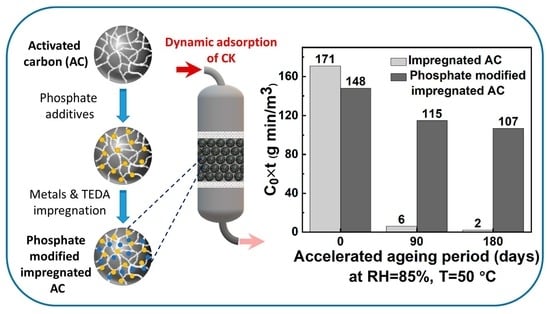
Phosphate Additives for Aging Inhibition of Impregnated Activated Carbon against Hazardous Gases
Abstract
:1. Introduction
2. Results and Discussion
2.1. CK Adsorption on IAC
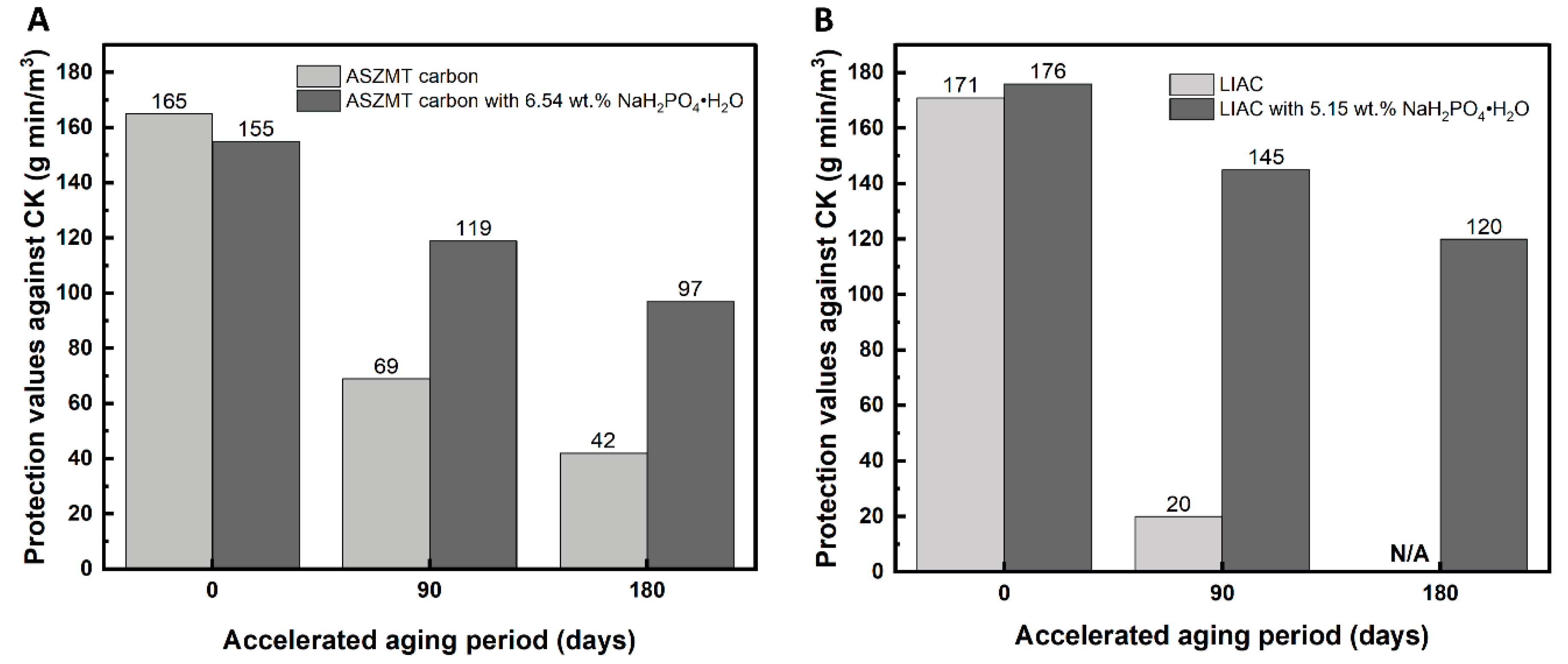
2.1.1. Pre-Humidified IAC
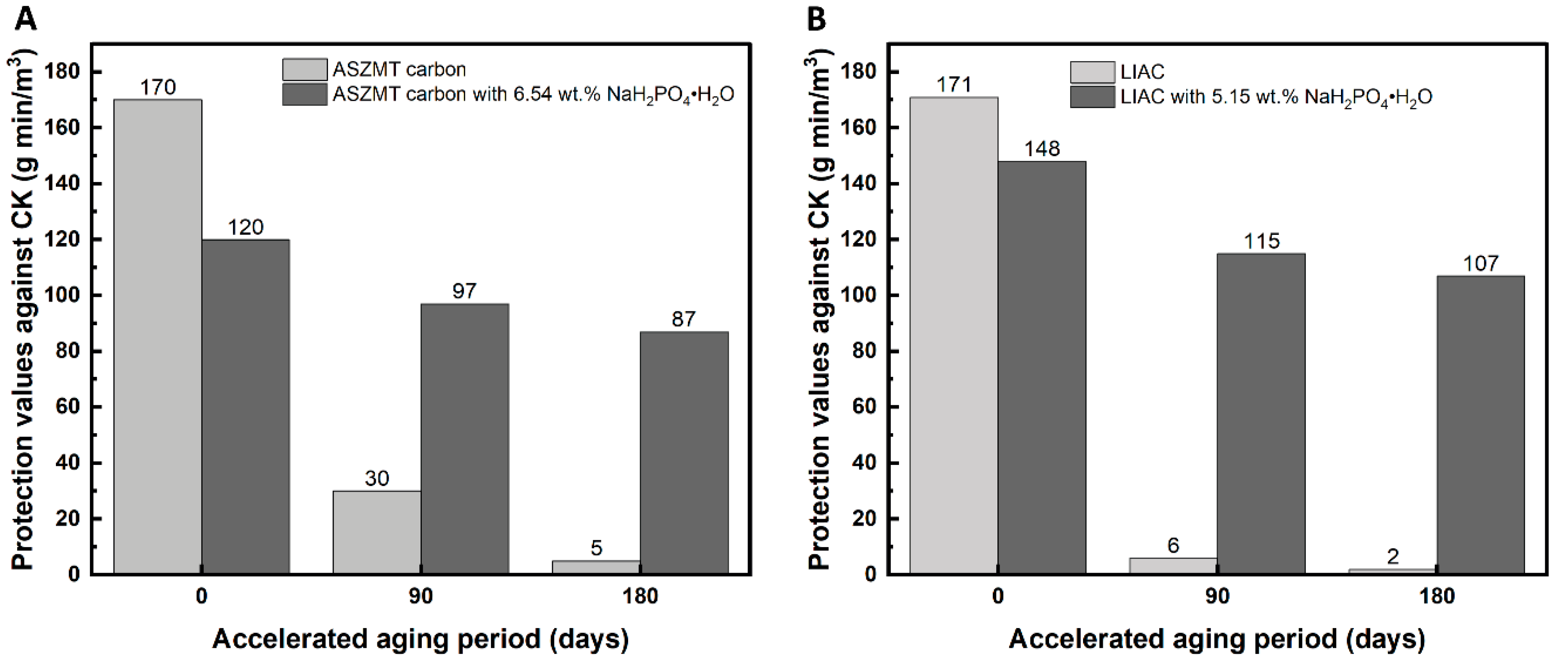
2.1.2. Effect of Phosphate Additive (NaH2PO4·H2O) on IAC Aging
2.1.3. Effect of Phosphate Concentration (NaH2PO4·H2O) on IAC Aging
2.1.4. Effect of Phosphate Type on LIAC Aging
2.2. The Effect of Phosphate on the Physical Adsorption of Toluene
2.3. Effect of a Phosphate Additive on the Textural Properties of IACs
2.4. Effect of a Phosphate Additive on Impregnated Metal Migration to the External Carbon Surface
3. Materials and Methods
3.1. Chemicals
3.2. Impregnated Activated Carbon Preparation
3.3. Addition of Phosphate Additives
3.4. Preparation of Activated Carbon Beds
3.5. IAC Pretreatments
3.5.1. Humidification of the Carbon Beds
3.5.2. Accelerated Aging
3.5.3. Carbon Bed Drying
3.6. CK Dynamic Adsorption
3.7. Toluene Dynamic Adsorption
3.8. Carbon Characterization
- I.
- Changes in the surface elemental composition of the carbon adsorbents were analyzed using energy-dispersive X-ray spectrometry (EDX) using a PhenomProX scanning electron microscope (SEM) produced by Thermo Fisher Scientific. All data were recorded using a 15 keV electron acceleration voltage. Since the metal distribution on the external surface of the carbon is heterogeneous even for a given granule, the elemental concentration was determined as the average of 10 different areas on different granules. The analyzed area in each measurement was 316 μm × 316 μm (magnification of 850×).
- II.
- The Brunauer‒Emmett‒Teller (BET) surface area and pore volume were obtained from N2 adsorption–desorption isotherms at 77 K using a NOVA 1200 e (Quantachrome) system. Before analysis, the samples were outgassed under vacuum at 80 °C. The micropore surface area was derived from a t-plot analysis of the adsorption isotherm. The outgassing was performed at a temperature of 80 °C because, at higher temperatures, a significant sublimation of TEDA from the IAC may occur. Furthermore, the sublimation rate of the TEDA from the IAC at high temperatures may vary for the different types of IACs.
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choma, J.; Jaroniec, M.; Jaroniec, M. Chapter 3 Characterization of Nanoporous Carbons by Using Gas Adsorption Isotherms. In Activated Carbon Surfaces in Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2006; Volume 7, pp. 107–158. [Google Scholar] [CrossRef]
- Wilson, R.E.; Whetzwl, J.C. Imtrgmated Carbon and Process of Making Same. U.S. Patent 1,519,740, 1924. Available online: https://patentimages.storage.googleapis.com/92/7d/ec/0f7014889caf4a/US1519470.pdf (accessed on 24 April 2023).
- Noyes, W.A.; Chief, J.R. Military Problems with Aerosols and Non-Persistent Gases; Summary Technical Report of the National Defence Research Committee (NDRC), Division 10; NDRC: Washington, DC, USA, 1946. [Google Scholar]
- Lodewyckx, P.; Lodewyckx, P. Chapter 10 Adsorption of Chemical Warfare Agents. In Activated Carbon Surfaces in Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2006; Volume 7, pp. 475–528. [Google Scholar] [CrossRef]
- Kiani, S.S.; Farooq, A.; Ahmad, M.; Irfan, N.; Nawaz, M.; Irshad, M.A. Impregnation on Activated Carbon for Removal of Chemical Warfare Agents (CWAs) and Radioactive Content. Environ. Sci. Pollut. Res. 2021, 28, 60477–60494. [Google Scholar] [CrossRef]
- Doughty, D.T.; Knebel, W.J.; Cobes, J.W. Chromium-Free Impregnated Activated Universal Respirator Carbon for Adsorption of Toxic Gases and/or Vapors. EP. Patent 0 405 404 B1, 1993. Available online: https://patentimages.storage.googleapis.com/b8/cd/d2/29c054f5b3f50a/EP0405404B1.pdf (accessed on 21 March 2023).
- Wang, S.; Nam, H.; Lee, D.; Nam, H. H2S Gas Adsorption Study Using Copper Impregnated on KOH Activated Carbon from Coffee Residue for Indoor Air Purification. J. Environ. Chem. Eng. 2022, 10, 108797. [Google Scholar] [CrossRef]
- Zulkefli, N.N.; Mathuray Veeran, L.S.; Noor Azam, A.M.I.; Masdar, M.S.; Wan Isahak, W.N.R. Effect of Bimetallic-Activated Carbon Impregnation on Adsorption–Desorption Performance for Hydrogen Sulfide (H2S) Capture. Materials 2022, 15, 5409. [Google Scholar] [CrossRef]
- Wang, X.; Jing, X.; Wang, F.; Ma, Y.; Cheng, J.; Wang, L.; Xu, K.; Cheng, C.; Ning, P. Coupling Catalytic Hydrolysis and Oxidation on Metal-Modified Activated Carbon for HCN Removal. RSC Adv. 2016, 6, 57108–57116. [Google Scholar] [CrossRef]
- Smith, J.W.H.; Romero, J.V.; Dahn, T.R.; Dunphy, K.; Croll, L.M.; Dahn, J.R. The Effect of Co-Impregnated Acids on the Performance of Zn-Based Broad Spectrum Respirator Carbons. J. Hazard. Mater. 2012, 235–236, 279–285. [Google Scholar] [CrossRef]
- Rossin, J.A.; Morrison, R.W. The Effects of Molybdenum on Stabilizing the Performance of an Experimental Copper/Zinc Impregnated, Activated Carbon. Carbon 1993, 31, 657–659. [Google Scholar] [CrossRef]
- Rossin, J.A.; Morrison, R.W. Spectroscopic Analysis and Performance of an Experimental Copper/Zinc Impregnated, Activated Carbon. Carbon 1991, 29, 887–892. [Google Scholar] [CrossRef]
- Rossin, J.; Petersen, E.; Tevault, D. Effects of Environnental Weathering on the Properties of ASC-Whetlerite. Carbon 1991, 29, 197–205. [Google Scholar]
- Ehrburger, P. Aging of Cupric Oxide Supported on Activated Carbon. J. Catal. 1986, 100, 429–436. [Google Scholar] [CrossRef]
- Brown, P.N.; Jayson, G.G.; Thompson, G.; Wilkinson, M.C. Effect of Ageing and Moisture on the Retention of Hydrogen Cyanide by Impregnated Activated Charcoals. Carbon 1989, 27, 821–833. [Google Scholar] [CrossRef]
- Oliver, T.M.; Jugoslav, K.; Aleksandar, P.; Nikola, D. Synthetic Activated Carbons for the Removal of Hydrogen Cyanide from Air. Chem. Eng. Process. Process Intensif. 2005, 44, 1181–1187. [Google Scholar] [CrossRef]
- Bischof, R.; Venetz, F. Impregnated Filter Material. U.S. Patent 10,625,104 B2, 29 November 2020. [Google Scholar]
- Lahaye, J.; Ehrburger, P.; Fangeat, R. Destruction of Cyanogen Chloride with 4 Pyridine Carboxylic Acid Impregnated Activated Carbon-I. The Physical-Chemistry of Interaction. Carbon 1987, 25, 227–231. [Google Scholar] [CrossRef]
- Deitz, V.R.; Karwacki, C.J. Influence of Acetoacetamide on the Chemisorption of Cyano-Containing Vapors by Whetlerites. Carbon 1993, 31, 237–238. [Google Scholar] [CrossRef]
- Deitz, V.R.; Karwacki, C.J. Chemisorption of Cyano-Containing Vapors by Metal-Ligand Structures Adsorbed by Activated Carbon. Carbon 1994, 32, 703–707. [Google Scholar] [CrossRef]
- Manoilova, L.V.; Chatzis, A.P.; Nickolov, R.N. Aging of the ASC Whetlerite Type Gas Mask Carbons during Storage and Operation-Overview. Secur. Futur. 2019, 3, 35–37. [Google Scholar]
- Rossin, J.A. X-ray Photoelectron Spectroscopy Surface Studies of Activated Carbon. 1989. Available online: https://apps.dtic.mil/sti/citations/ADA209393 (accessed on 24 April 2023).
- Mahle, J.J.; Peterson, G.W.; Schindler, B.J.; Smith, P.B.; Rossin, J.A.; Wagner, G.W. Role of TEDA as an Activated Carbon Impregnant for the Removal of Cyanogen Chloride from Air Streams: Synergistic Effect with Cu(Ii). J. Phys. Chem. C 2010, 114, 20083–20090. [Google Scholar] [CrossRef]
- Sastry, M.S.; Kesavadas, T.; Rao, G.S.; Sastryt, M.D. Phosphate Coordination in Copper(II) Complexes. Proc. Indian Acad. Sci. (Chem. Sci.) 1984, 93, 843–848. [Google Scholar] [CrossRef]
- Ziemniak, S.E.; Jones, M.E.; Combs, K.E.S. Copper(II) Oxide Solubility Behavior in Aqueous Sodium Phosphate Solutions at Elevated Temperatures. J. Solut. Chem. 1992, 21, 179–200. [Google Scholar] [CrossRef]
- Morrow, J.R.; Trogler, W.C. Hydrolysis of Phosphate Triesters with Copper(II) Catalysts. Inorg. Chem. 1989, 28, 2330–2333. [Google Scholar] [CrossRef]
- Munkaila, S.; Dahal, R.; Kokayi, M.; Jackson, T.; Bastakoti, B.P. Hollow Structured Transition Metal Phosphates and Their Applications. Chem. Record. 2022, 22, e202200084. [Google Scholar] [CrossRef]
- Park, S.H.; McClain, S.; Tian, Z.R.; Suib, S.L.; Karwacki, C. Surface and Bulk Measurements of Metals Deposited on Activated Carbon. Chem. Mater. 1997, 9, 176–183. [Google Scholar] [CrossRef]
- Smith, J.W.H.; Westreich, P.; Croll, L.M.; Reynolds, J.H.; Dahn, J.R. Understanding the Role of Each Ingredient in a Basic Copper Carbonate Based Impregnation Recipe for Respirator Carbons. J. Colloid Interface Sci. 2009, 337, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.W.H.; Romero, J.V.; Dahn, T.R.; Dunphy, K.; Sullivan, B.; Mallay, M.; Croll, L.M.; Reynolds, J.H.; Andress, C.; Dahn, J.R. The Effect of Heating Temperature and Nitric Acid Treatments on the Performance of Cu- and Zn-Based Broad Spectrum Respirator Carbons. J. Colloid Interface Sci. 2011, 364, 178–194. [Google Scholar] [CrossRef]
- Afandizadeh, S.; Foumeny, E.A. Design of Packed Bed Reactors: Guides to Catalyst Shape, Size, and Loading Selection. Appl. Therm. Eng. 2001, 21, 669–682. [Google Scholar] [CrossRef]





| ASZMT without Phosphate | ASZMT with 6.54 wt.% NaH2PO4·H2O | LIAC without Phosphate | LIAC with 5.15 wt.% NaH2PO4·H2O |
|---|---|---|---|
| 347 | 394 | 331 | 356 |
| ASZMT without Phosphate | ASZMT with 6.54 wt.% NaH2PO4·H2O | LIAC without Phosphate | LIAC with 5.15 wt.% NaH2PO4·H2O | |
|---|---|---|---|---|
| Surface area (m2/g) (BET) | 687 | 699 | 734 | 696 |
| % microporosity | 80.1 | 84.0 | 81.1 | 81.1 |
| Micropore volume (cm3/g) | 0.295 | 0.305 | 0.298 | 0.288 |
| IAC Type | Cu | Zn | Mo |
|---|---|---|---|
| ASZMT–new | 8.4 | 23.8 | 3.7 |
| ASZMT–aged 3 M | 23.4 | 37.5 | 4.2 |
| ASZMT–aged 6 M | 23.2 | 40.7 | 2.9 |
| ASZMT with 6.54 wt.% NaH2PO4·H2O–new | 6.3 | 13.1 | 2.8 |
| ASZMT with 6.54% NaH2PO4·H2O–aged 3 M | 8.9 | 15.6 | 3.0 |
| ASZMT with 6.54% NaH2PO4·H2O–aged 6 M | 8.8 | 17.5 | 2.6 |
| LIAC–new | 8.9 | 28.5 | 2.5 |
| LIAC–aged 3 M | 20.8 | 30.9 | 5.2 |
| LIAC–aged 6 M | 19.6 | 26.5 | 5.1 |
| LIAC with 5.15 wt.% NaH2PO4·H2O–new | 13.3 | 23.5 | 1.7 |
| LIAC with 5.15% NaH2PO4·H2O–aged 3 M | 21.8 | 22.3 | 2.6 |
| LIAC with 5.15% NaH2PO4·H2O–aged 6 M | 25.3 | 28.5 | 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nir, I.; Shepelev, V.; Pevzner, A.; Marciano, D.; Rosh, L.; Amitay-Rosen, T.; Rotter, H. Phosphate Additives for Aging Inhibition of Impregnated Activated Carbon against Hazardous Gases. Int. J. Mol. Sci. 2023, 24, 13000. https://doi.org/10.3390/ijms241613000
Nir I, Shepelev V, Pevzner A, Marciano D, Rosh L, Amitay-Rosen T, Rotter H. Phosphate Additives for Aging Inhibition of Impregnated Activated Carbon against Hazardous Gases. International Journal of Molecular Sciences. 2023; 24(16):13000. https://doi.org/10.3390/ijms241613000
Chicago/Turabian StyleNir, Ido, Vladislav Shepelev, Alexander Pevzner, Daniele Marciano, Lilach Rosh, Tal Amitay-Rosen, and Hadar Rotter. 2023. "Phosphate Additives for Aging Inhibition of Impregnated Activated Carbon against Hazardous Gases" International Journal of Molecular Sciences 24, no. 16: 13000. https://doi.org/10.3390/ijms241613000
APA StyleNir, I., Shepelev, V., Pevzner, A., Marciano, D., Rosh, L., Amitay-Rosen, T., & Rotter, H. (2023). Phosphate Additives for Aging Inhibition of Impregnated Activated Carbon against Hazardous Gases. International Journal of Molecular Sciences, 24(16), 13000. https://doi.org/10.3390/ijms241613000