Sialic-Acid-Related Enzymes of B Cells and Monocytes as Novel Markers to Discriminate Improvement Categories and to Fulfill Two Remission Definitions in Rheumatoid Arthritis
Abstract
1. Introduction
2. Results
2.1. AUC of B-Cell and Monocyte ST and Neu Levels and Ratios against Two Remission Definitions
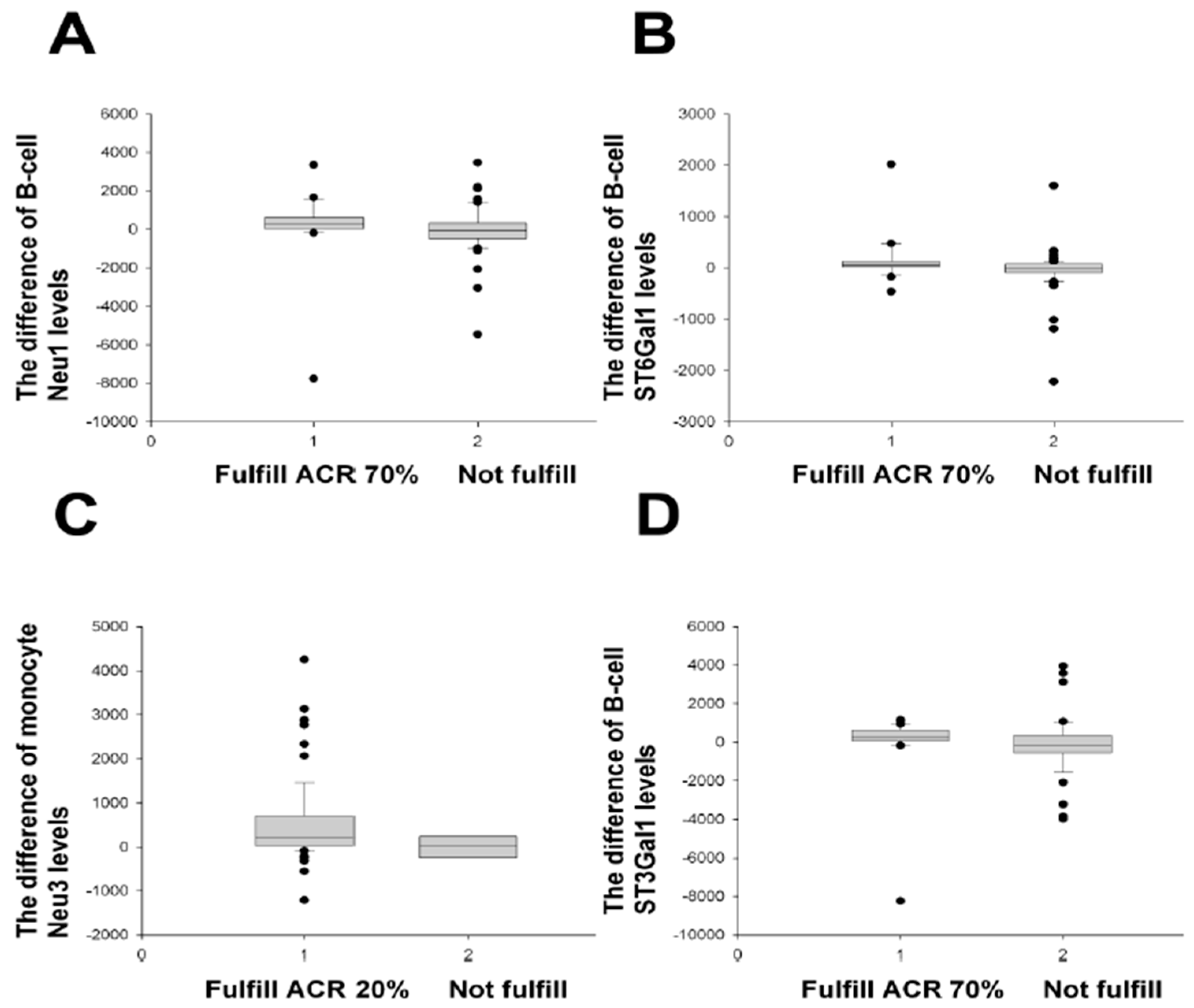
2.2. Treatment Responses Using Different Response (Improvement) Criteria
2.3. Treatment Responses in RA Patients with Positive Rheumatoid Factor
2.4. Treatment Responses in RA Patients with Positive Anti-Cyclic Citrullinated Peptide (Anti-CCP) Antibodies
2.5. Treatment Responses in RA Patients with the Use of Biologics
2.6. Comparison of Enzyme Levels between Different Categories of DAS28-ESR and DAS28-MCP-1 Scores
3. Discussion
4. Materials and Methods
4.1. Patient Enrollment
4.2. Cell Staining and Flow Cytometric Detection
- (a)
- ST3Gal1: rabbit polyclonal IgG anti-ST3Gal1 (control: rabbit IgG) plus allophycocyanin (APC)-goat antibody against rabbit IgG
- (b)
- Neu3: rabbit polyclonal IgG anti-Neu3 (control: rabbit IgG) plus APC-goat antibody against rabbit IgG
- (c)
- ST6Gal1: mouse monoclonal IgM anti-ST6Gal1 (control: mouse IgM) plus APC-monoclonal rat antibody against mouse IgM
- (d)
- Neu1: rabbit polyclonal IgG anti-Neu1 (control: rabbit IgG) plus APC-goat antibody against rabbit IgG
4.3. Determination of Rheumatoid Factor and Anti-Cyclic Citrullinated Peptide Antibodies
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pransen, J.; Moens, H.B.; Speyer, I.; van Riel, P.L.C.M. Effectiveness of systemic monitoring of rheumatoid arthritis disease activity in daily practice: A multicentre, cluster randomized controlled trial. Ann. Rheum. Dis. 2005, 64, 1294–1298. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.J.; Harrison, A.A.; Highton, J.; Chapma, P.; Stamp, L.; Dockerty, J.; McQueen, F.; Jones, P.B.B.; Ching, D.; Porter, D.; et al. Disease activity score 28-ESR bears a similar relationship to treatment decisions across different rheumatologists, but misclassification is to frequent to replace physician judgement. Rheumatology 2008, 47, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Kuga, Y.; Kaneko, A.; Nishino, J.; Eto, Y.; Chiba, N.; Yasuda, M.; Saisho, K.; Shimada, K.; Tohma, S. Disease activity score28 (DAS28) using C-reactive protein underestimates disease activity and overestimates EULAR response criteria compared with DAS 28 using erythrocyte sedimentation rate in a large observational cohort of rheumatoid arthritis patients in Japan. Ann. Rheum. Dis. 2007, 66, 1221–1226. [Google Scholar]
- Wolfe, F. The many myths of erythrocyte sedimentation rate and C-reactive protein. J. Rheumatol. 2008, 36, 1568–1569. [Google Scholar] [CrossRef] [PubMed]
- Pope, J.E.; Thorne, C.; Haraoui, B.P.; Truong, D.; Psaradellis, E.; Sampalis, J.S. Does C-reactive protein add value in active rheumatoid arthritis? Results form the optimization of Humira trial. Scand. J. Rheumatol. 2011, 40, 232–233. [Google Scholar] [CrossRef]
- Liou, L.B.; Tsai, W.P.; Chang, C.J.; Chao, W.J.; Chen, M.H. Blood Monocyte Chemotactic Protein-1 (MCP-1) and Adapted Disease Activity Score28-MCP-1: Favorable Indicators for Rheumatoid Arthritis Activity. PLoS ONE 2013, 8, e55346. [Google Scholar] [CrossRef]
- Inoue, E.; Yamanaka, H.; Hara, M.; Tomatsu, T.; Kamatani, N. Comparison of Disease Activity Score (DAS)28-erythrocyte sedimentation rate and DAS28-C-reactive protein threshold values. Ann. Rheum. Dis. 2007, 66, 407–409. [Google Scholar] [CrossRef]
- Liou, L.B.; Fang, Y.F.; Tan, C.F.; Lai, J.H.; Jang, S.S.; Tsai, P.H.; Yeh, T.C. A new laboratory surrogate (Monocyte Chemotactic Protein-1) for Disease Activity Score28: A favourable indicator for remission in rheumatoid arthritis. Sci. Rep. 2020, 10, 8238. [Google Scholar] [CrossRef]
- Greenmyer, J.R.; Stacy, J.M.; Sahmoun, A.E.; Beal, J.R.; Diri, E. DAS28-CRP Cutoffs for High Disease Activity and Remission Are Lower Than DAS28-ESR in Rheumatoid Arthritis. ACR Open Rheumatol. 2020, 2, 507–511. [Google Scholar] [CrossRef]
- Gorczyca, W.; Wieczore, Z.; Lisowsκκi, J. Cell surface sialic acid affects immunoglobulin binding to macrophages. FEBS Lett. 1989, 259, 99–102. [Google Scholar] [CrossRef]
- Fushima, K.; Taasai, S. Suppressive role of sialylated N-glycans in Fc receptor-mediated phagocytosis by macrophages. Biochem. Biophys. Res. Commun. 1993, 192, 333–337. [Google Scholar] [CrossRef]
- Jenner, J.; Kerst, G.; Handgretinger, R.; Muller, I. Increased α-2,6-sialylation of surface proteins on tolerogenic, immature dendritic cells and regulatory T cells. Exp. Hematol. 2006, 34, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Ohmi, Y.; Ise, W.; Harazono, A.; Takakura, D.; Fukuyama, H.; Baba, Y.; Narazaki, M.; Shoda, H.; Takahashi, N.; Ohkawa, Y.; et al. Sialylation converts arthritogenic IgG into inhibitors of collagen-induced arthritis. Nat. Commun. 2016, 7, 11205. [Google Scholar] [CrossRef]
- Wang, Y.; Khan, A.; Antonopoulos, A.; Nouche, L.; Buckley, C.D.; Filar, A.; Raza, K.; Li, K.P.; Tolusso, B.; Gremese, E.; et al. Loss of α2-6 sialylation promotes the transformation of synovial firboblasts into a pro-inflammatory phenotype in arthrtitis. Nat. Commun. 2021, 12, 2343. [Google Scholar] [CrossRef] [PubMed]
- Harduin-Lepers, A.; Vallejo-Ruiz, V.; Krzewinski-Recchi, M.A.; Samyn-Petit, B.; Julien, S.; Delannoy, P. The human sialyltransferases family. Biochimie 2001, 83, 727–737. [Google Scholar] [CrossRef]
- Lehoux, S.; Groux-Degroote, S.; Cazet, A.; Dhaenens, C.-M.; Maurage, C.-A.; Caillet-Boudin, M.-L.; Delannoy, P.; Krzewinski-Recchi, M.-A. Transcriptional regulation of the human ST6GAL2 gene in cerebral cortex and neuronal cells. Glycoconj. J. 2010, 27, 99–114. [Google Scholar] [CrossRef]
- Videira, P.A.; Correia, M.; Malagolini, N.; Crespo, H.J.; Ligeiro, D.; Calais, F.M.; Helder Trindade, H.; Dall’Olio, F. ST3Gal.I sialyltransferase relevance in bladder cancer tissues and cell lines. BMC Cancer 2009, 9, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Picco, G.; Julien, S.; Brockhausen, I.; Beatson, R.; Antonopoulos, A.; Haslam, S.; Mandel, U.; Dell, A.; Pinder, S.; Taylor-Papadimitriou, J.; et al. Over-expression of ST3Gal-I promotes mammary tumorigenesis. Glycobiology 2010, 20, 1241–1250. [Google Scholar] [CrossRef]
- Van Dyken, S.J.; Green, R.S.; Marth, J.D. Structural and mechanistic features of protein O glycosylation linked to CD8+ T-cell apoptosis. Mol. Cell. Biol. 2007, 27, 1096–1111. [Google Scholar] [CrossRef][Green Version]
- Barb, A.W.; Brady, E.K.; Prestegard, J.H. Branch Specific Sialylation of IgG-Fc Glycans by ST6Gal-I. Biochemistry 2009, 48, 9705–9707. [Google Scholar] [CrossRef]
- Basset, C.; Durand, V.; Mimassi, N.; Pennec, Y.L.; Youinou, P.; Dueymes, M. Enhanced sialyltransferase activity in B lymphocytes from patients with primary Sjögren’s syndrome. Scand. J. Immunol. 2000, 51, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Liou, L.B.; Jang, S.S. α2,3-Sialyltransferase 1 and Neuraminidase 3 of Monocytes Correlate with Disease Activity Measures in Patients with Rheumatoid Arthritis. J. Chin. Med. Assoc. 2019, 82, 179–185. [Google Scholar] [CrossRef]
- Chen, X.P.; Enioutina, E.Y.; Daynes, R.A. The control of IL-4 gene expression in activated murine T lymphocytes: A novel role for neu-1 sialidase. J. Immunol. 1997, 158, 3070–3080. [Google Scholar] [CrossRef]
- Seyrantepe, V.; Iannello, A.; Liang, F.; Kanshin, E.; Jayanth, P.; Samarani, S.; Szewczuk, M.R.; Ahmad, A.; Pshezhetsky, A.V. Regulation of phagocytosis in macrophages by neuraminidase 1. J. Biol. Chem. 2010, 285, 206–215. [Google Scholar] [CrossRef]
- Naraparaju, V.R.; Yamamoto, N. Roles of beta-galactosidase of B lymphocytes and sialidase of T lymphocytes in inflammation-primed activation of macrophages. Immunol. Lett. 1994, 43, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yamaguchi, K.; Wada, T.; Hata, K.; Zhao, X.J.; Fujimoto, T.; Miyagi, T. A close association of the ganglioside-specific sialidase Neu3 with caveolin in membrane microdomains. J. Biol. Chem. 2002, 277, 26252–26259. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Nimmerjahn, F.; Ravetch, J.V. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 2006, 313, 670–673. [Google Scholar] [CrossRef]
- Liou, L.B.; Huang, C.C. Sialyltransferase and Neuraminidase Levels/Ratios and Sialic Acid Levels in Peripheral Blood B Cells Correlate with Measures of Disease Activity in Patients with Systemic Lupus Erythematosus and Rheumatoid Arthritis: A Pilot Study. PLoS ONE 2016, 11, e0151669. [Google Scholar] [CrossRef]
- Makinen, H.; Kautianen, H.; Hannonen, P.; Sokka, T. Is DAS28 an appropriate tool to assess remission in rheumatoid arthritis? Ann. Rheum. Dis. 2005, 64, 1410–1413. [Google Scholar] [CrossRef]
- Felson, D.T.; Smolen, J.S.; Wells, G.; Zhang, B.; van Tuyl, L.H.D.; Funovits, J.; Aletaha, D.; Allaart, C.F.; Bathon, J.; Bombardieri, S.; et al. American College of Rheumatology/European League against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheumatol. 2011, 63, 573–586. [Google Scholar] [CrossRef]
- Felson, D.T.; Anderson, J.J.; Boers, M.; Bombardier, C.; Furst, D.; Goldsmith, C.; Katz, L.M.; Lightfoot, R., Jr.; Paulus, H.; Strand, V.; et al. American College of Rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheumatol. 1995, 38, 727–735. [Google Scholar] [CrossRef] [PubMed]
- van Gestel, A.M.; Prevoo, M.L.; van‘t Hof, M.A.; van Rijswijk, M.H.; van de Putte, L.B.; van Riel, P.L. Development and validation of the European Leaque Against Rheumatism response criteria for rheumatoid arthritis: Comparison with the Preliminary American College of Rheumatology and the World Health Organization/International Leaque Against Rheumatism Criteria. Arthritis Rheumatol. 1996, 39, 34–40. [Google Scholar]
- Aletaha, D.; Martinez-Avila, J.; Kvien, T.J.; Smolen, J.S. Definition of treatment response in rheumatoid arthritis based on the simplified and the clinical disease activity index. Ann. Rheum. Dis. 2012, 71, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Prevoo, M.L.; van‘t Hof, M.A.; Kuper, H.H.; van Leeuwen, M.A.; van de Putte, L.B.; van Riel, P.L. Modified disease activity scores that include twenty-eight-joint counts development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheumatol. 1995, 38, 44–48. [Google Scholar] [CrossRef]
- Pincus, T. The American College of Rheumatology (ACR) Core Data Set and derivative patient only indices to assess rheumatoid arthritis. Clin. Exp. Rheumatol. 2005, 23 (Suppl. S39), S109–S113. [Google Scholar]
- Crowson, C.S. Rheumatoid arthritis and heart disease: The chicken and the egg. J. Int. Med. 2010, 268, 552–554. [Google Scholar] [CrossRef]
- Cerezo, L.A.; Kuklová, M.; Hulejová, H.; Vernerová, Z.; Kaspříková, N.; Veigl, D.; Pavelka, K.; Vencovský, J.; Šenolt, L. Progranulin Is Associated with Disease Activity in Patients with Rheumatoid Arthritis. Mediat. Inflamm. 2015, 2015, 740357. [Google Scholar]
- Castro-Villega, C.; Castro-Villegas, C.; Pérez-Sánchez, C.; Escudero, A.; Filipescu, I.; Verdu, M.; Ruiz-Limón, P.; Aguirre, M.A.; Jiménez-Gomez, Y.; Font, P.; et al. Circulating miRNAs as potential biomarers of therapy effectiveness in rheumatoid arthritis patients treated with anti-TNFα. Arthritis. Res. Therapy 2015, 17, 49. [Google Scholar] [CrossRef]
- Ha, Y.J.; Kang, E.J.; Lee, S.W.; Lee, S.K.; Park, Y.B.; Song, J.S.; Choi, S.T. Usefulness of Serum Leucine-Rich Alpha-2 Glycoprotein as a Disease Activity Biomarker in Patients with Rheumatoid Arthritis. J. Korean Med. Sci. 2014, 29, 1199–1204. [Google Scholar] [CrossRef]
- Anand, D.; Kumar, U.; Kanjila, M.; Kaur, S.; Das, N. Leucocyte complement receptor 1 (CR1/CD35) transcript and its correlation with the clinical disease activity in rheumatoid arthritis patients. Clin. Exp. Immunol. 2014, 176, 327–335. [Google Scholar] [CrossRef]
- van Gestel, A.M.; Anderson, J.J.; van Riel, P.L.; Boers, M.; Haagsma, C.J.; Rich, B.; Wells, G.; Lange, M.L.; Felson, D.T. ACR and EULAR improvement criteria has comparable validity in rheumatoid arthritis trials. J. Rheumatol. 1999, 199, 705–711. [Google Scholar]
- Liou, L.B.; Huang, C.C. Reverse expression of α2,6-sialic acid ratios on IgG, IgM, and IgG/IgM autoantibodies correlates with mouse arthritis and rheumatoid arthritis disease activity. J. Chin. Med. Assoc. 2020, 83, 1079–1086. [Google Scholar] [CrossRef]
- Bartsch, Y.C.; Rahmöller, J.; Mertes, M.M.M.; Eiglmeier, S.; Lorenz, F.K.M.; Stoehr, A.D.; Braumann, D.; Lorenz, A.K.; Winkler, A.; Lilienthal, G.M.; et al. Sialylated autoantigen-reactive IgG antibodies attenuate disease development in autoimmune model of lupus nephritis and rheumatoid arthritis. Front. Immunol. 2018, 9, 1183. [Google Scholar] [CrossRef] [PubMed]
- Fickentscher, C.; Magorivska, I.; Janko, C.; Biermann, M.; Bilyy, R.; Nalli, C.; Tincani, A.; Medeghini, V.; Meini, A.; Nimmerjahn, F.; et al. The pathogenecity of anti-b2GP1-IgG autoantibodies depends on Fc glycosylation. J. Immunol. Res. 2015, 2015, 638129. [Google Scholar] [CrossRef] [PubMed]
- Orr, C.K.; Najm, A.; Young, F.; McGarry, T.; Biniecka, M.; Fearon, U.; Veale, D.J. The Utility and Limitations of CRP, ESR and DAS28-CRP in Appraising Disease Activity in Rheumatoid Arthritis. Front. Med. 2018, 5, 185. [Google Scholar] [CrossRef] [PubMed]
- Harigai, M.; Hara, M.; Yoshimura, T.; Leonard, E.J.; Inoue, K.; Kashiwazaki, S. Monocyte chemoattractant protein-1 (MCP-1) in inflammatory joint diseases and its involvement in the cytokine network of rheumatoid synovium. Clin. Immunol. Immunopathol. 1993, 69, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.E.; Kunkel, S.L.; Harlow, L.A.; Johnson, B.; Evanoff, H.L.; Haines, G.K.; Burdick, M.D.; Pope, R.M.; Strieter, R.M. Enhanced production of monocyte chemoattractant protein-1 in rheumatoid arthritis. J. Clin. Investig. 1992, 90, 772–779. [Google Scholar] [CrossRef]
- Gong, J.H.; Ratkay, L.G.; Waterfield, J.D.; Clark-Lewis, I. An antagonist of monocyte chemoattractant protein 1 (MCP-1) inhibits arthritis in the MRL-lpr mouse model. J. Exp. Med. 1997, 186, 131–137. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; de Wit, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann. Rheum. Dis. 2010, 69, 964–975. [Google Scholar] [CrossRef]
- Keystone, E.C.; Genovese, M.C.; Klareskog, L.; Hsia, E.C.; Hall, S.T.; Miranda, P.; Pazdur, J.; Bae, S.-C.; Palmer, W.W.; Zrubek, J.; et al. Golimumab, a human antibody to tumor necrosis factor a given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: The GO-FORWARD study. Ann. Rheum. Dis. 2009, 68, 789–796. [Google Scholar] [CrossRef]
- Yamanaka, H.; Tanaka, Y.; Inoue, E.; Hoshi, D.; Momohara, S.; Hanami, K.; Yunoue, N.; Saito, K.; Amano, K.; Kameda, H.; et al. Efficacy and tolerability of tocilizumab in rheumatoid arthritis patients seen in daily clinical practice in Japan: Results from a retrospective study (REACTION study). Mod. Rheumatol. 2011, 21, 122–133. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fuss, I.J.; Kanof, M.E.; Smith, P.D.; Zola, H. Unit 7.1 Isolation of Whole Mononuclear Cells from Peripheral Blood and Cord Blood, Current Protocols in Immunology, Vol. II; John-Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 0-471-52276-7. [Google Scholar]
- Mannik, M. Rheumatoid factors in the pathogenesis of rheumatoid arthritis. J. Rheumatol. Suppl. 1992, 32, 46–49. [Google Scholar] [PubMed]
- Tanaka, Y. Rheumatoid arthritis. Inflamm. Regen. 2020, 40, 20. [Google Scholar] [CrossRef] [PubMed]




| Median (or Mean ± SD) | Quartiles (25–75%) or Range | |
|---|---|---|
| Patient number (female: male) | 100 (80:20) | |
| Total visits | 312 | |
| Age | 55.4 ± 13.0 | 22–79 |
| Disease duration (months from initial arthritic symptoms) | 58.5 | 5.0–114.8 |
| Swollen joint count | 6.5 | 5.0–10.0 |
| Tender joint count | 8.0 | 5.0–12.5 |
| Global health | 80 | 50–80 |
| Morning stiffness (min) | 60 | 22.5–172.5 |
| ESR (mm/h) | 28.0 | 17.0–44.8 |
| Rheumatoid factor (IU/mL) | 59.9 | 15.2–296.5 |
| Anti-CCP (mg/mL) | 279.8 | 50.8–336.9 |
| DAS28-ESR | 5.7 ± 1.0 | 3.4–8.4 |
| HAQ-DI | 1.00 | 0.00–3.33 |
| Area under the Curve | Asymptotic Significance | Asymptotic 95% Confidence Interval | |
|---|---|---|---|
| Modified ARA remission | |||
| Monocyte ST6/Neu1 ratios | 0.634 | p = 0.013 | 0.532–0.736 |
| B-cell ST3Gal1 | 0.658 | p = 0.003 | 0.568–0.749 |
| B-cell Neu3 | 0.641 | p = 0.009 | 0.549–0.734 |
| B-cell ST6Gal1 | 0.648 | p = 0.006 | 0.554–0.742 |
| B-cell Neu1 | 0.674 | p = 0.001 | 0.538–0.764 |
| CRP (mg/L) | 0.331 | p = 0.002 | 0.256–0.406 |
| ESR (mm/h) | 0.356 | p = 0.004 | 0.283–0.428 |
| 2011 ACR/EULAR remission | |||
| Monocyte ST6/Neu1 ratios | 0.672 | p = 0.001 | 0.583–0.762 |
| B-cell ST3Gal1 | 0.633 | p = 0.009 | 0.537–0.729 |
| B-cell Neu3 | 0.662 | p = 0.001 | 0.572–0.751 |
| B-cell Neu1 | 0.682 | p < 0.001 | 0.593–0.772 |
| CRP (mg/L) | 0.368 | p = 0.014 | 0.290–0.445 |
| ESR (mm/h) | 0.416 | p = 0.097 | 0.330–0.502 |
| B-Cell ST3 | B-Cell Neu3 | B-Cell ST6 | B-Cell Neu1 | B-Cell ST3/Neu3 | B-Cell ST6/Neu1 | |
|---|---|---|---|---|---|---|
| M0 − M3 | - | - | - | - | - | - |
| M0 − M12 | - | - | - | 0.014 | - | - |
| M0 − M15 | - | - | - | - | - | - |
| B-Cell ST3 | B-Cell Neu3 | B-Cell ST6 | B-Cell Neu1 | Mono ST3 | Mono Neu3 | Mono ST6 | Mono Neu1 | B-Cell ST3/Neu3 | B-Cell ST6/Neu1 | Mono ST3/Neu3 | Mono ST6/Neu1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M0 − M3 | - | 0.047 # | - | 0.018 # | - | - | - | - | - | - | - | - |
| M0 − M12 | 0.003 * | 0.013 *; 0.020 # | 0.007* | 0.001 *; 0.035 # | 0.028 @ | 0.001 * | 0.023 * | <0.001 * | 0.014 * | - | 0.044 @ | 0.007 @ |
| M0 − M15 | - | - | - | - | - | - | - | - | - | - | - | - |
| B-Cell ST3 | B-Cell Neu3 | B-Cell ST6 | B-Cell Neu1 | B-Cell ST3/Neu3 | B-Cell ST6/Neu1 | |
|---|---|---|---|---|---|---|
| M0 − M3 | - | - | - | - | - | - |
| M0 − M12 | 0.016 | 0.016 | - | 0.016 | - | - |
| M0 − M15 | - | - | - | - | - | - |
| B-Cell ST3 | B-Cell Neu3 | B-Cell ST6 | B-Cell Neu1 | B-Cell ST3/Neu3 | B-Cell ST6/Neu1 | |
|---|---|---|---|---|---|---|
| M0 − M3 | - | - | - | - | - | - |
| M0 − M12 | - | - | - | 0.037 | - | - |
| M0 − M15 | - | - | - | - | - | - |
| B-Cell ST3 | B-Cell Neu3 | B-Cell ST6 | B-Cell Neu1 | B-Cell ST3/Neu3 | B-Cell ST6/Neu1 | |
|---|---|---|---|---|---|---|
| M0 − M3 | - | - | - | - | - | - |
| M0 − M12 | - | - | - | 0.028 | - | - |
| M0 − M15 | - | - | - | - | - | - |
| Enzyme(s) | Fulfill the Criterion for Differentiating RA Activity Improvement | Fulfill Two Remission Definitions |
|---|---|---|
| B-cell Neu1 | ACR, EULAR, and SDAI | Yes |
| B-cell ST6Gal1 | ACR and SDAI | Only modified ARA remission |
| B-cell Neu3 | SDAI | Yes |
| B-cell ST3Gal1 | SDAI | Yes |
| Monocyte ST6Gal1/ Neu1 ratios | SDAI | Yes |
| Enzyme(s) | Fulfill the Criterion for Differentiating RA Activity Improvement in which Criterion/Subgroup |
|---|---|
| B-cell Neu1 | ACR/RF, EULAR/RF, SDAI/RF; EULAR/anti-CCP, SDAI/anti-CCP; ACR/biologics, EULAR/biologics, SDAI/biologics |
| B-cell ST6Gal1 | ACR/RF, SDAI/RF; ACR/anti-CCP, SDAI/anti-CCP |
| B-cell ST6Gal1/ Neu1 ratios | ACR/biologics |
| B-cell Neu3 | EULAR/RF, SDAI/RF; SDAI/anti-CCP; SDAI/biologics |
| B-cell ST3Gal1 | ACR/RF, EULAR/RF, SDAI/RF; SDAI/anti-CCP |
| Monocyte Neu1 | SDAI/RF; SDAI/anti-CCP |
| Moncoyte ST6Gal1 | SDAI/RF; ACR/anti-CCP, SDAI/anti-CCP |
| Moncyte ST6Gal1/ Neu1 ratios | SDAI/RF; SDAI/anti-CCP; ACR/biologics |
| Monocyte Neu3 | ACR/RF, SDAI/RF; SDAI/anti-CCP |
| Monocyte ST3Gal1 /Neu3 ratios | ACR/anti-CCP; ACR/biologics, SDAI/biologics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liou, L.-B.; Tsai, P.-H.; Fang, Y.-F.; Chen, Y.-F.; Chen, C.-C.; Lai, J.-H. Sialic-Acid-Related Enzymes of B Cells and Monocytes as Novel Markers to Discriminate Improvement Categories and to Fulfill Two Remission Definitions in Rheumatoid Arthritis. Int. J. Mol. Sci. 2023, 24, 12998. https://doi.org/10.3390/ijms241612998
Liou L-B, Tsai P-H, Fang Y-F, Chen Y-F, Chen C-C, Lai J-H. Sialic-Acid-Related Enzymes of B Cells and Monocytes as Novel Markers to Discriminate Improvement Categories and to Fulfill Two Remission Definitions in Rheumatoid Arthritis. International Journal of Molecular Sciences. 2023; 24(16):12998. https://doi.org/10.3390/ijms241612998
Chicago/Turabian StyleLiou, Lieh-Bang, Ping-Han Tsai, Yao-Fan Fang, Yen-Fu Chen, Chih-Chieh Chen, and Jenn-Haung Lai. 2023. "Sialic-Acid-Related Enzymes of B Cells and Monocytes as Novel Markers to Discriminate Improvement Categories and to Fulfill Two Remission Definitions in Rheumatoid Arthritis" International Journal of Molecular Sciences 24, no. 16: 12998. https://doi.org/10.3390/ijms241612998
APA StyleLiou, L.-B., Tsai, P.-H., Fang, Y.-F., Chen, Y.-F., Chen, C.-C., & Lai, J.-H. (2023). Sialic-Acid-Related Enzymes of B Cells and Monocytes as Novel Markers to Discriminate Improvement Categories and to Fulfill Two Remission Definitions in Rheumatoid Arthritis. International Journal of Molecular Sciences, 24(16), 12998. https://doi.org/10.3390/ijms241612998






