Genome-Wide Exploration and Expression Analysis of the CNGC Gene Family in Eggplant (Solanum melongena L.) under Cold Stress, with Functional Characterization of SmCNGC1a
Abstract
:1. Introduction
2. Results
2.1. Identification of the CNGC Genes in Eggplant
2.2. Phylogenetic Analysis of SmCNGC Proteins
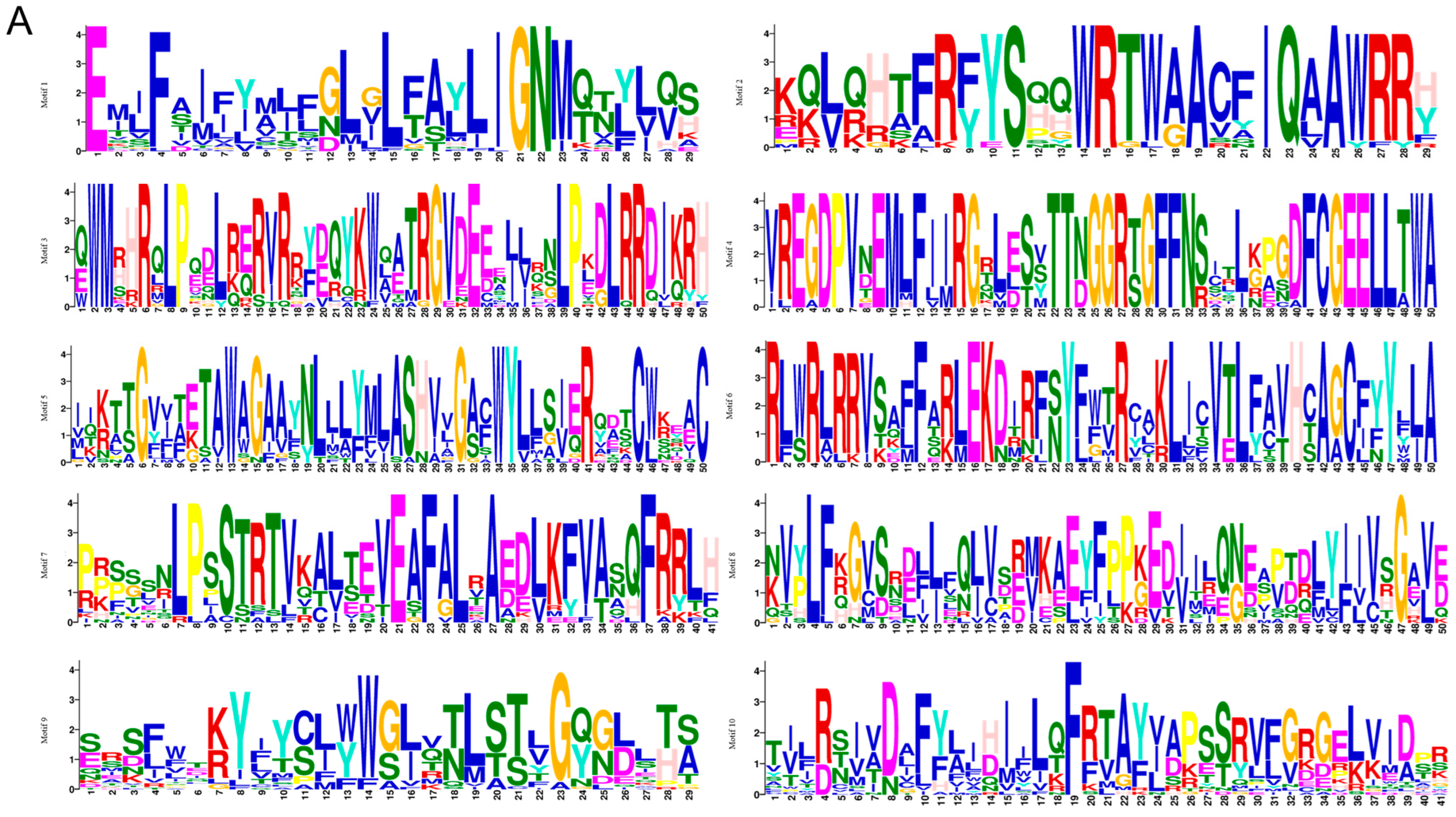
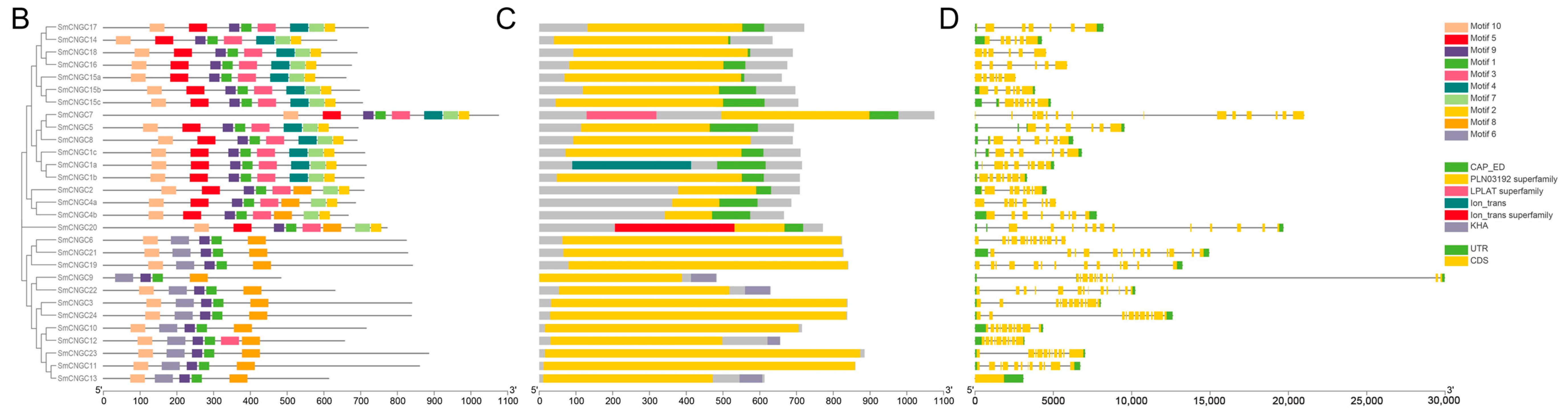
2.3. SmCNGC Gene Structures and the Conserved Motifs Analyses
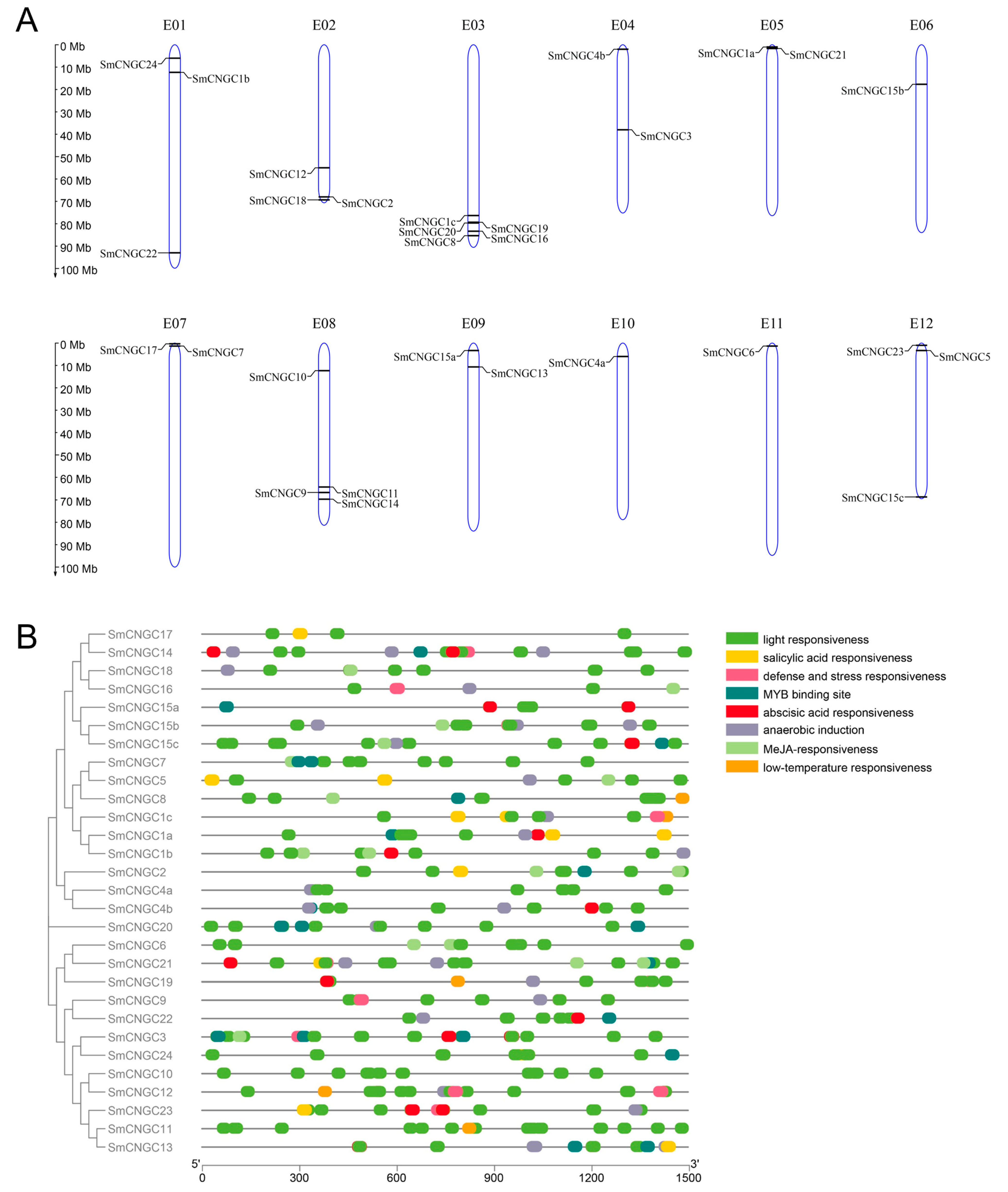
2.4. Chromosome Localization and Cis-Acting Elements Prediction of SmCNGCs
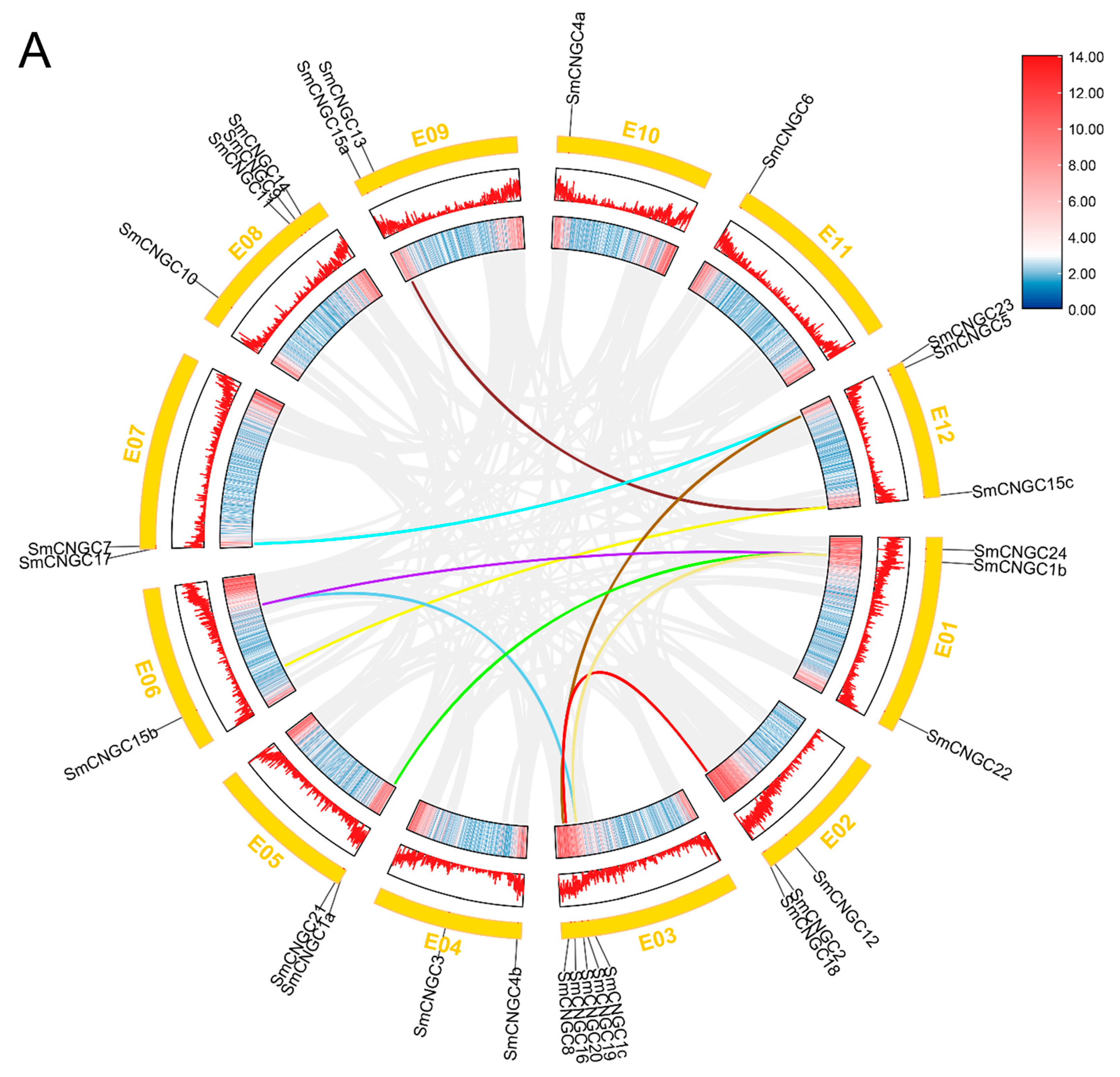
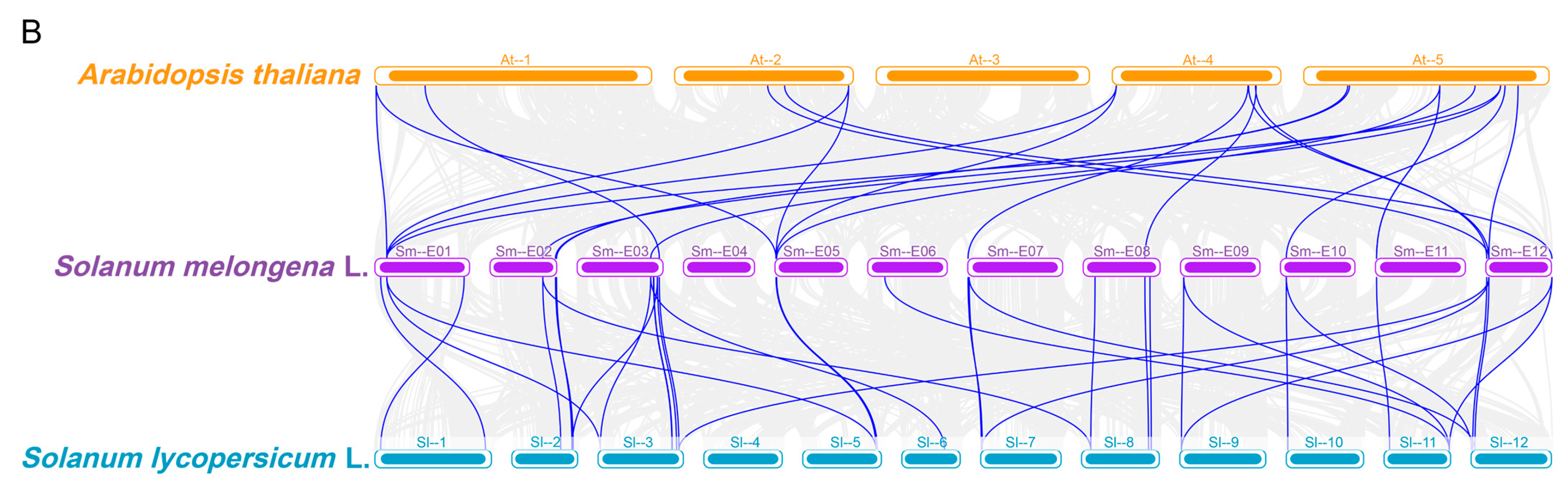
2.5. Collinearity Analysis of SmCNGCs
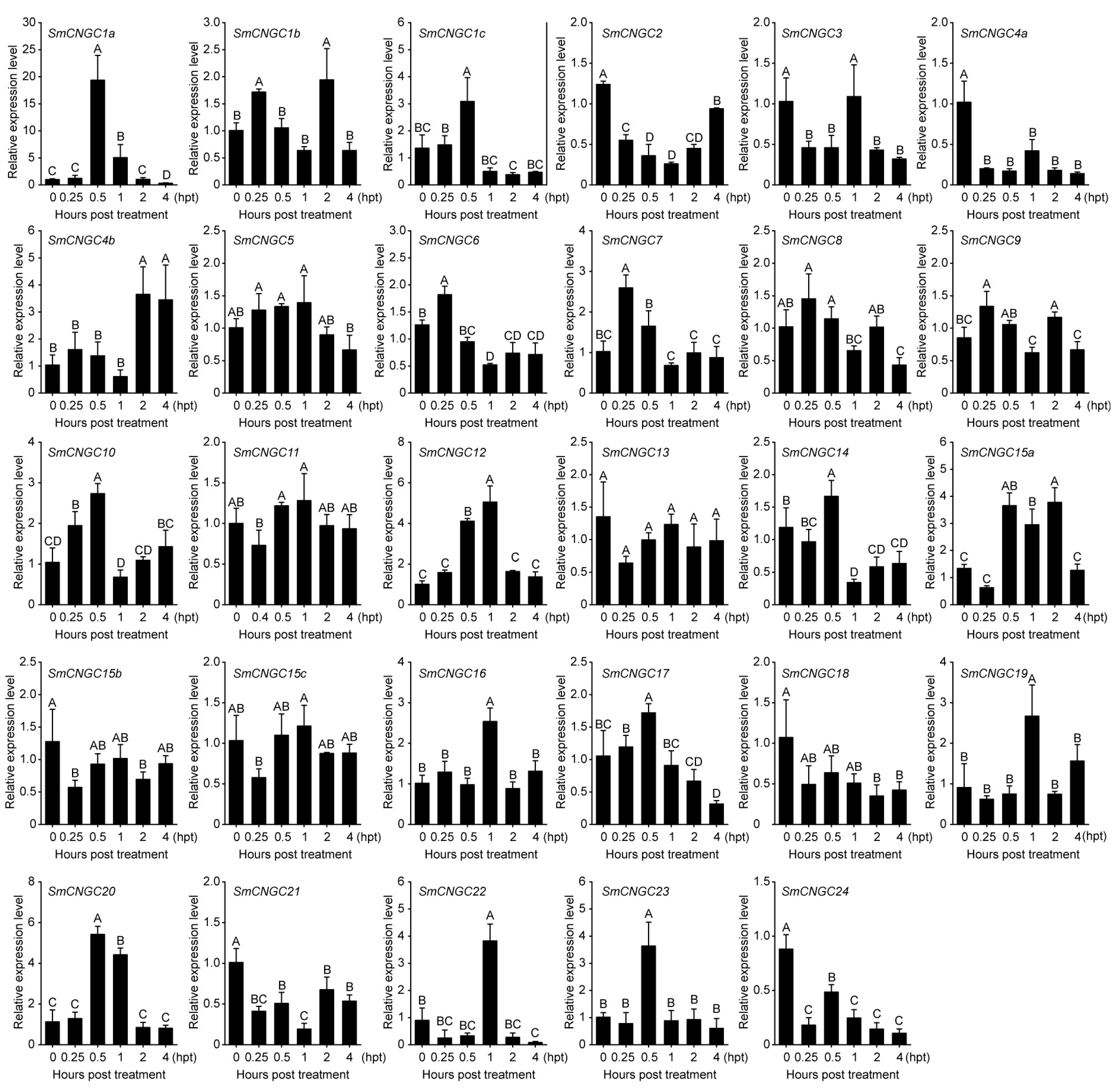
2.6. Expression Patterns of SmCNGC Genes in Cold Stress
2.7. Expression Patterns of SmCNGC1a under Various Stress Conditions
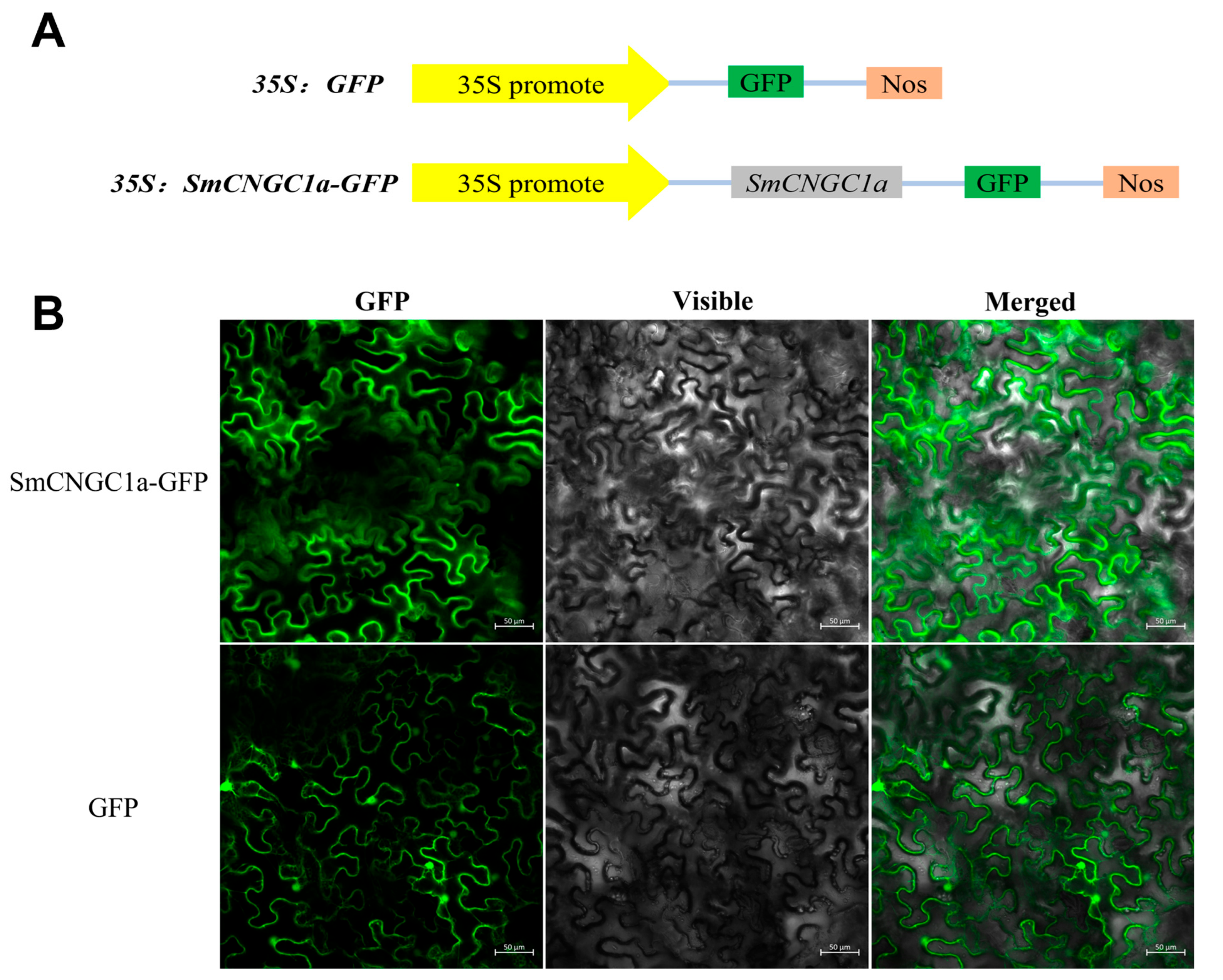
2.8. Subcellular Localization and Tissue-Specific Expression Patterns of SmCNGC1a
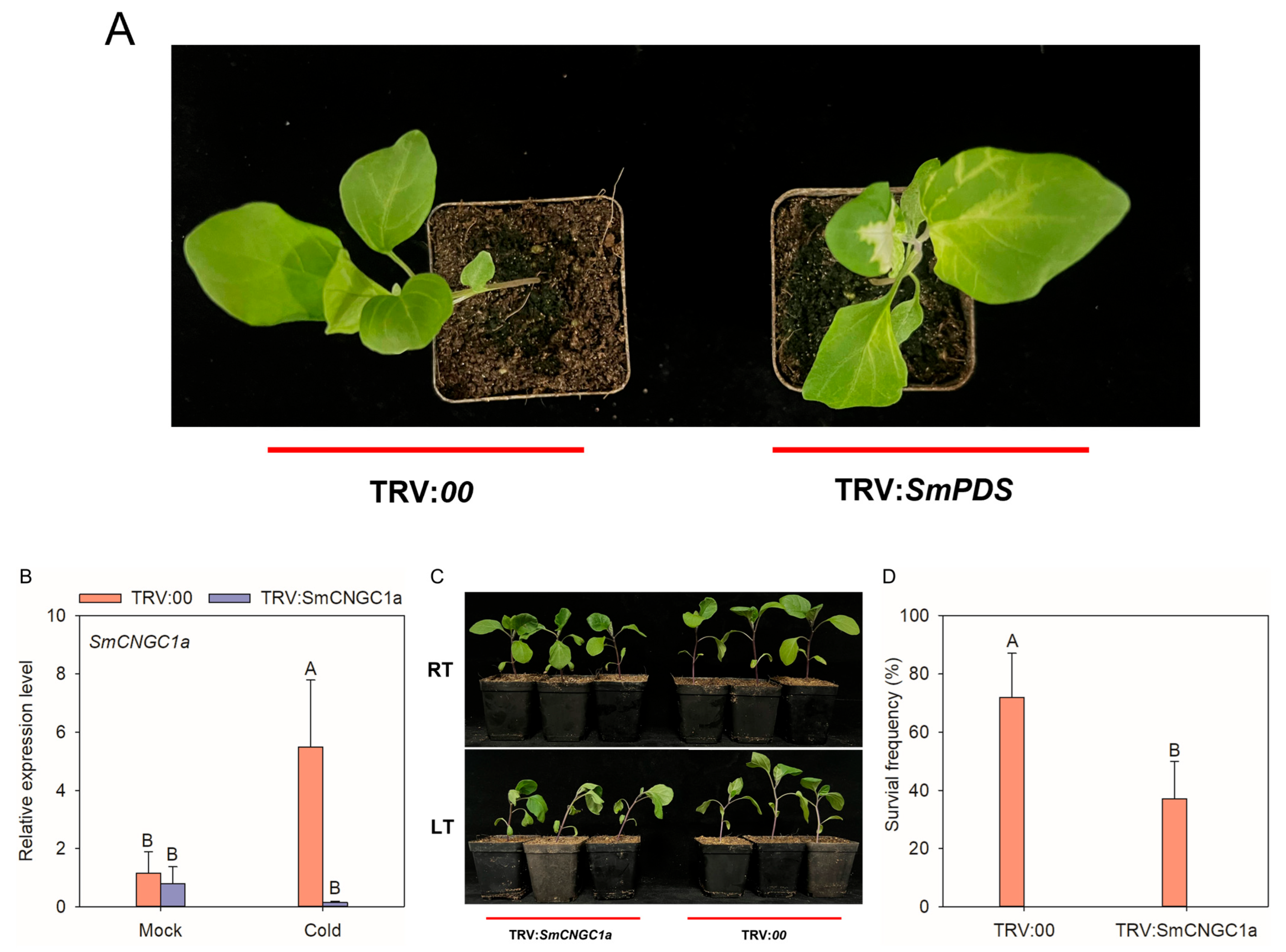
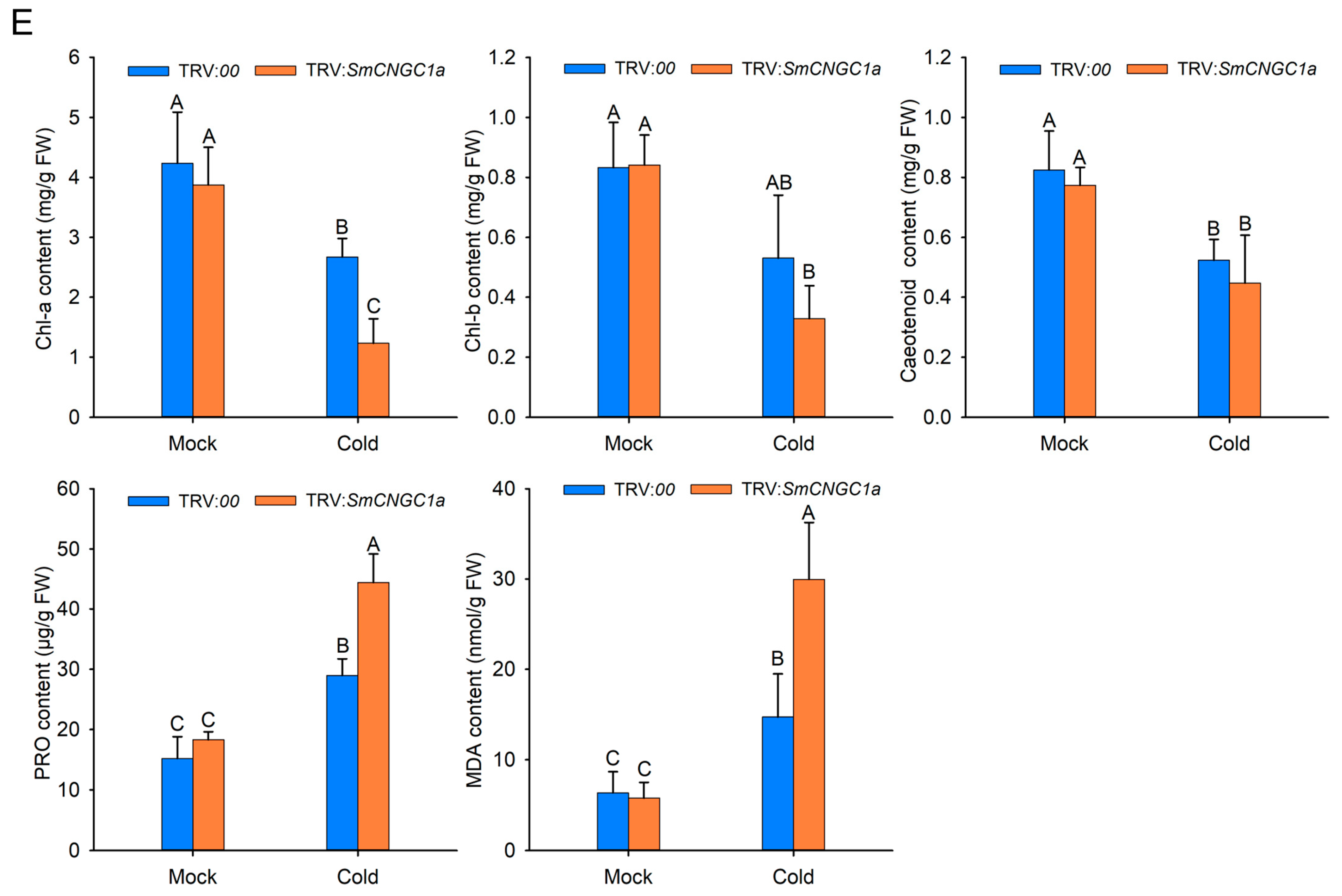
2.9. Silencing of SmCNGC1a Reduced Eggplant Tolerance to Cold Stress
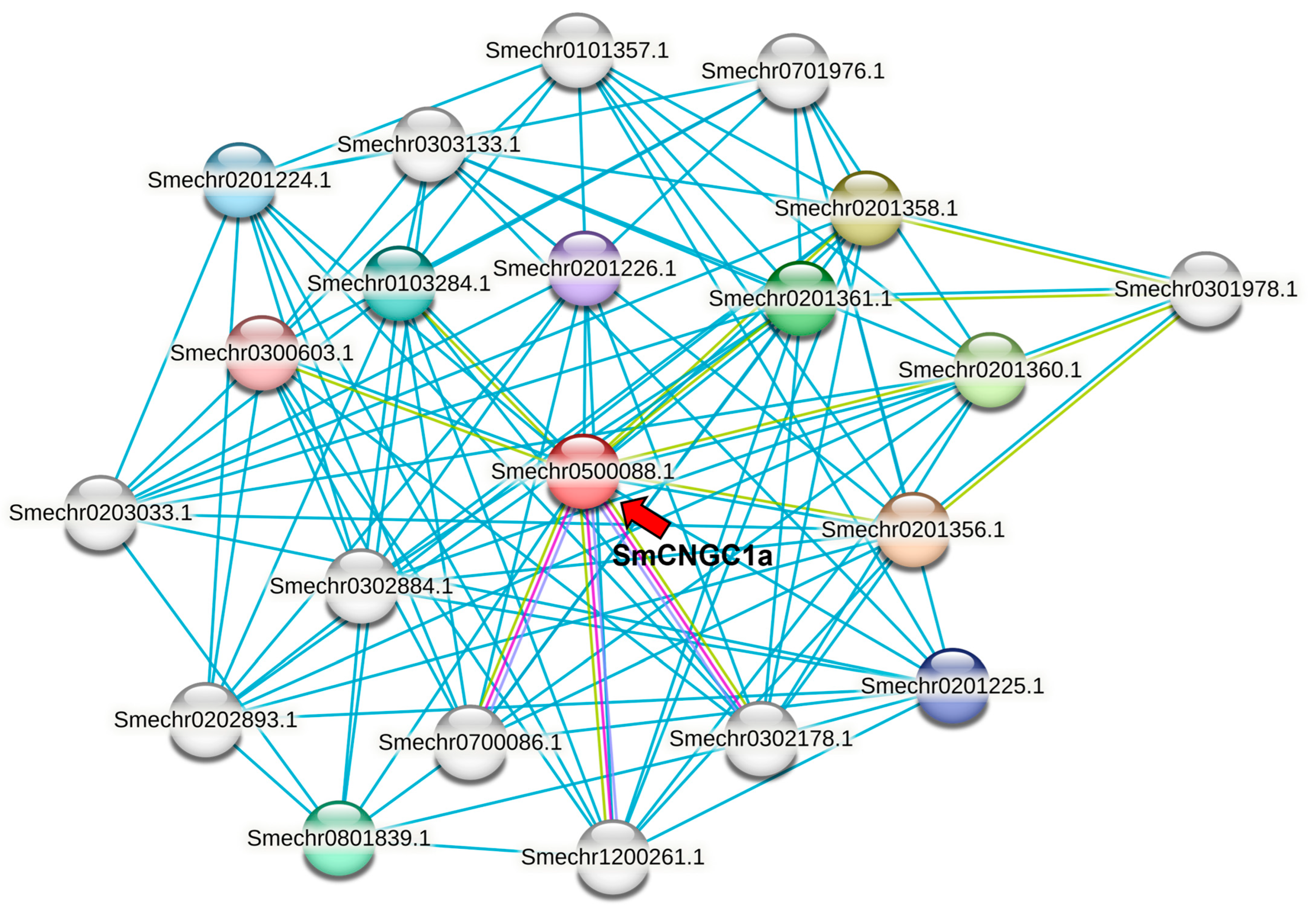
2.10. Analysis Interaction Network of SmCNGC1a in Eggplant
3. Discussion
4. Materials and Methods
4.1. Identification of CNGC Gene Family in Eggplant Genome
4.2. Multiple Sequence Alignment and Phylogenetic Analysis
4.3. Gene Structures and Conserved Motifs Analysis
4.4. Gene Distribution and Cis-Acting Elements Prediction
4.5. Collinearity Analysis
4.6. Plant Materials and Treatments
4.7. Subcellular Localization
4.8. Functional Analysis of SmCNGC1a Based on VIGS Method
4.9. Gene Expression Analysis by qPCR
4.10. Physiological Parameter Determination
4.11. Statistic Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pebriana, E.; Maghfoer, M.D.; Widaryanto, E. Effect of grafting using wild eggplant as rootstock on growth and yield of four eggplant (Solanum melongena L.) cultivars. Biosci. Res. 2018, 15, 337–347. [Google Scholar]
- Kadivec, M.; Kopjar, M.; Znidarcic, D.; Pozrl, T. Potential of Eggplant Peel as by-Product. Acta Aliment. 2015, 44, 126–131. [Google Scholar] [CrossRef]
- Hanson, J.B. Impairment of Respiration, Ion Accumulation, and Ion Retention in Root Tissue Treated with Ribonuclease and Ethylenediamine Tetraacetic Acid. Plant Physiol. 1960, 35, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Dodd, A.N.; Kudla, J.; Sanders, D. The language of calcium signaling. Annu. Rev. Plant Biol. 2010, 61, 593–620. [Google Scholar] [CrossRef]
- Kudla, J.; Batistic, O.; Hashimoto, K. Calcium signals: The lead currency of plant information processing. Plant Cell 2010, 22, 541–563. [Google Scholar] [CrossRef]
- Guo, K.M.; Babourina, O.; Christopher, D.A.; Borsic, T.; Rengel, Z. The cyclic nucleotide-gated channel AtCNGC10 transports Ca2+ and Mg2+ in Arabidopsis. Physiol. Plant 2010, 139, 303–312. [Google Scholar] [CrossRef]
- Maser, P.; Thomine, S.; Schroeder, J.I.; Ward, J.M.; Hirschi, K.; Sze, H.; Talke, I.N.; Amtmann, A.; Maathuis, F.J.; Sanders, D.; et al. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 2001, 126, 1646–1667. [Google Scholar] [CrossRef]
- Yuen, C.C.Y.; Christopher, D.A. The group IV-A cyclic nucleotide-gated channels, CNGC19 and CNGC20, localize to the vacuole membrane in Arabidopsis thaliana. Aob Plants 2013, 5, plt012. [Google Scholar] [CrossRef]
- Talke, I.N.; Blaudez, D.; Maathuis, F.J.; Sanders, D. CNGCs: Prime targets of plant cyclic nucleotide signalling? Trends Plant Sci. 2003, 8, 286–293. [Google Scholar] [CrossRef]
- Ma, W.; Berkowitz, G.A. Ca2+ conduction by plant cyclic nucleotide gated channels and associated signaling components in pathogen defense signal transduction cascades. New. Phytol. 2011, 190, 566–572. [Google Scholar] [CrossRef]
- Spalding, E.P.; Harper, J.F. The ins and outs of cellular Ca2+ transport. Curr. Opin. Plant Biol. 2011, 14, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Zelman, A.K.; Dawe, A.; Gehring, C.; Berkowitz, G.A. Evolutionary and structural perspectives of plant cyclic nucleotide-gated cation channels. Front. Plant Sci. 2012, 3, 95. [Google Scholar] [CrossRef] [PubMed]
- Young, E.C.; Krougliak, N. Distinct structural determinants of efficacy and sensitivity in the ligand-binding domain of cyclic nucleotide-gated channels. J. Biol. Chem. 2004, 279, 3553–3562. [Google Scholar] [CrossRef]
- Schuurink, R.C.; Shartzer, S.F.; Fath, A.; Jones, R.L. Characterization of a calmodulin-binding transporter from the plasma membrane of barley aleurone. Proc. Natl. Acad. Sci. USA 1998, 95, 1944–1949. [Google Scholar] [CrossRef]
- Arazi, T.; Kaplan, B.; Fromm, H. A high-affinity calmodulin-binding site in a tobacco plasma-membrane channel protein coincides with a characteristic element of cyclic nucleotide-binding domains. Plant Mol. Biol. 2000, 42, 591–601. [Google Scholar] [CrossRef]
- Kohler, C.; Neuhaus, G. Characterisation of calmodulin binding to cyclic nucleotide-gated ion channels from Arabidopsis thaliana. FEBS Lett. 2000, 471, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Saand, M.A.; Xu, Y.P.; Li, W.; Wang, J.P.; Cai, X.Z. Cyclic nucleotide gated channel gene family in tomato: Genome-wide identification and functional analyses in disease resistance. Front. Plant Sci. 2015, 6, 303. [Google Scholar] [CrossRef]
- Dietrich, P.; Moeder, W.; Yoshioka, K. Plant Cyclic Nucleotide-Gated Channels: New Insights on Their Functions and Regulation. Plant Physiol. 2020, 184, 27–38. [Google Scholar] [CrossRef]
- Tian, W.; Hou, C.; Ren, Z.; Wang, C.; Zhao, F.; Dahlbeck, D.; Hu, S.; Zhang, L.; Niu, Q.; Li, L.; et al. A calmodulin-gated calcium channel links pathogen patterns to plant immunity. Nature 2019, 572, 131–135. [Google Scholar] [CrossRef]
- Jarratt-Barnham, E.; Wang, L.; Ning, Y.; Davies, J.M. The Complex Story of Plant Cyclic Nucleotide-Gated Channels. Int. J. Mol. Sci. 2021, 22, 874. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, Y.; Tian, W.; Dong, M.; Zhu, H.; Luan, S.; Li, L. Arabidopsis CNGC14 Mediates Calcium Influx Required for Tip Growth in Root Hairs. Mol. Plant 2017, 10, 1004–1006. [Google Scholar] [CrossRef] [PubMed]
- Brost, C.; Studtrucker, T.; Reimann, R.; Denninger, P.; Czekalla, J.; Krebs, M.; Fabry, B.; Schumacher, K.; Grossmann, G.; Dietrich, P. Multiple cyclic nucleotide-gated channels coordinate calcium oscillations and polar growth of root hairs. Plant J. 2019, 99, 910–923. [Google Scholar] [CrossRef] [PubMed]
- Zeb, Q.; Wang, X.; Hou, C.; Zhang, X.; Dong, M.; Zhang, S.; Zhang, Q.; Ren, Z.; Tian, W.; Zhu, H.; et al. The interaction of CaM7 and CNGC14 regulates root hair growth in Arabidopsis. J. Integr. Plant Biol. 2020, 62, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Q.; Yang, Y.; Zhang, A.; Fei, C.F.; Gu, L.L.; Sun, S.J.; Xu, W.; Wang, L.; Liu, H.; Wang, Y.F. Three CNGC Family Members, CNGC5, CNGC6, and CNGC9, Are Required for Constitutive Growth of Arabidopsis Root Hairs as Ca2+-Permeable Channels. Plant Commun. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Moon, J.Y.; Belloeil, C.; Ianna, M.L.; Shin, R. Arabidopsis CNGC Family Members Contribute to Heavy Metal Ion Uptake in Plants. Int. J. Mol. Sci. 2019, 20, 413. [Google Scholar] [CrossRef]
- Tunc-Ozdemir, M.; Tang, C.; Ishka, M.R.; Brown, E.; Groves, N.R.; Myers, C.T.; Rato, C.; Poulsen, L.R.; McDowell, S.; Miller, G.; et al. A cyclic nucleotide-gated channel (CNGC16) in pollen is critical for stress tolerance in pollen reproductive development. Plant Physiol. 2013, 161, 1010–1020. [Google Scholar] [CrossRef]
- Tunc-Ozdemir, M.; Rato, C.; Brown, E.; Rogers, S.; Mooneyham, A.; Frietsch, S.; Myers, C.T.; Poulsen, L.R.; Malho, R.; Harper, J.F. Cyclic nucleotide gated channels 7 and 8 are essential for male reproductive fertility. PLoS ONE 2013, 8, e55277. [Google Scholar] [CrossRef]
- Pan, Y.; Chai, X.; Gao, Q.; Zhou, L.; Zhang, S.; Li, L.; Luan, S. Dynamic Interactions of Plant CNGC Subunits and Calmodulins Drive Oscillatory Ca2+ Channel Activities. Dev. Cell 2019, 48, 710–725.e715. [Google Scholar] [CrossRef]
- Gao, Q.F.; Fei, C.F.; Dong, J.Y.; Gu, L.L.; Wang, Y.F. Arabidopsis CNGC18 is a Ca2+-permeable channel. Mol. Plant 2014, 7, 739–743. [Google Scholar] [CrossRef]
- Gao, Q.F.; Gu, L.L.; Wang, H.Q.; Fei, C.F.; Fang, X.; Hussain, J.; Sun, S.J.; Dong, J.Y.; Liu, H.; Wang, Y.F. Cyclic nucleotide-gated channel 18 is an essential Ca2+ channel in pollen tube tips for pollen tube guidance to ovules in Arabidopsis. Proc. Natl. Acad. Sci. USA 2016, 113, 3096–3101. [Google Scholar] [CrossRef]
- Shi, J.; Zuo, J.; Xu, D.; Gao, L.; Wang, Q. Effect of low-temperature conditioning combined with methyl jasmonate treatment on the chilling resistance of eggplant (Solanum melongena L.) fruit. J. Food Sci. Technol. 2019, 56, 4658–4666. [Google Scholar] [CrossRef] [PubMed]
- Hong-Bo, S.; Li-Ye, C.; Ming-An, S.; Shi-Qing, L.; Ji-Cheng, Y. Bioengineering plant resistance to abiotic stresses by the global calcium signal system. Biotechnol. Adv. 2008, 26, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, Z.; Kakar, K.U.; Saand, M.A.; Shu, Q.Y. Cyclic nucleotide-gated ion channel gene family in rice, identification, characterization and experimental analysis of expression response to plant hormones, biotic and abiotic stresses. BMC Genom. 2014, 15, 853. [Google Scholar] [CrossRef] [PubMed]
- Kakar, K.U.; Nawaz, Z.; Kakar, K.; Ali, E.; Almoneafy, A.A.; Ullah, R.; Ren, X.L.; Shu, Q.Y. Comprehensive genomic analysis of the CNGC gene family in Brassica oleracea: Novel insights into synteny, structures, and transcript profiles. BMC Genom. 2017, 18, 869. [Google Scholar] [CrossRef]
- Nawaz, Z.; Kakar, K.U.; Ullah, R.; Yu, S.; Zhang, J.; Shu, Q.Y.; Ren, X.L. Genome-wide identification, evolution and expression analysis of cyclic nucleotide-gated channels in tobacco (Nicotiana tabacum L.). Genomics 2019, 111, 142–158. [Google Scholar] [CrossRef]
- Sivankalyani, V.; Sela, N.; Feygenberg, O.; Zemach, H.; Maurer, D.; Alkan, N. Transcriptome Dynamics in Mango Fruit Peel Reveals Mechanisms of Chilling Stress. Front. Plant Sci. 2016, 7, 1579. [Google Scholar] [CrossRef]
- Wang, L.; Li, M.; Liu, Z.; Dai, L.; Zhang, M.; Wang, L.; Zhao, J.; Liu, M. Genome-wide identification of CNGC genes in Chinese jujube (Ziziphus jujuba Mill.) and ZjCNGC2 mediated signalling cascades in response to cold stress. BMC Genom. 2020, 21, 191. [Google Scholar] [CrossRef]
- Li, Q.; Yang, S.; Ren, J.; Ye, X.; Jiang, X.; Liu, Z. Genome-wide identification and functional analysis of the cyclic nucleotide-gated channel gene family in Chinese cabbage. 3 Biotech 2019, 9, 114. [Google Scholar] [CrossRef]
- Gao, F.; Han, X.; Wu, J.; Zheng, S.; Shang, Z.; Sun, D.; Zhou, R.; Li, B. A heat-activated calcium-permeable channel—Arabidopsis cyclic nucleotide-gated ion channel 6—Is involved in heat shock responses. Plant J. 2012, 70, 1056–1069. [Google Scholar] [CrossRef]
- Katano, K.; Kataoka, R.; Fujii, M.; Suzuki, N. Differences between seedlings and flowers in anti-ROS based heat responses of Arabidopsis plants deficient in cyclic nucleotide gated channel 2. Plant Physiol. Biochem. 2018, 123, 288–296. [Google Scholar] [CrossRef]
- Finka, A.; Cuendet, A.F.; Maathuis, F.J.; Saidi, Y.; Goloubinoff, P. Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired thermotolerance. Plant Cell 2012, 24, 3333–3348. [Google Scholar] [CrossRef]
- Cui, Y.; Lu, S.; Li, Z.; Cheng, J.; Hu, P.; Zhu, T.; Wang, X.; Jin, M.; Wang, X.; Li, L.; et al. Cyclic Nucleotide-Gated Ion Channels 14 and 16 Promote Tolerance to Heat and Chilling in Rice. Plant Physiol. 2020, 183, 1794–1808. [Google Scholar] [CrossRef]
- Jin, L.; Mackey, D.M. Measuring Callose Deposition, an Indicator of Cell Wall Reinforcement, during Bacterial Infection in Arabidopsis. Methods Mol. Biol. 2017, 1578, 195–205. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, S.R.; Laver, D.R.; Walker, N.A. Anion channel activity in the Chara plasma membrane: Co-operative subunit phenomena and a model. J. Exp. Bot. 1997, 48, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.; Silvestre, M.; Wells, C.I.; Sanderson, J.L.; Ferrer, C.A.; Ong, H.W.; Lang, Y.; Richardson, W.; Silvaroli, J.A.; Bashore, F.M.; et al. Discovery and characterization of a specific inhibitor of serine-threonine kinase cyclin dependent kinase-like 5 (CDKL5) demonstrates role in hippocampal CA1 physiology. bioRxiv 2023. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Shen, L.; Zhao, E.; Liu, R.; Yang, X. Transcriptome Analysis of Eggplant under Salt Stress: AP2/ERF Transcription Factor SmERF1 Acts as a Positive Regulator of Salt Stress. Plants 2022, 11, 2205. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fang, Z.; Zhao, D.; Tao, J. Effects of High-Temperature Stress on Photosynthetic Characteristics and Antioxidant Enzyme System of Paeonia ostii. Phyton 2022, 91, 599–615. [Google Scholar] [CrossRef]



| Gene Name | Gene ID | Number of Amino Acid/aa | Molecular Weight | Theoretical pI | Instability Index | Aliphatic Index | Grand Average of Hydropathicity | Prediction of Subcellular Localization |
|---|---|---|---|---|---|---|---|---|
| SmCNGC1a | Smechr0500088.1 | 714 | 82,462.57 | 9.51 | 53.73 | 90.17 | −0.208 | cytomembrane |
| SmCNGC1b | Smechr0101357.1 | 708 | 81,926.22 | 9.32 | 48.72 | 93.4 | −0.071 | cytomembrane |
| SmCNGC1c | Smechr0302178.1 | 710 | 81,869.6 | 8.9 | 50.11 | 92.46 | −0.132 | cytomembrane |
| SmCNGC2 | Smechr0202893.1 | 708 | 81,371.83 | 9.65 | 53.34 | 92.78 | −0.034 | cytomembrane |
| SmCNGC3 | Smechr0400998.1 | 838 | 96,160.87 | 7.32 | 32.86 | 93.05 | −0.198 | cytomembrane |
| SmCNGC4a | Smechr1000491.1 | 685 | 80,107.48 | 8.28 | 46.96 | 90.31 | −0.169 | cytomembrane |
| SmCNGC4b | Smechr0400207.1 | 665 | 77,317.03 | 8.98 | 45.03 | 92.77 | −0.075 | cytomembrane |
| SmCNGC5 | Smechr1200261.1 | 692 | 78,774.97 | 9.19 | 51.32 | 92.76 | −0.114 | cytomembrane |
| SmCNGC6 | Smechr1100117.1 | 823 | 94,278.62 | 6.38 | 40.34 | 98.54 | −0.131 | cytomembrane |
| SmCNGC7 | Smechr0700086.1 | 1074 | 122,281.25 | 9.3 | 53.27 | 90.8 | −0.079 | cytomembrane |
| SmCNGC8 | Smechr0303133.1 | 689 | 79,669.91 | 8.78 | 43.35 | 91.13 | −0.183 | cytomembrane |
| SmCNGC9 | Smechr0801568.1 | 482 | 55,399.66 | 8.21 | 37.08 | 96.24 | −0.188 | cytoplasm |
| SmCNGC10 | Smechr0800622.1 | 714 | 82,222.56 | 5.82 | 38.97 | 95.03 | −0.123 | cytoplasm |
| SmCNGC11 | Smechr0801458.1 | 859 | 96,677.4 | 6.6 | 40.65 | 98.43 | −0.055 | cytomembrane |
| SmCNGC12 | Smechr0201396.1 | 655 | 75,003.7 | 7.84 | 39.83 | 93.45 | −0.092 | cytomembrane |
| SmCNGC13 | Smechr0900702.1 | 612 | 69,109.84 | 7.32 | 46.02 | 99.1 | 0.033 | cytomembrane |
| SmCNGC14 | Smechr0801673.1 | 634 | 72,997.99 | 8.67 | 47.05 | 91.85 | −0.097 | cytomembrane |
| SmCNGC15a | Smechr0900248.1 | 659 | 76,020.09 | 8.73 | 49.81 | 93.38 | −0.033 | cytomembrane |
| SmCNGC15b | Smechr0600577.1 | 696 | 80,257.66 | 9.24 | 50.58 | 91.18 | −0.19 | cytomembrane |
| SmCNGC15c | Smechr1201893.1 | 704 | 80,655.83 | 9.26 | 53.81 | 88.82 | −0.217 | cytomembrane |
| SmCNGC16 | Smechr0302884.1 | 674 | 77,929.8 | 8.31 | 47.48 | 89.39 | −0.157 | cytomembrane |
| SmCNGC17 | Smechr0700014.1 | 720 | 83,011.82 | 9.32 | 41.97 | 93.74 | −0.174 | cytomembrane |
| SmCNGC18 | Smechr0203033.1 | 689 | 79,438.12 | 7.17 | 49.98 | 88.78 | −0.15 | cytomembrane |
| SmCNGC19 | Smechr0302451.1 | 840 | 95,490.13 | 6.12 | 39.05 | 97.96 | −0.151 | cytomembrane |
| SmCNGC20 | Smechr0302478.1 | 771 | 88,894.76 | 9.32 | 49.87 | 89.29 | −0.191 | cytomembrane |
| SmCNGC21 | Smechr0500154.1 | 827 | 94,588.85 | 6.62 | 39.78 | 94.79 | −0.13 | cytomembrane |
| SmCNGC22 | Smechr0103753.1 | 629 | 72,010.19 | 8.16 | 44.82 | 99.63 | −0.018 | nucleus |
| SmCNGC23 | Smechr1200076.1 | 884 | 99,814.77 | 6.47 | 39.65 | 96.24 | −0.111 | chloroplast |
| SmCNGC24 | Smechr0100701.1 | 837 | 95,413.1 | 6.61 | 33.24 | 95.97 | −0.13 | cytomembrane |
| Website | URL |
|---|---|
| Eggplant Genome Database | http://eggplant-hq.cn/Eggplant/home/index (accessed on 3 March 2023) |
| InterPro protein family database | https://www.ebi.ac.uk/interpro/ (accessed on 3 March 2023) |
| NCBI CDD | https://www.ncbi.nlm.nih.gov/cdd/ (accessed on 3 March 2023) |
| Expasy ProtParam tool | https://web.expasy.org/protparam/ (accessed on 7 April 2023) |
| WoLF PSORT subcellular localization prediction tool | https://wolfpsort.hgc.jp/ (accessed on 7 April 2023) |
| TAIR | https://www.arabidopsis.org/ (accessed on 16 March 2023) |
| Tomato genome database | https://solgenomics.net/ (accessed on 16 March 2023) |
| Evolview v3 | https://www.evolgenius.info/evolview/ (accessed on 16 March 2023) |
| MEME | http://memesuite.org/tools/meme/ (accessed on 30 March 2023) |
| MapGene2Chromosome v2.1 | http://mg2c.iask.in/mg2c_v2.1/ (accessed on 30 March 2023) |
| PlantCARE | http://bioinformatics.psb.ugent.be/webtools/plantcare/html/ (accessed on 30 March 2023) |
| Gene Name | Gene ID | Forward Primer (5’ –> 3’) | Reverse Primer (5’ –> 3’) |
|---|---|---|---|
| SmCNGC1a | Smechr0500088.1 | AACCAACGTTTAGCTCGTTGA | TAGAGGATGCATGCGAATTG |
| SmCNGC1b | Smechr0101357.1 | AAAGCCACCAATCTGCTCAT | AGGAAAGGGATGCACATTGA |
| SmCNGC1c | Smechr0302178.1 | CGGCAAATTTGGAGTGTTCT | TTTGGCCAGAAGGCAACTTA |
| SmCNGC2 | Smechr0202893.1 | CAACCTGATAACAGCGACGA | TCACAACTGGTGGAATGGAA |
| SmCNGC3 | Smechr0400998.1 | GGAAGTGAAATATTCATCATATGGTTT | CCACCTCTCTCACCGTACCT |
| SmCNGC4a | Smechr1000491.1 | GGACAAGGATGTGGATGAGG | ACACGACCACGACCACTACA |
| SmCNGC4b | Smechr0400207.1 | GCTCGAGTGATCTGATTGTTGA | TCCAAAATAAGTGATACCGATCC |
| SmCNGC5 | Smechr1200261.1 | TTGTTGATCTTTTGAGCTTTGC | TTTACACAATCGGTGTATATAAAACTC |
| SmCNGC6 | Smechr1100117.1 | TGGAGGTCGAGCAGAGTATG | TTTGCCGGCTAATTTTTCTC |
| SmCNGC7 | Smechr0700086.1 | GAGTCGAGTTTGAGGGCTTG | TCGCAGTCTTGCTGATGAAC |
| SmCNGC8 | Smechr0303133.1 | TTGGAGGGCAAAAAGAAAAG | TGGTTACATGCCCACCAGTA |
| SmCNGC9 | Smechr0801568.1 | CTGAAGGATCTGGATTCTTTGC | TCATCTTGACATCTTAACTTATGGA |
| SmCNGC10 | Smechr0800622.1 | GGACATGGAAAGCAAACCAA | CGTCCACAACTTTCACCTTC |
| SmCNGC11 | Smechr0801458.1 | ATTGCTTGTGGACAATGGTG | TCACCTCCATACCGGATGAT |
| SmCNGC12 | Smechr0201396.1 | GGGATTTGGAGGTTTTGGTT | TGTCCATCACCTTTCTCTTGC |
| SmCNGC13 | Smechr0900702.1 | TTTCAGAAATGTATCTGATTGACC | CTCAATGACTAGAATTCCGCTGT |
| SmCNGC14 | Smechr0801673.1 | AGCTGGCCAAAGAACTTTACA | TGTTGATCATCCTCGGGTTC |
| SmCNGC15a | Smechr0900248.1 | CCTCGAGGAGGTCCTATAAACA | CCATGGGATAACTTGCATCC |
| SmCNGC15b | Smechr0600577.1 | CAGTTGTAACTTGTAAGATAAGATGGA | ATGGCACAAAAGCTGCAGTA |
| SmCNGC15c | Smechr1201893.1 | CAAATGTGGAAGGGTGTTTT | GTTTCTCTTCCCCCTCTTGC |
| SmCNGC16 | Smechr0302884.1 | CAGGGAAAGTCGTTTTGGAA | GGAAGCAGCAAAAACAGAGG |
| SmCNGC17 | Smechr0700014.1 | TGGGAGGAAAAGCAGACAGT | CCTTTTTAGGCCTCCCAAAC |
| SmCNGC18 | Smechr0203033.1 | GGTGGCGTCAGATTTTTGAT | TGACGAAAGGGACGAAGAAG |
| SmCNGC19 | Smechr0302451.1 | GCCAAAGAAGTTCAGGCAGA | AGTAATTCCGCAGCCATTTG |
| SmCNGC20 | Smechr0302478.1 | TTGGTCGAGAGCCTGAGAAT | TACGCCAACCATTTCGTTCT |
| SmCNGC21 | Smechr0500154.1 | AATCGTCGAGAAGCAGCAGT | GAGGCCATTGATGACGTTTT |
| SmCNGC22 | Smechr0103753.1 | AAAAACAGAGGAAACAAATATAATGAA | TGCTATCATGTTCATCTCATTACCA |
| SmCNGC23 | Smechr1200076.1 | TGGAGCAGCACAAGAAATTG | TTGCCGATCATAAGGTGAAA |
| SmCNGC24 | Smechr0100701.1 | TGCAAATGAGCCATTCATACA | TGCTACTCCCATGGCTATCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Du, L.; Shen, L.; He, J.; Xia, X.; Zhang, L.; Yang, X. Genome-Wide Exploration and Expression Analysis of the CNGC Gene Family in Eggplant (Solanum melongena L.) under Cold Stress, with Functional Characterization of SmCNGC1a. Int. J. Mol. Sci. 2023, 24, 13049. https://doi.org/10.3390/ijms241713049
Jiang Z, Du L, Shen L, He J, Xia X, Zhang L, Yang X. Genome-Wide Exploration and Expression Analysis of the CNGC Gene Family in Eggplant (Solanum melongena L.) under Cold Stress, with Functional Characterization of SmCNGC1a. International Journal of Molecular Sciences. 2023; 24(17):13049. https://doi.org/10.3390/ijms241713049
Chicago/Turabian StyleJiang, Zheng, Lihui Du, Lei Shen, Jie He, Xin Xia, Longhao Zhang, and Xu Yang. 2023. "Genome-Wide Exploration and Expression Analysis of the CNGC Gene Family in Eggplant (Solanum melongena L.) under Cold Stress, with Functional Characterization of SmCNGC1a" International Journal of Molecular Sciences 24, no. 17: 13049. https://doi.org/10.3390/ijms241713049





