J.; Kopsky, D.J.; Wordliczek, J.; Dobrogowski, J.; Przeklasa-Muszyńska, A. Topical Treatments and Their Molecular/Cellular Mechanisms in Patients with Peripheral Neuropathic Pain-Narrative Review. Pharmaceutics 2021, 13, 450. [Google Scholar] [CrossRef] [PubMed]
- Flexion Therapeutics and Xenon Pharmaceuticals Announce Flexion’s Acquisition of an Investigational NaV1.7 Inhibitor for the Treatment of Post-Operative Pain|Xenon Pharmaceuticals Inc. Available online: https://investor.xenon-pharma.com/news-releases/news-release-details/flexion-therapeutics-and-xenon-pharmaceuticals-announce-flexions (accessed on 27 June 2023).
- Study to Evaluate the Safety and Tolerability of FX301 in Patients Undergoing Bunionectomy—Full Text View—ClinicalTrials.Gov. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04826328 (accessed on 27 June 2023).
- Hong, B.; Sun, J.; Zheng, H.; Le, Q.; Wang, C.; Bai, K.; He, J.; He, H.; Dong, Y. Effect of Tetrodotoxin Pellets in a Rat Model of Postherpetic Neuralgia. Mar. Drugs 2018, 16, 195. [Google Scholar] [CrossRef] [PubMed]
- Huerta, M.; de la Nava, J.; Artacho-Cordón, A.; Nieto, F.R. Efficacy and Security of Tetrodotoxin in the Treatment of Cancer-Related Pain: Systematic Review and Meta-Analysis. Mar. Drugs 2023, 21, 316. [Google Scholar] [CrossRef]
- Grosser, T.; Theken, K.N.; FitzGerald, G.A. Cyclooxygenase Inhibition: Pain, Inflammation, and the Cardiovascular System. Clin. Pharmacol. Ther. 2017, 102, 611–622. [Google Scholar] [CrossRef]
- El-Malah, A.A.; Gineinah, M.M.; Deb, P.K.; Khayyat, A.N.; Bansal, M.; Venugopala, K.N.; Aljahdali, A.S. Selective COX-2 Inhibitors: Road from Success to Controversy and the Quest for Repurposing. Pharmaceuticals 2022, 15, 827. [Google Scholar] [CrossRef]
- Perrone, M.G.; Scilimati, A.; Simone, L.; Vitale, P. Selective COX-1 Inhibition: A Therapeutic Target to Be Reconsidered. Curr. Med. Chem. 2010, 17, 3769–3805. [Google Scholar] [CrossRef]
- Araldi, D.; Ferrari, L.F.; Lotufo, C.M.; Vieira, A.S.; Athié, M.C.P.; Figueiredo, J.G.; Duarte, D.B.; Tambeli, C.H.; Ferreira, S.H.; Parada, C.A. Peripheral Inflammatory Hyperalgesia Depends on the COX Increase in the Dorsal Root Ganglion. Proc. Natl. Acad. Sci. USA 2013, 110, 3603–3608. [Google Scholar] [CrossRef]
- Zhu, X.; Eisenach, J.C. Cyclooxygenase-1 in the Spinal Cord Is Altered after Peripheral Nerve Injury. Anesthesiology 2003, 99, 1175–1179. [Google Scholar] [CrossRef]
- Zhu, X.; Conklin, D.; Eisenach, J.C. Cyclooxygenase-1 in the Spinal Cord Plays an Important Role in Postoperative Pain. Pain 2003, 104, 15–23. [Google Scholar] [CrossRef]
- Sun, W.; Yang, F.; Wang, Y.; Fu, H.; Yang, Y.; Li, C.-L.; Wang, X.-L.; Lin, Q.; Chen, J. Contribution of Large-Sized Primary Sensory Neuronal Sensitization to Mechanical Allodynia by Upregulation of Hyperpolarization-Activated Cyclic Nucleotide Gated Channels via Cyclooxygenase 1 Cascade. Neuropharmacology 2017, 113, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, C.; Izumimoto, N.; Morimoto, T.; Suzuki, T.; Kainoh, M. Analgesic Effects of TRK-700 on the Fibromyalgia and Neuropathic Pain Models in Rodents. J. Pharmacol. Sci. 2017, 133, S186. [Google Scholar]
- Kostich, W.; Hamman, B.D.; Li, Y.W.; Naidu, S.; Dandapani, K.; Feng, J.; Easton, A.; Bourin, C.; Baker, K.; Allen, J.; et al. Inhibition of AAK1 Kinase as a Novel Therapeutic Approach to Treat Neuropathic Pain. J. Pharmacol. Exp. Ther. 2016, 358, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Chen, L.; Kostich, W.A.; Hamman, B.; Allen, J.; Easton, A.; Bourin, C.; Gulianello, M.; Lippy, J.; Nara, S.; et al. Discovery of ( S)-1-((2′,6-Bis(Difluoromethyl)-[2,4′-Bipyridin]-5-Yl)Oxy)-2,4-Dimethylpentan-2-Amine (BMS-986176/LX-9211): A Highly Selective, CNS Penetrable, and Orally Active Adaptor Protein-2 Associated Kinase 1 Inhibitor in Clinical Trials for the Treatment of Neuropathic Pain. J. Med. Chem. 2022, 65, 4457–4480. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, J. LX9211 A Selective Inhibitor of AAK1 (Adapter-Associated Kinase) for Neuropathic Pain? Some Thoughts on Selectivity and Specificity. Austin. Neurol. 2018, 3, 1013. [Google Scholar]
- Bundrant, L.A.; Hunt, T.L.; Banks, P.; Gopinathan, S.; Boehm, K.A.; Kassler-Taub, K.; Tyle, P.; Wilson, A.; Warner, C.; Wason, S. Results of Two Phase 1, Randomized, Double-Blind, Placebo-Controlled, Studies (Ascending Single-Dose and Multiple-Dose Studies) to Determine the Safety, Tolerability, and Pharmacokinetics of Orally Administered LX9211 in Healthy Participants. Clin. Ther. 2021, 43, 1029–1050. [Google Scholar] [CrossRef]
- Lexicon Announces Planned Advancement of LX9211 Into Late-Stage Development. Available online: https://www.lexpharma.com/media-center/news/2023-06-23-lexicon-announces-planned-advancement-of-lx9211-into-late-stage-development (accessed on 8 August 2023).
- Harpur, C.M.; West, A.C.; A Le Page, M.; Lam, M.; Hodges, C.; Oseghale, O.; Gearing, A.J.; Tate, M.D. Naturally Derived Cytokine Peptides Limit Virus Replication and Severe Disease during Influenza A Virus Infection. Clin. Transl. Immunol. 2023, 12, e1443. [Google Scholar] [CrossRef]
- Huang, C.; Chen, M.; Pang, D.; Bi, D.; Zou, Y.; Xia, X.; Yang, W.; Luo, L.; Deng, R.; Tan, H.; et al. Developmental and Activity-Dependent Expression of LanCL1 Confers Antioxidant Activity Required for Neuronal Survival. Dev. Cell 2014, 30, 479–487. [Google Scholar] [CrossRef]
- Tan, H.; Chen, M.; Pang, D.; Xia, X.; Du, C.; Yang, W.; Cui, Y.; Huang, C.; Jiang, W.; Bi, D.; et al. LanCL1 Promotes Motor Neuron Survival and Extends the Lifespan of Amyotrophic Lateral Sclerosis Mice. Cell Death Differ. 2020, 27, 1369–1382. [Google Scholar] [CrossRef]
- Lai, K.Y.; Galan, S.R.G.; Zeng, Y.; Zhou, T.H.; He, C.; Raj, R.; Riedl, J.; Liu, S.; Chooi, K.P.; Garg, N.; et al. LanCLs Add Glutathione to Dehydroamino Acids Generated at Phosphorylated Sites in the Proteome. Cell 2021, 184, 2680–2695.e26. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, X.; Fang, Q.; Zhan, H.; Wang, X.; Huang, X.; Fan, T.; Liu, W.; Wu, W. LANCL1 as the Key Immune Marker in Neuropathic Pain. Neural Plast. 2022, 2022, 9762244. [Google Scholar] [CrossRef] [PubMed]
- Tubau-Juni, N.; Hontecillas, R.; Leber, A.; Maturavongsadit, P.; Chauhan, J.; Bassaganya-Riera, J. First-in-Class Topical Therapeutic Omilancor Ameliorates Disease Severity and Inflammation through Activation of LANCL2 Pathway in Psoriasis. Sci. Rep. 2021, 11, 19827. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Hontecillas, R.; Philipson, C.; Bassaganya-Riera, J. Lanthionine Synthetase Component C-like Protein 2: A New Drug Target for Inflammatory Diseases and Diabetes. Curr. Drug. Targets 2014, 15, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Leber, A.; Hontecillas, R.; Zoccoli-Rodriguez, V.; Colombel, J.F.; Chauhan, J.; Ehrich, M.; Farinola, N.; Bassaganya-Riera, J. The Safety, Tolerability, and Pharmacokinetics Profile of BT-11, an Oral, Gut-Restricted Lanthionine Synthetase C-Like 2 Agonist Investigational New Drug for Inflammatory Bowel Disease: A Randomized, Double-Blind, Placebo-Controlled Phase I Clinical Trial. Inflamm. Bowel Dis. 2020, 26, 643–652. [Google Scholar] [CrossRef]
- Maixner, D.W.; Christy, D.; Kong, L.; Viatchenko-Karpinski, V.; Horner, K.A.; Hooks, S.B.; Weng, H.R. Phytohormone Abscisic Acid Ameliorates Neuropathic Pain via Regulating LANCL2 Protein Abundance and Glial Activation at the Spinal Cord. Mol. Pain 2022, 18, 17448069221107781. [Google Scholar] [CrossRef]
- Kwon, D.; Park, G. Effect of Intra-Articular Injection of AOD9604 with or without Hyaluronic Acid in Rabbit Osteoarthritis Model. Ann. Clin. Lab. Sci. 2015, 45, 426–432. [Google Scholar]
- Moré, M.I.; Kenley, D. Safety and Metabolism of AOD9604, a Novel Nutraceutical Ingredient for Improved Metabolic Health. J. Endocrinol. Metab. 2014, 4, 64–77. [Google Scholar] [CrossRef][Green Version]
- Stier, H.; Vos, E.; Kenley, D. Safety and Tolerability of the Hexadecapeptide AOD9604 in Humans. J. Endocrinol. Metab. 2013, 3, 7–15. [Google Scholar] [CrossRef][Green Version]
- Parsons, C.G. NMDA Receptors as Targets for Drug Action in Neuropathic Pain. Eur. J. Pharmacol. 2001, 429, 71–78. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Chen, S.R.; Pan, H.L. Targeting N-Methyl-D-Aspartate Receptors for Treatment of Neuropathic Pain. Expert Rev. Clin. Pharmacol. 2011, 4, 379. [Google Scholar] [CrossRef]
- Collins, S.; Sigtermans, M.J.; Dahan, A.; Zuurmond, W.W.A.; Perez, R.S.G.M. NMDA Receptor Antagonists for the Treatment of Neuropathic Pain. Pain Med. 2010, 11, 1726–1742. [Google Scholar] [CrossRef] [PubMed]
- Aiyer, R.; Mehta, N.; Gungor, S.; Gulati, A. A Systematic Review of NMDA Receptor Antagonists for Treatment of Neuropathic Pain in Clinical Practice. Clin. J. Pain 2018, 34, 450–467. [Google Scholar] [CrossRef] [PubMed]
- Niesters, M.; Martini, C.; Dahan, A. Ketamine for Chronic Pain: Risks and Benefits. Br. J. Clin. Pharmacol. 2014, 77, 357. [Google Scholar] [CrossRef] [PubMed]
- Fedgchin, M.; Trivedi, M.; Daly, E.J.; Melkote, R.; Lane, R.; Lim, P.; Vitagliano, D.; Blier, P.; Fava, M.; Liebowitz, M.; et al. Efficacy and Safety of Fixed-Dose Esketamine Nasal Spray Combined With a New Oral Antidepressant in Treatment-Resistant Depression: Results of a Randomized, Double-Blind, Active-Controlled Study (TRANSFORM-1). Int. J. Neuropsychopharmacol. 2019, 22, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Lee, P.B.; Oh, T.K. Is Magnesium Sulfate Effective for Pain in Chronic Postherpetic Neuralgia Patients Comparing with Ketamine Infusion Therapy? J. Clin. Anesth. 2015, 27, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Sawynok, J.; Zinger, C. Topical Amitriptyline and Ketamine for Post-Herpetic Neuralgia and Other Forms of Neuropathic Pain. Expert Opin. Pharmacother. 2016, 17, 601–609. [Google Scholar] [CrossRef]
- Tao, J.C.; Huang, B.; Luo, G.; Zhang, Z.Q.; Xin, B.Y.; Yao, M. Trigeminal Extracranial Thermocoagulation along with Patient-Controlled Analgesia with Esketamine for Refractory Postherpetic Neuralgia after Herpes Zoster Ophthalmicus: A Case Report. World J. Clin. Cases 2022, 10, 4220–4225. [Google Scholar] [CrossRef]
- Pasternak, G.W.; Pan, Y.X. Mu Opioids and Their Receptors: Evolution of a Concept. Pharmacol. Rev. 2013, 65, 1257. [Google Scholar] [CrossRef]
- Watson, C.P.N.; Babul, N. Efficacy of Oxycodone in Neuropathic Pain: A Randomized Trial in Postherpetic Neuralgia. Neurology 1998, 50, 1837–1841. [Google Scholar] [CrossRef]
- Mcnicol, E.D.; Ferguson, M.C.; Hudcova, J. Patient Controlled Opioid Analgesia versus Non-Patient Controlled Opioid Analgesia for Postoperative Pain. Cochrane Database Syst. Rev. 2015, 2015, CD003348. [Google Scholar] [CrossRef]
- Kawai, S.; Sobajima, S.; Jinnouchi, M.; Nakano, H.; Ohtani, H.; Sakata, M.; Adachi, T. Efficacy and Safety of Tramadol Hydrochloride Twice-Daily Sustained-Release Bilayer Tablets with an Immediate-Release Component for Chronic Pain Associated with Knee Osteoarthritis: A Randomized, Double-Blind, Placebo-Controlled, Treatment-Withdrawal Study. Clin. Drug Investig. 2022, 42, 403. [Google Scholar] [CrossRef] [PubMed]
- Barker, P.A.; Mantyh, P.; Arendt-Nielsen, L.; Viktrup, L.; Tive, L. Nerve Growth Factor Signaling and Its Contribution to Pain. J. Pain Res. 2020, 13, 1223–1241. [Google Scholar] [CrossRef]
- Wise, B.L.; Seidel, M.F.; Lane, N.E. The Evolution of Nerve Growth Factor Inhibition in Clinical Medicine. Nat. Rev. Rheumatol. 2020, 17, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, M.C.; Tive, L.A.; Abramson, S.B.; Vignon, E.; Verburg, K.M.; West, C.R.; Smith, M.D.; Hungerford, D.S. When Is Osteonecrosis Not Osteonecrosis?: Adjudication of Reported Serious Adverse Joint Events in the Tanezumab Clinical Development Program. Arthritis Rheumatol. 2016, 68, 382–391. [Google Scholar] [CrossRef] [PubMed]

| Main Pharmacological Target | Cite | Drug | Clinical Trial Code | Phase | Route and Dosage | Completion Date |
|---|---|---|---|---|---|---|
| * AT2R antagonist | [24] | Olodanrigan (EMA401) | NCT03094195 | II | Oral: 25, 100, 300 mg BID | 7 March 2019 |
| VGCC α2δ subunit inhibitor | [13] | Crisugabalin (HSK16149) | NCT05140863 | III | Oral: 4, 20 mg BID | 5 January 2023 |
| VGCC α2δ subunit inhibitor | [14] | Mirogabalin (DS-5565) | NCT02318719 | III | Oral: 15, 20, 30 mg | 25 May 2017 |
| VGCC α2δ subunit inhibitor | [15] | Mirogabalin (DS-5565) | NCT02318719 | III | Oral: 15, 20, 30 mg | 25 May 2017 |
| VGCC α2δ subunit inhibitor | [16] | Pregabalin (controlled release) | NCT01270828 | III | Oral: 82.5 to 660 mg QD | November 2014 |
| VGCC α2δ subunit inhibitor | [17] | Pregabalin | NCT01455428 | IV | Oral: 300 mg/day BID | January 2014 |
| VGCC α2δ subunit inhibitor | [18] | Pregabalin (YHD1119) | NCT02985216 | III | Oral: 150, 300, 600 mg | 2 May 2018 |
| VGSC blocker (Nav1.6/1.7) | [19] | Funapide (TV-45070; XEN402; XPF-002) | NCT01195636 | IIa | Topical: ointment 8% BID | March 2011 |
| VGSCs blocker | [20] | Lidocaine | ChiCTR 1800017762 | III | Intravenous: 4 mg/kg QD | June 2021 |
| COX-1 inhibitor | [25] | TRK-700 | NCT02701374 | II | Oral: not specified | July 2017 |
| * AAK1 inhibitor | [26] | LX9211 | NCT04662281 | II | Oral: not specified QD | 21 December 2022 |
| * LANCL ligand | [27] | LAT8881 (AOD9604) | NCT03865953 | II | Oral: 30 mg BID | 3 May 2020 |
| Unknown | [28] | SR419 | NCT05357677 | II | Oral: 30 mg TID | 18 January 2023 |
| NMDAR antagonist | [29] | Esketamine | NCT04664530 | IV | Intranasal: 5–35 mg TID | 1 December 2023 |
| Mu opioid agonist and SNRI | [21] | Sustained-release tramadol (NZ-687) | JapicCTI-163341 | III | Oral: 100–400 mg/day BID | 7 December 2022 |
| Mu opioid agonist | [22] | Oxycodone | ACTRN 12615000013561 | IIa | Transdermal: 23.4 mg in 3 d | 18 April 2017 |
| Mu opioid agonist | [23] | Hydromorphone (PCA) | ChiCTR 1800019880 | IV | Intravenous PCA: 1 mg/mL | 30 June 2020 |
| * mAB anti-NGF | [30] | Fulranumab (JNJ-42160443) | NCT00964990 | II | Subcutaneous: 1, 3, or 10 mg every 28 d | 2 May 2016 |
| Drug | Main Result (Primary Outcome) | Other Information (Primary Outcome) |
|---|---|---|
| Olodanrigan (EMA401) [24] | NRS TD:
| Reduction in all doses for LS means (change from baseline in the 24 h average weekly mean NRS pain score over 12 weeks) |
| Crisugabalin (HSK16149) [13] |
| Preclinical evidence compared to pregabalin:
|
| Mirogabalin (DS-5565) [14,15] | LS in ADPS mean versus placebo was:
| Responders rate (≥30% ADPS):
|
| Pregabalin (CR) [16] | LTR criteria (p < 0.0001):
| Time to loss of therapeutic response (LTR):
|
| Pregabalin [17] | Reduction in mean pain score:
| Proportion of patients with ≥30% mean pain reduction (p = 0.0007):
|
| Pregabalin (YHD1119) [18] | LS mean in DPRS score at the end:
| SR pregabalin was not inferior to IR pregabalin in reducing pain intensity (p non-inferiority < 0.0001) |
| Funapide (TV-45070; XEN402; XPF-002) [19] | LS mean change in mean daily pain score:
|
|
| Lidocaine [20] |
|
|
| TRK-700 [25] |
| Primary outcome: change in average NRS from baseline to week 8 |
| LX9211 [26] |
| Primary outcome: change from baseline (week 2) to week 6 in ADPS (11-point NRS) |
| LAT8881 (AOD9604) [27] | Primary outcome: mean change in pain intensity scores (NPRS) during 4 weeks:
| 30% responder rate was not significant:
|
| SR419 [28] |
| Primary outcome: proportion of subjects who rate their pain as “much improved” or “very much improved” (PGIC) |
| Esketamine [29] |
| NRS will be measured at baseline and once for the period of drug administration |
| Sustained-release tramadol (NZ-687) [21] | Proportion of patients with an inadequate analgesic effect (double-blind period):
| Cumulative retention rate:
|
| Oxycodone [22] |
| Reduced average pain scores in high level paresthesia subpopulation (post hoc) (p < 0.05) |
| Hydromorphone (PCA) [23] | NRS 1, 4 and 12 weeks after treatment:
| NRS scores in between groups had a significant difference in 1, 4 and 12 weeks after treatment (all p < 0.001) |
| Fulranumab (JNJ-42160443) [30] | No significant TD or dose–response was observed in responders (≥30% improvement):
| For ≥50% improvement difference vs. placebo
|
| Drug | Overall Safety Information (vs. Placebo) | Most Frequent AEs |
|---|---|---|
| Olodanrigan (EMA401) [24] | Patients that experienced ≥1 AE:
| Diarrhea:
|
| Crisugabalin (HSK16149) [13] | No publications available for PHN Safety information extracted from a Phase II/III DPN trial | Dizziness Somnolence (All transient and mild, no treatment needed) |
| Mirogabalin (DS-5565) [14,15] | Total discontinuation:
| Dizziness Somnolence Weight increase Edema (All mild/moderate, more frequent for DS-5565 groups in a dose-dependent manner and resolved without treatment) |
| Pregabalin (CR) [16] | Patients that experienced ≥1 AE:
| Edema:
|
| Pregabalin [17] | Patients that experienced ≥1 AE:
| Nasopharyngitis:
|
| Pregabalin (YHD1119) [18] | Patients that experienced ≥1 AE:
| Dizziness:
|
| Funapide (TV-45070; XEN402; XPF-002) [19] | Patients that experienced ≥1 AE (non-DR):
| General disorders and application site-related (e.g., pain and pruritus)
|
| Lidocaine [20] |
| Somnolence:
|
| TRK-700 [25] | No publications available | Without CNS alterations (All in rat models of DPN and fibromyalgia) |
| LX9211 [26] | Data not published Safety information extracted from two Phase I studies Patients that experienced ≥1 AE:
| Headache:
|
| LAT8881 (AOD9604) [27] | Patients that experienced ≥1 AE:
| Upper RTI (nasopharyngitis) Headache (In both groups) |
| SR419 [28] | Data not published | Data not published |
| Esketamine [29] | Data not published No differences between esketamine and morphine (study for postoperative pain [31]) | A tendency to a higher frequency of nystagmus in esketamine A tendency to a higher frequency of dry mouth in morphine |
| SR tramadol (NZ-687) [21] | Total AEs:
| Nausea:
|
| Oxycodone [22] | Total AEs:
| General disorders and administration site conditions:
|
| Hydromorphone (PCA) [23] | Incidence 1 week after treatment:
| Dizziness and somnolence Nausea and vomiting Sweating Constipation Urinary retention Drowsiness (All transient and improved after treatment) |
| Fulranumab (JNJ-42160443) [30] | Patients that experienced ≥1 AE:
| Osteoarthritis: >10% difference between JNJ-42160443 10 mg and placebo groups Headache, arthralgia, back pain and motor-related (also in placebo, no TD) (All mild/moderate) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huerta, M.Á.; Garcia, M.M.; García-Parra, B.; Serrano-Afonso, A.; Paniagua, N. Investigational Drugs for the Treatment of Postherpetic Neuralgia: Systematic Review of Randomized Controlled Trials. Int. J. Mol. Sci. 2023, 24, 12987. https://doi.org/10.3390/ijms241612987
Huerta MÁ, Garcia MM, García-Parra B, Serrano-Afonso A, Paniagua N. Investigational Drugs for the Treatment of Postherpetic Neuralgia: Systematic Review of Randomized Controlled Trials. International Journal of Molecular Sciences. 2023; 24(16):12987. https://doi.org/10.3390/ijms241612987
Chicago/Turabian StyleHuerta, Miguel Á., Miguel M. Garcia, Beliu García-Parra, Ancor Serrano-Afonso, and Nancy Paniagua. 2023. "Investigational Drugs for the Treatment of Postherpetic Neuralgia: Systematic Review of Randomized Controlled Trials" International Journal of Molecular Sciences 24, no. 16: 12987. https://doi.org/10.3390/ijms241612987
APA StyleHuerta, M. Á., Garcia, M. M., García-Parra, B., Serrano-Afonso, A., & Paniagua, N. (2023). Investigational Drugs for the Treatment of Postherpetic Neuralgia: Systematic Review of Randomized Controlled Trials. International Journal of Molecular Sciences, 24(16), 12987. https://doi.org/10.3390/ijms241612987


_Putnam.png)






