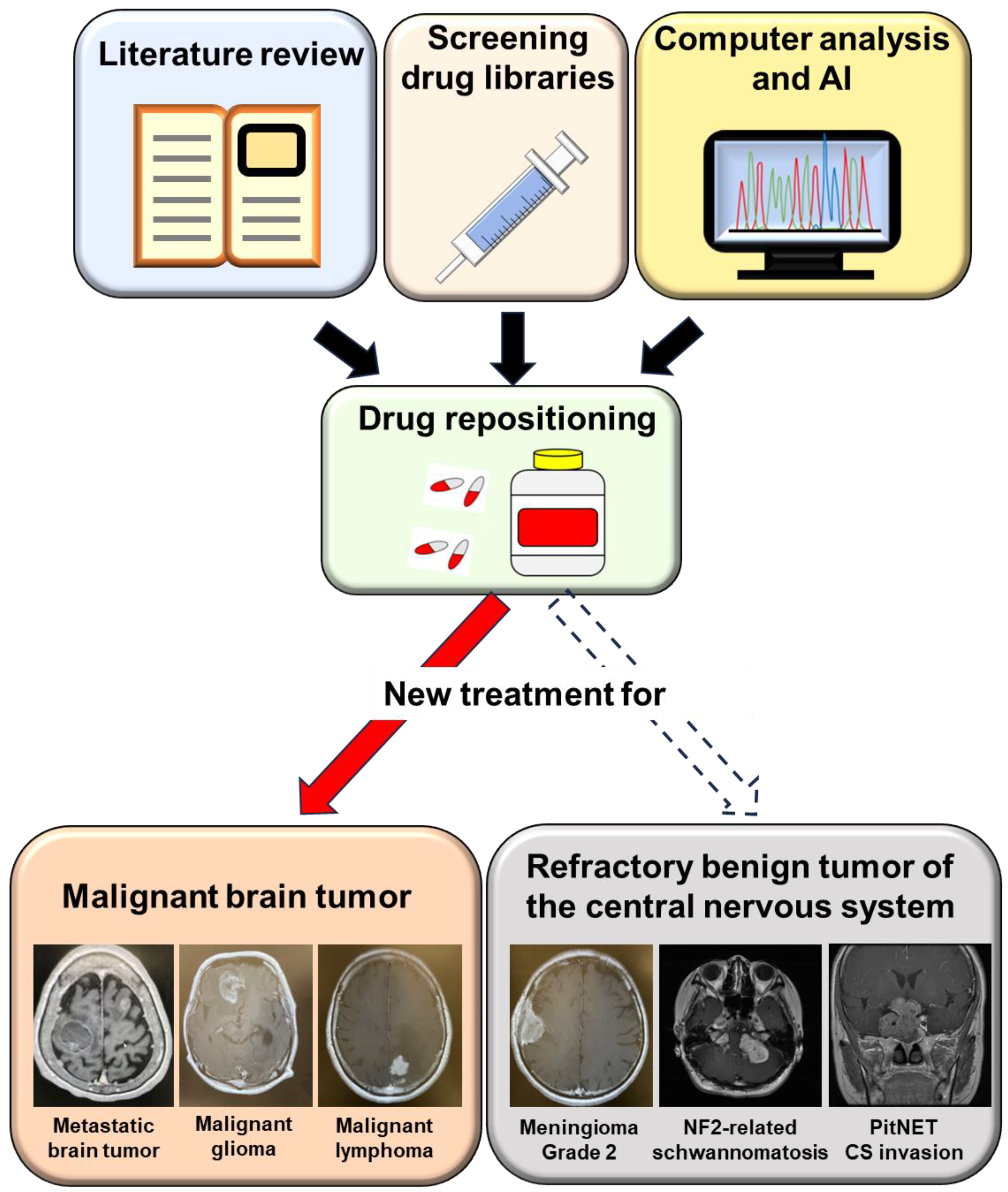
Drug Repositioning for Refractory Benign Tumors of the Central Nervous System
Abstract
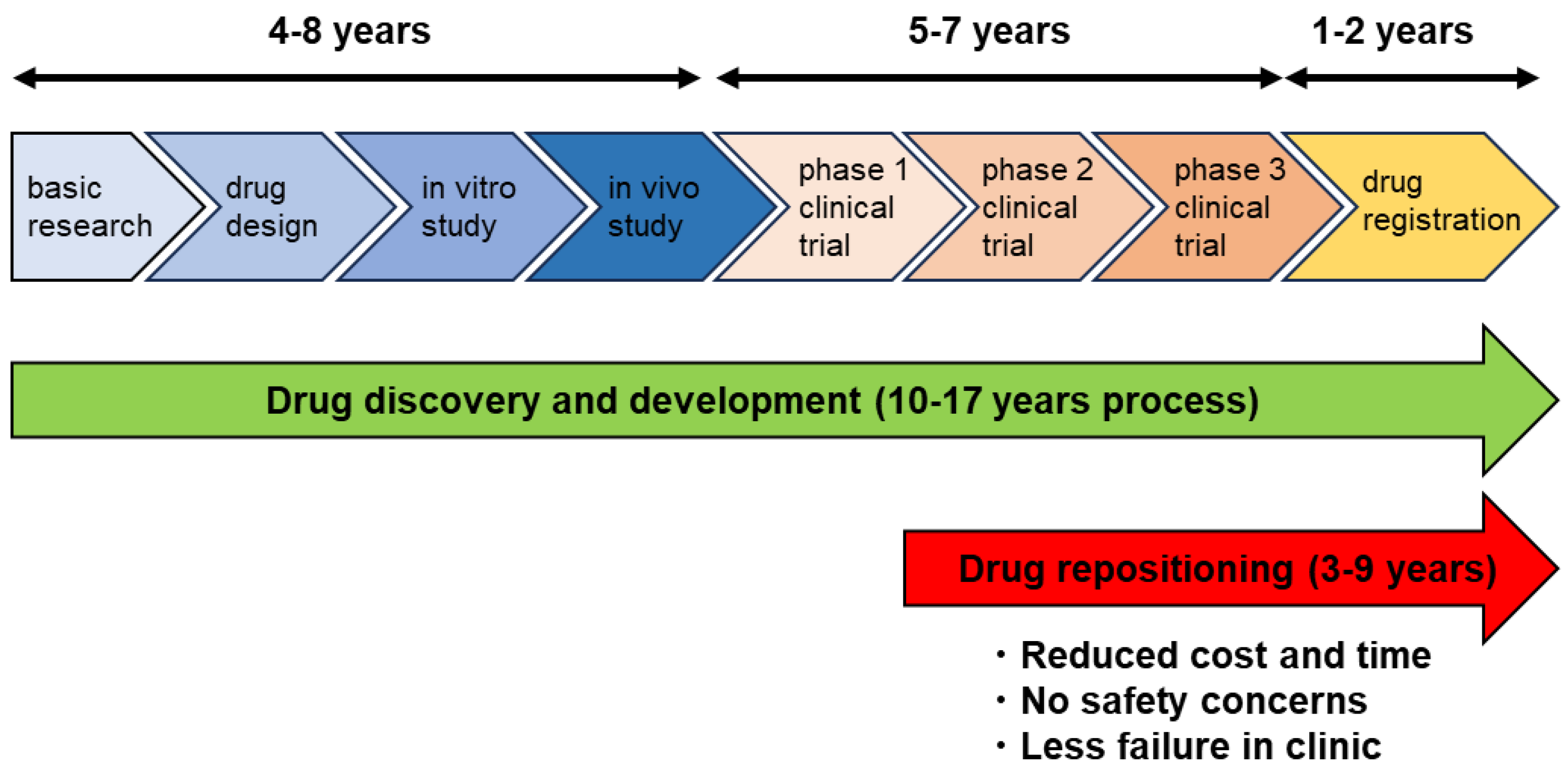
:1. Introduction
2. Meningioma
2.1. Antiseizure Drugs
2.2. Anthelminthic Drugs
2.3. Antidiabetic Drugs
2.4. Antihypertensive Drugs
2.5. Antihyperlipidemic Drugs
3. Schwannoma
3.1. Anti-Inflammatory Drugs
3.2. Abortion Pills
4. Pituitary Neuroendocrine Tumor
4.1. Antidiabetic Drugs
4.2. Antabuse
4.3. Nonsteroidal Anti-Estrogenic Agent
4.4. Other Drugs (Combination Therapy)
5. Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jourdan, J.P.; Bureau, R.; Rochais, C.; Dallemagne, P. Drug repositioning: A brief overview. J. Pharm. Pharmacol. 2020, 72, 1145–1151. [Google Scholar] [CrossRef]
- Paul, S.M.; Mytelka, D.S.; Dunwiddie, C.T.; Persinger, C.C.; Munos, B.H.; Lindborg, S.R.; Schacht, A.L. How to improve R&D productivity: The pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discov. 2010, 9, 203–214. [Google Scholar] [PubMed]
- Hay, M.; Thomas, D.W.; Craighead, J.L.; Economides, C.; Rosenthal, J. Clinical development success rates for investigational drugs. Nat. Biotechnol. 2014, 32, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Nosengo, N. Can you teach old drugs new tricks? Nature 2016, 534, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, F.; Zhao, Y.; Moreira, V.M.; Tagliaferri, R.; Kere, J.; D’Amato, M.; Greco, D. Drug repositioning: A machine-learning approach through data integration. J. Cheminform. 2013, 5, 30. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Aggarwal, S.; Verma, S.S.; Aggarwal, S.; Gupta, S.C. Drug repurposing for breast cancer therapy: Old weapon for new battle. Semin. Cancer Biol. 2021, 68, 8–20. [Google Scholar] [CrossRef]
- Masuda, T.; Tsuruda, Y.; Matsumoto, Y.; Uchida, H.; Nakayama, K.I.; Mimori, K. Drug repositioning in cancer: The current situation in Japan. Cancer Sci. 2020, 111, 1039–1046. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, L.; Xie, N.; Nice, E.C.; Zhang, T.; Cui, Y.; Huang, C. Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 113. [Google Scholar] [CrossRef]
- de Robles, P.; Fiest, K.M.; Frolkis, A.D.; Pringsheim, T.; Atta, C.; St Germaine-Smith, C.; Day, L.; Lam, D.; Jette, N. The worldwide incidence and prevalence of primary brain tumors: A systematic review and meta-analysis. Neuro-Oncol. 2015, 17, 776–783. [Google Scholar] [CrossRef]
- Miller, K.D.; Ostrom, Q.T.; Kruchko, C.; Patil, N.; Tihan, T.; Cioffi, G.; Fuchs, H.E.; Waite, K.A.; Jemal, A.; Siegel, R.L.; et al. Brain and other central nervous system tumor statistics, 2021. CA Cancer J. Clin. 2021, 71, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, F.; Takeshima, H.; Yamashita, S.; Yokogami, K.; Watanabe, T.; Ohta, H.; Miyazaki Brain Tumor Research Group. Epidemiologic Study of Primary Brain Tumors in Miyazaki Prefecture: A Regional 10-year Survey in Southern Japan. Neurol. Med.-Chir. 2021, 61, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Ghasemi, H.; Fatahian, R.; Mansouri, K.; Dokaneheifard, S.; Shiri, M.H.; Hemmati, M.; Mohammadi, M. The global prevalence of primary central nervous system tumors: A systematic review and meta-analysis. Eur J Med Res 2023, 28, 39. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, C.; Philbrick, B.D.; Adamson, D.C. Meningioma: A Review of Epidemiology, Pathology, Diagnosis, Treatment, and Future Directions. Biomedicines 2021, 9, 319. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.A.; Huang, L.; Ramanathan, D.; Lopez-Gonzalez, M.; Pillai, P.; De Los Reyes, K.; Kumal, M.; Boling, W. Review of Atypical and Anaplastic Meningiomas: Classification, Molecular Biology, and Management. Front. Oncol. 2020, 10, 565582. [Google Scholar] [CrossRef] [PubMed]
- Vagnoni, L.; Aburas, S.; Giraffa, M.; Russo, I.; Chiarella, V.; Paolini, S.; Tini, P.; Minniti, G. Radiation therapy for atypical and anaplastic meningiomas: An overview of current results and controversial issues. Neurosurg. Rev. 2022, 45, 3019–3033. [Google Scholar] [CrossRef]
- Tamura, R. Current Understanding of Neurofibromatosis Type 1, 2, and Schwannomatosis. Int. J. Mol. Sci. 2021, 22, 5850. [Google Scholar] [CrossRef]
- Kruyt, I.J.; Verheul, J.B.; Hanssens, P.E.J.; Kunst, H.P.M. Gamma Knife radiosurgery for treatment of growing vestibular schwannomas in patients with neurofibromatosis Type 2: A matched cohort study with sporadic vestibular schwannomas. J. Neurosurg. 2018, 128, 49–59. [Google Scholar] [CrossRef]
- Plotkin, S.R.; Duda, D.G.; Muzikansky, A.; Allen, J.; Blakeley, J.; Rosser, T.; Campian, J.L.; Clapp, D.W.; Fisher, M.J.; Tonsgard, J.; et al. Multicenter, Prospective, Phase II and Biomarker Study of High-Dose Bevacizumab as Induction Therapy in Patients with Neurofibromatosis Type 2 and Progressive Vestibular Schwannoma. J. Clin. Oncol. 2019, 37, 3446–3454. [Google Scholar] [CrossRef]
- Plotkin, S.R.; Allen, J.; Dhall, G.; Campian, J.L.; Clapp, D.W.; Fisher, M.J.; Jain, R.K.; Tonsgard, J.; Ullrich, N.J.; Thomas, C.; et al. Multicenter, prospective, phase 2 study of maintenance bevacizumab for children and adults with NF2-related schwannomatosis and progressive vestibular schwannoma. Neuro-Oncol. 2023, 25, 1498–1506. [Google Scholar] [CrossRef]
- Tamura, R.; Tanaka, T.; Miyake, K.; Yoshida, K.; Sasaki, H. Bevacizumab for malignant gliomas: Current indications, mechanisms of action and resistance, and markers of response. Brain Tumor Pathol. 2017, 34, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Trouillas, J.; Jaffrain-Rea, M.L.; Vasiljevic, A.; Raverot, G.; Roncaroli, F.; Villa, C. How to Classify the Pituitary Neuroendocrine Tumors (PitNET)s in 2020. Cancers 2020, 12, 514. [Google Scholar] [CrossRef] [PubMed]
- Guaraldi, F.; Zoli, M.; Righi, A.; Gibertoni, D.; Marino Picciola, V.; Faustini-Fustini, M.; Morandi, L.; Bacci, A.; Pasquini, E.; Mazzatenta, D.; et al. A practical algorithm to predict postsurgical recurrence and progression of pituitary neuroendocrine tumours (PitNET)s. Clin. Endocrinol. 2020, 93, 36–43. [Google Scholar] [CrossRef]
- Asa, S.L.; Casar-Borota, O.; Chanson, P.; Delgrange, E.; Earls, P.; Ezzat, S.; Grossman, A.; Ikeda, H.; Inoshita, N.; Karavitaki, N.; et al. From pituitary adenoma to pituitary neuroendocrine tumor (PitNET): An International Pituitary Pathology Club proposal. Endocr.-Relat. Cancer 2017, 24, C5–C8. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; de Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A common classification framework for neuroendocrine neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, R.V.; Kovacs, K.; Young, W.F., Jr.; Farrel, W.E.; Asa, S.L.; Truillas, J.; Kontogeorgos, G.; Sano, T.; Scheithauer, B.W.; Horvath, E.; et al. Pituitary tumors. Introduction. In WHO Classification of Tumours, Pathology and Genetics of Tumours of Endocrine Organs; DeLellis, R.A., Lloyd, R.V., Heitz, P.U., Eng, C., Eds.; IARC Press: Lyon, France, 2004; Volume 8, pp. 10–13. [Google Scholar]
- Heaney, A. Management of aggressive pituitary adenomas and pituitary carcinomas. J. Neuro-Oncol. 2014, 117, 459–468. [Google Scholar] [CrossRef]
- Kasuki, L.; Raverot, G. Definition and diagnosis of aggressive pituitary tumors. Rev. Endocr. Metab. Disord. 2020, 21, 203–208. [Google Scholar] [CrossRef]
- Tamura, R.; Ohara, K.; Morimoto, Y.; Kosugi, K.; Oishi, Y.; Sato, M.; Yoshida, K.; Toda, M. PITX2 Expression in Non-functional Pituitary Neuroendocrine Tumor with Cavernous Sinus Invasion. Endocr. Pathol. 2019, 30, 81–89. [Google Scholar] [CrossRef]
- Zhu, M.M.; Li, H.L.; Shi, L.H.; Chen, X.P.; Luo, J.; Zhang, Z.L. The pharmacogenomics of valproic acid. J. Hum. Genet. 2017, 62, 1009–1014. [Google Scholar] [CrossRef]
- Chiou, H.-Y.C.; Lai, W.-K.; Huang, L.-C.; Huang, S.-M.; Chueh, S.-H.; Ma, H.-I.; Hueng, D.-Y. Valproic acid promotes radiosensitization in meningioma stem-like cells. Oncotarget 2015, 6, 9959–9969. [Google Scholar] [CrossRef]
- Münst, G.J.; Karlaganis, G.; Bircher, J. Plasma concentrations of mebendazole during treatment of echinococcosis: Preliminary results. Eur. J. Clin. Pharmacol. 1980, 17, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Guerini, A.E.; Triggiani, L.; Maddalo, M.; Bonù, M.L.; Frassine, F.; Baiguini, A.; Alghisi, A.; Tomasini, D.; Borghetti, P.; Pasinetti, N.; et al. Mebendazole as a candidate for drug repurposing in oncology: An extensive review of current literature. Cancers 2019, 11, 1284. [Google Scholar] [CrossRef] [PubMed]
- Larsen, A.R.; Bai, R.Y.; Chung, J.H.; Borodovsky, A.; Rudin, C.M.; Riggins, G.J.; Bunz, F. Repurposing the antihelmintic mebendazole as a hedgehog inhibitor. Mol. Cancer Ther. 2015, 14, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.-Y.; Staedtke, V.; Rudin, C.M.; Bunz, F.; Riggins, G.J. Effective treatment of diverse medulloblastoma models with mebendazole and its impact on tumor angiogenesis. Neuro-Oncol. 2015, 17, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, J.; Ramesh, R.; Chada, S.; Gomyo, Y.; Roth, J.A.; Mukhopadhyay, T. The anthelmintic drug mebendazole induces mitotic arrest and apoptosis by depolymerizing tubulin in non-small cell lung cancer cells. Mol. Cancer Ther. 2002, 1, 1201–1209. [Google Scholar]
- Skibinski, C.G.; Williamson, T.; Riggins, G.J. Mebendazole and radiation in combination increase survival through anticancer mechanisms in an intracranial rodent model of malignant meningioma. J. Neuro-Oncol. 2018, 140, 529–538. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 569–589. [Google Scholar] [CrossRef]
- Bao, B.; Azmi, A.S.; Ali, S.; Zaiem, F.; Sarkar, F.H. Metformin may function as anti-cancer agent via targeting cancer stem cells: The potential biological significance of tumor-associated miRNAs in breast and pancreatic cancers. Ann. Transl. Med. 2014, 2, 59. [Google Scholar]
- Saini, N.; Yang, X. Metformin as an anti-cancer agent: Actions and mechanisms targeting cancer stem cells. Acta Biochim. Biophys. Sin. 2018, 50, 133–143. [Google Scholar] [CrossRef]
- Rena, G.; Pearson, E.R.; Sakamoto, K. Molecular mechanism of action of metformin: Old or new insights? Diabetologia 2013, 56, 1898–1906. [Google Scholar] [CrossRef]
- Guo, L.; Cui, J.; Wang, H.; Medina, R.; Zhang, S.; Zhang, X.; Zhuang, Z.; Lin, Y. Metformin enhances anti-cancer effects of cisplatin in meningioma through AMPK-mTOR signaling pathways. Mol. Ther. Oncolytics. 2020, 20, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, H.A.; Iliopoulos, D.; Tsichlis, P.N.; Struhl, K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009, 69, 7507–7511. [Google Scholar] [CrossRef]
- Wandee, J.; Prawan, A.; Senggunprai, L.; Kongpetch, S.; Tusskorn, O.; Kukongviriyapan, V. Metformin enhances cisplatin induced inhibition of cholangiocarcinoma cells via AMPK-mTOR pathway. Life Sci. 2018, 207, 172–183. [Google Scholar] [CrossRef]
- Shivapathasundram, G.; Wickremesekera, A.C.; Brasch, H.D.; van Schaijik, B.; Marsh, R.W.; Tan, S.T.; Itinteang, T. Expression of components of the renin-angiotensin system by the putative stem cell population within WHO grade I meningioma. Front. Surg. 2019, 6, 23. [Google Scholar] [CrossRef]
- Masaaki, K.; Yuki, S.; Daisuke, O.; Keisuke, M.; Akira, N.; Takashi, T. (Pro)renin receptor is crucial for glioma development via the Wnt/β-catenin signaling pathway. J. Neurosurg. 2017, 127, 819–828. [Google Scholar]
- Gross, S.; Mallu, P.; Joshi, H.; Schultz, B.; Go, C.; Soboloff, J. Ca(2+) as a therapeutic target in cancer. Adv. Cancer Res. 2020, 148, 233–317. [Google Scholar] [PubMed]
- Roth, I.M.; Wickremesekera, A.C.; Wickremesekera, S.K.; Davis, P.F.; Tan, S.T. Therapeutic targeting of cancer stem cells via modulation of the renin-angiotensin system. Front. Oncol. 2019, 9, 745. [Google Scholar] [CrossRef] [PubMed]
- Antal, L.; Martin-Caraballo, M. T-type calcium channels in cancer. Cancers 2019, 11, 134. [Google Scholar] [CrossRef]
- Cheng, Q.; Chen, A.; Du, Q.; Liao, Q.; Shuai, Z.; Chen, C.; Yang, X.; Hu, Y.; Zhao, J.; Liu, S.; et al. Novel insights into ion channels in cancer stem cells. Int. J. Oncol. 2018, 53, 1435–1441. [Google Scholar] [CrossRef]
- Jensen, R.L.; Origitano, T.C.; Lee, Y.S.; Weber, M.; Wurster, R.D. In vitro growth inhibition of growth factor-stimulated meningioma cells by calcium channel antagonists. Neurosurgery 1995, 36, 365–374. [Google Scholar] [CrossRef]
- Jensen, R.L.; Lee, Y.S.; Guijrati, M.; Origitano, T.C.; Wurster, R.D.; Reichman, O.H. Inhibition of in vitro meningioma proliferation after growth factor stimulation by calcium channel antagonists: Part II—Additional growth factors, growth factor receptor immunohistochemistry, and intracellular calcium measurements. Neurosurgery 1995, 37, 937–947. [Google Scholar] [CrossRef]
- Jensen, R.L.; Wurster, R.D. Calcium channel antagonists inhibit growth of subcutaneous xenograft meningiomas in nude mice. Surg. Neurol. 2001, 55, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Ragel, B.; Couldwell, W.; Wurster, R.; Jensen, R. Chronic suppressive therapy with calcium channel antagonists for refractory meningiomas. Neurosurg. Focus 2007, 23, E10. [Google Scholar] [CrossRef] [PubMed]
- Ragel, B.; Jensen, R. New approaches for the treatment of refractory meningiomas. Cancer Control 2003, 10, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Karsy, M.; Hoang, N.; Barth, T.; Burt, L.; Dunson, W.; Gillespie, D.; Jensen, R.L. Combined hydroxyurea and verapamil in the clinical treatment of refractory meningioma: Human and orthotopic xenograft studies. World Neurosurg. 2016, 86, 210–219. [Google Scholar] [CrossRef]
- Johnson, M.D.; Woodard, A.; Okediji, E.J.; Toms, S.A.; Allen, G.S. Lovastatin is a potent inhibitor of meningioma cell proliferation: Evidence for inhibition of a mitogen associated protein kinase. J. Neuro-Oncol. 2002, 56, 133–142. [Google Scholar] [CrossRef]
- Wu, H.; Jiang, H.; Lu, D.; Xiong, Y.; Qu, C.; Zhou, D.; Mahmood, A.; Chopp, M. Effect of simvastatin on glioma cell proliferation, migration, and apoptosis. Neurosurgery 2009, 65, 1087–1097. [Google Scholar] [CrossRef]
- Afzali, M.; Vatankhah, M.; Ostad, S.N. Investigation of simvastatin-induced apoptosis and cell cycle arrest in cancer stem cells of MCF-7. J. Cancer Res. Ther. 2016, 12, 725–730. [Google Scholar]
- Dilwali, S.; Kao, S.Y.; Fujita, T.; Landegger, L.D.; Stankovic, K.M. Nonsteroidal anti-inflammatory medications are cytostatic against human vestibular schwannomas. Transl. Res. 2015, 166, 1–11. [Google Scholar] [CrossRef]
- Nakanishi, M.; Rosenberg, D.W. Multifaceted roles of PGE2 in inflammation and cancer. Semin. Immunopathol. 2013, 35, 123–137. [Google Scholar] [CrossRef]
- Kandathil, C.K.; Dilwali, S.; Wu, C.C.; Ibrahimov, M.; McKenna, M.J.; Lee, H.; Stankovic, K.M. Aspirin intake correlates with halted growth of sporadic vestibular schwannoma in vivo. Otol. Neurotol. 2014, 35, 353–357. [Google Scholar] [CrossRef]
- Guerrant, W.; Kota, S.; Troutman, S.; Mandati, V.; Fallahi, M.; Stemmer-Rachamimov, A.; Kissil, J.L. YAP mediates tumorigenesis in neurofibromatosis type 2 by promoting cell survival and proliferation through a COX-2-EGFR signaling axis. Cancer Res. 2016, 76, 3507–3519. [Google Scholar] [CrossRef] [PubMed]
- Wahle, B.M.; Hawley, E.T.; He, Y.; Smith, A.E.; Yuan, J.; Masters, A.R.; Jones, D.R.; Gehlhausen, J.R.; Park, S.J.; Conway, S.J.; et al. Chemopreventative celecoxib fails to prevent schwannoma formation or sensorineural hearing loss in genetically engineered murine model of neurofibromatosis type 2. Oncotarget 2018, 9, 718–725. [Google Scholar] [CrossRef] [PubMed]
- MacKeith, S.; Wasson, J.; Baker, C.; Guifoyle, M.; John, D.; Donnelly, N.; Mannion, R.; Jefferies, S.; Axon, P.; Tysome, J.R. Aspirin does not prevent growth of vestibular schwannomas: A case-control study. Laryngoscope 2018, 128, 2139–2144. [Google Scholar] [CrossRef] [PubMed]
- Behling, F.; Ries, V.; Skardelly, M.; Gepfner-Tuma, I.; Schuhmann, M.; Ebner, F.H.; Tabatabai, G.; Bornemann, A.; Schittenhelm, J.; Tatagiba, M. COX2 expression is associated with proliferation and tumor extension in vestibular schwannoma but is not influenced by acetylsalicylic acid intake. Acta Neuropathol. Commun. 2019, 7, 105. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.B.; O’Connell, B.P.; Wanna, G.B.; Bennett, M.L.; Rivas, A.; Thompson, R.C.; Haynes, D.S. Vestibular schwannoma growth with aspirin and other nonsteroidal anti-inflammatory drugs. Otol. Neurotol. 2017, 38, 1158–1164. [Google Scholar] [CrossRef]
- Muller, D.N.; Heissmeyer, V.; Dechend, R.; Hampich, F.; Park, J.K.; Fiebeler, A.; Shagdarsuren, E.; Theuer, J.; Elger, M.; Pilz, B.; et al. Aspirin inhibits NF-kappa B and protects from angiotensin II-induced organ damage. FASEB J. 2001, 15, 1822–1824. [Google Scholar] [CrossRef]
- Gehlhausen, J.R.; Hawley, E.; Wahle, B.M.; He, Y.; Edwards, D.; Rhodes, S.D.; Lajiness, J.D.; Staser, K.; Chen, S.; Yang, X.; et al. A proteasome-resistant fragment of NIK mediates oncogenic NF-kappa B signaling in schwannomas. Hum. Mol. Genet. 2019, 28, 572–583. [Google Scholar] [CrossRef]
- Van Gompel, J.J.; Agazzi, S.; Carlson, M.L.; Adewumi, D.A.; Hadjipanayis, C.G.; Uhm, J.H.; Olson, J.J. Congress of neurological surgeons systematic review and evidence-based guidelines on emerging therapies for the treatment of patients with vestibular schwannomas. Neurosurgery 2018, 82, E52–E54. [Google Scholar] [CrossRef]
- Sagers, J.E.; Brown, A.S.; Vasilijic, S.; Lewis, R.M.; Sahin, M.I.; Landegger, L.D.; Perlis, R.H.; Kohane, I.S.; Welling, D.B.; Patel, C.J.; et al. Computational repositioning and preclinical validation of mifepristone for human vestibular schwannoma. Sci. Rep. 2018, 8, 5437. [Google Scholar] [CrossRef]
- Nakanishi, H.; Kawashima, Y.; Kurima, K.; Chae, J.J.; Ross, A.M.; Pinto-Patarroyo, G.; Patel, S.K.; Muskett, J.A.; Ratay, J.S.; Chattaraj, P.; et al. NLRP3 mutation and cochlear autoinflammation cause syndromic and nonsyndromic hearing loss DFNA34 responsive to anakinra therapy. Proc. Natl. Acad. Sci. USA 2017, 114, E7766–E7775. [Google Scholar] [CrossRef] [PubMed]
- Sagers, J.E.; Sahin, M.I.; Moon, I.; Ahmed, S.G.; Stemmer-Rachamimov, A.; Brenner, G.J.; Stankovic, K.M. NLRP3 inflammasome activation in human vestibular schwannoma: Implications for tumor-induced hearing loss. Hear. Res. 2019, 381, 107770. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.O.; Katz, A.; Stein, R.A. Mifepristone: A Safe Method of Medical Abortion and Self-Managed Medical Abortion in the Post-Roe Era. Am. J. Ther. 2022, 29, e534–e543. [Google Scholar] [CrossRef] [PubMed]
- Sonam, D.; Briët, M.C.; Kao, S.-Y. Preclinical validation of anti-nuclear factor-kappa B therapy to inhibit human vestibular schwannoma growth. Mol. Oncol. 2015, 9, 1359–1370. [Google Scholar]
- Toda, M.; Tamura, R.; Toda, M. Recent Progress in Stem Cell Research of the Pituitary Gland and Pituitary Adenoma. Endocrines 2020, 1, 49–57. [Google Scholar] [CrossRef]
- Florio, T.; Barbieri, F. The status of the art of human malignant glioma management: The promising role of targeting tumor-initiating cells. Drug Discov. Today 2012, 17, 1103–1110. [Google Scholar] [CrossRef]
- Zahra, M.H.; Afify, S.M.; Hassan, G.; Nawara, H.M.; Kumon, K.; Seno, A.; Seno, M. Metformin suppresses self-renewal and stemness of cancer stem cell models derived from pluripotent stem cells. Cell Biochem Funct 2021, 39, 896–907. [Google Scholar] [CrossRef]
- An, J.; Pei, X.; Zang, Z.; Zhou, Z.; Hu, J.; Zheng, X.; Zhang, Y.; He, J.; Duan, L.; Shen, R.; et al. Metformin inhibits proliferation and growth hormone secretion of GH3 pituitary adenoma cells. Oncotarget 2017, 8, 37538–37549. [Google Scholar] [CrossRef]
- Wurth, R.; Thellung, S.; Bajetto, A.; Mazzanti, M.; Florio, T.; Barbieri, F. Drug-repositioning opportunities for cancer therapy: Novel molecular targets for known compounds. Drug Discov. Today 2016, 21, 190–199. [Google Scholar] [CrossRef]
- Yamato, A.; Nagano, H.; Gao, Y.; Matsuda, T.; Hashimoto, N.; Nakayama, A.; Yamagata, K.; Yokoyama, M.; Gong, Y.; Shi, X.; et al. Proteogenomic landscape and clinical characterization of GH-producing pituitary adenomas/somatotroph pituitary neuroendocrine tumors. Commun. Biol. 2022, 5, 1304. [Google Scholar] [CrossRef]
- Mertens, F.M.; Gremeaux, L.; Chen, J.; Fu, Q.; Willems, C.; Roose, H. Pituitary tumors contain a side population with tumor stem cell-associated characteristics. Endocr.-Relat. Cancer 2015, 22, 481–504. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Yuan, X.; Tunici, P.; Liu, G.; Fan, X.; Xu, M. Isolation of tumour stem-like cells from benign tumours. Br. J. Cancer 2009, 101, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Vankelecom, H.; Roose, H. The stem cell connection of pituitary tumors. Front. Endocrinol. 2017, 8, 339. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xiao, Z.; Chen, W.; Yang, J.; Li, T.; Fan, B. Disulfiram sensitizes pituitary adenoma cells to temozolomide by regulating O6-methylguanine-DNA methyltransferase expression. Mol. Med. Rep. 2015, 12, 2313–2322. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Sestak, I.; Cawthorn, S.; Hamed, H.; Holli, K.; Howell, A.; Forbes, J.F.; IBIS-I Investigators. Tamoxifen for prevention of breast cancer: Extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 2015, 16, 67–75. [Google Scholar] [CrossRef]
- Tamura, R.; Tanaka, T.; Yamamoto, Y.; Akasaki, Y.; Sasaki, H. Dual role of macrophage in tumor immunity. Immunotherapy 2018, 10, 899–909. [Google Scholar] [CrossRef]
- Lv, T.; Zhang, Z.; Yu, H.; Ren, S.; Wang, J.; Li, S.; Sun, L. Tamoxifen Exerts Anticancer Effects on Pituitary Adenoma Progression via Inducing Cell Apoptosis and Inhibiting Cell Migration. Int. J. Mol. Sci. 2022, 23, 2664. [Google Scholar] [CrossRef]
- Gilis-Januszewska, A.; Bogusławska, A.; Rzepka, E.; Ziaja, W.; Hubalewska-Dydejczyk, A. Individualized medical treatment options in Cushing disease. Front. Endocrinol. 2022, 13, 1060884. [Google Scholar] [CrossRef]
- Yu, H.; Ren, S.; Wang, J.; Lv, T.; Sun, L.; Du, G. Bexarotene combined with lapatinib for the treatment of Cushing’s disease: Evidence based on drug repositioning and experimental confirmation. Signal Transduct. Target. Ther. 2020, 5, 175. [Google Scholar] [CrossRef]
- Morita, A.; Tateishi, C.; Muramatsu, S.; Kubo, R.; Yonezawa, E.; Kato, H.; Nishida, E.; Tsuruta, D. Efficacy and safety of bexarotene combined with photo(chemo)therapy for cutaneous T-cell lymphoma. J. Dermatol. 2020, 47, 443–451. [Google Scholar] [CrossRef]
- Tremont-Lukats, I.W.; Ratilal, B.O.; Armstrong, T.; Gilbert, M.R. Antiepileptic drugs for preventing seizures in people with brain tumors. Cochrane Database Syst. Rev. 2008, 2008, CD004424. [Google Scholar] [CrossRef] [PubMed]
- Oprea, T.I.; Overington, J.P. Computational and practical aspects of drug repositioning. Assay Drug Dev. Technol. 2015, 13, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Oprea, T.I.; Mestres, J. Drug Repurposing: Far Beyond New Targets for Old Drugs. AAPS J. 2012, 14, 759–763. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamura, R. Drug Repositioning for Refractory Benign Tumors of the Central Nervous System. Int. J. Mol. Sci. 2023, 24, 12997. https://doi.org/10.3390/ijms241612997
Tamura R. Drug Repositioning for Refractory Benign Tumors of the Central Nervous System. International Journal of Molecular Sciences. 2023; 24(16):12997. https://doi.org/10.3390/ijms241612997
Chicago/Turabian StyleTamura, Ryota. 2023. "Drug Repositioning for Refractory Benign Tumors of the Central Nervous System" International Journal of Molecular Sciences 24, no. 16: 12997. https://doi.org/10.3390/ijms241612997




