Gasdermin D (GSDMD) Is Upregulated in Psoriatic Skin—A New Potential Link in the Pathogenesis of Psoriasis
Abstract
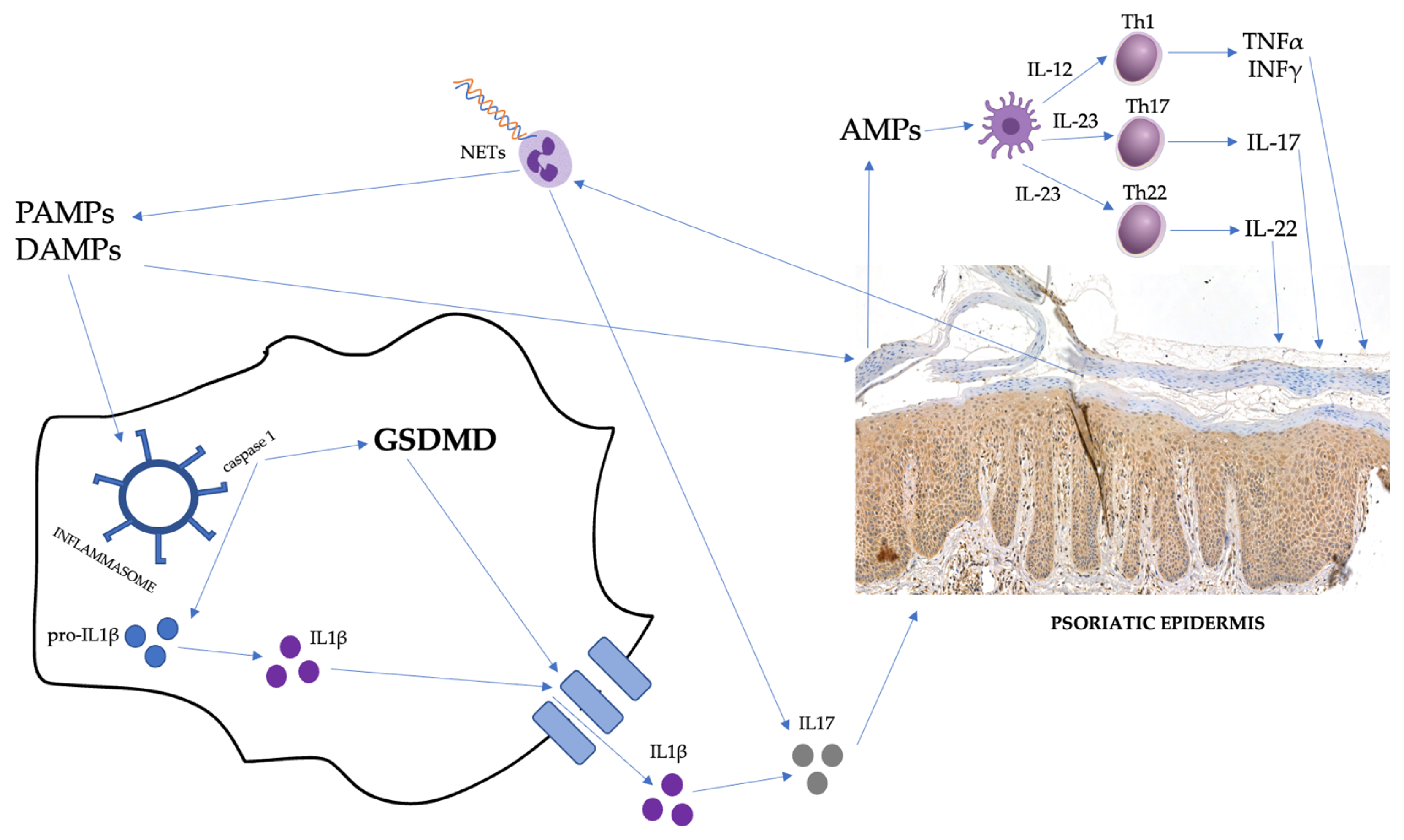
:1. Introduction
2. Results
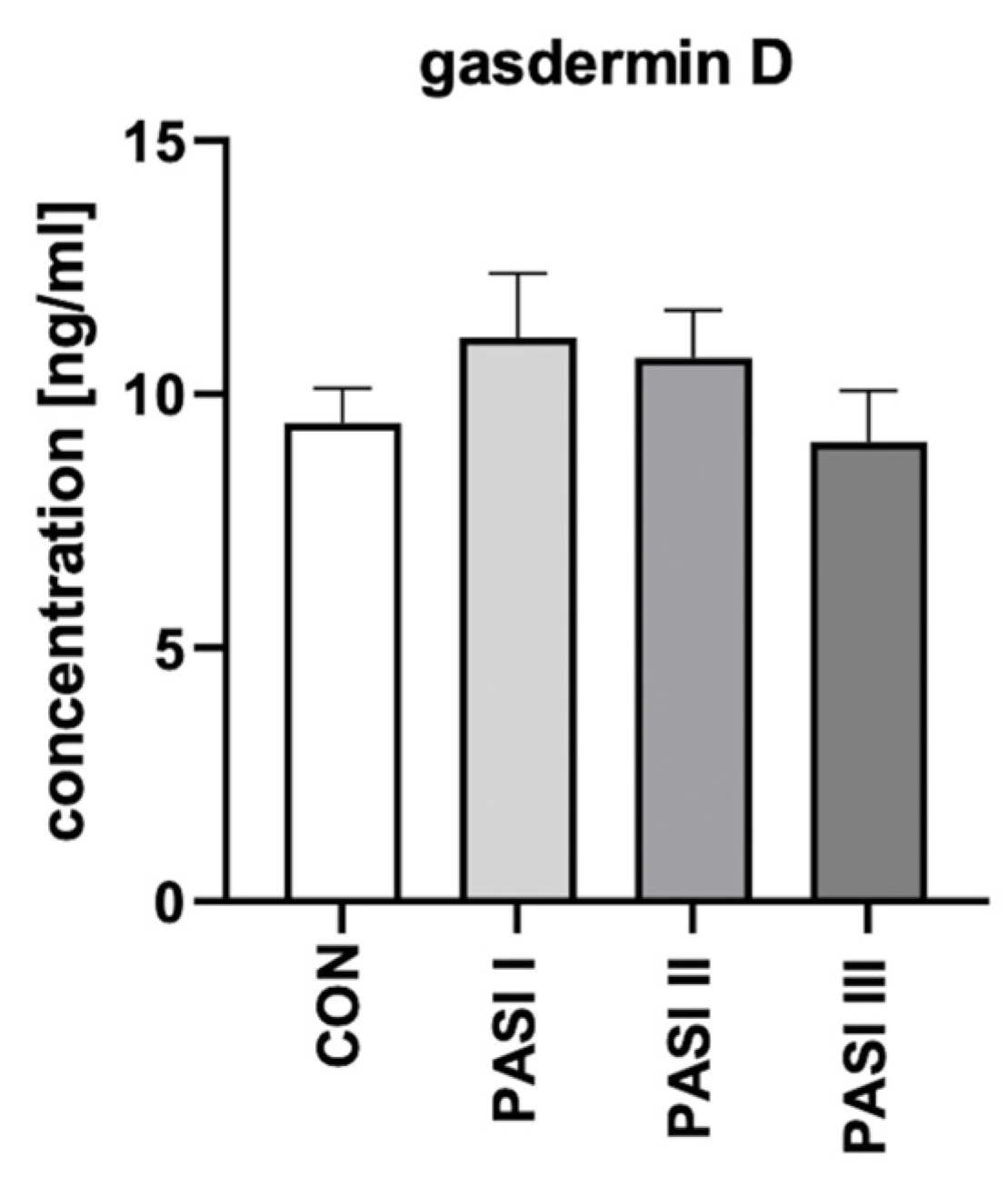
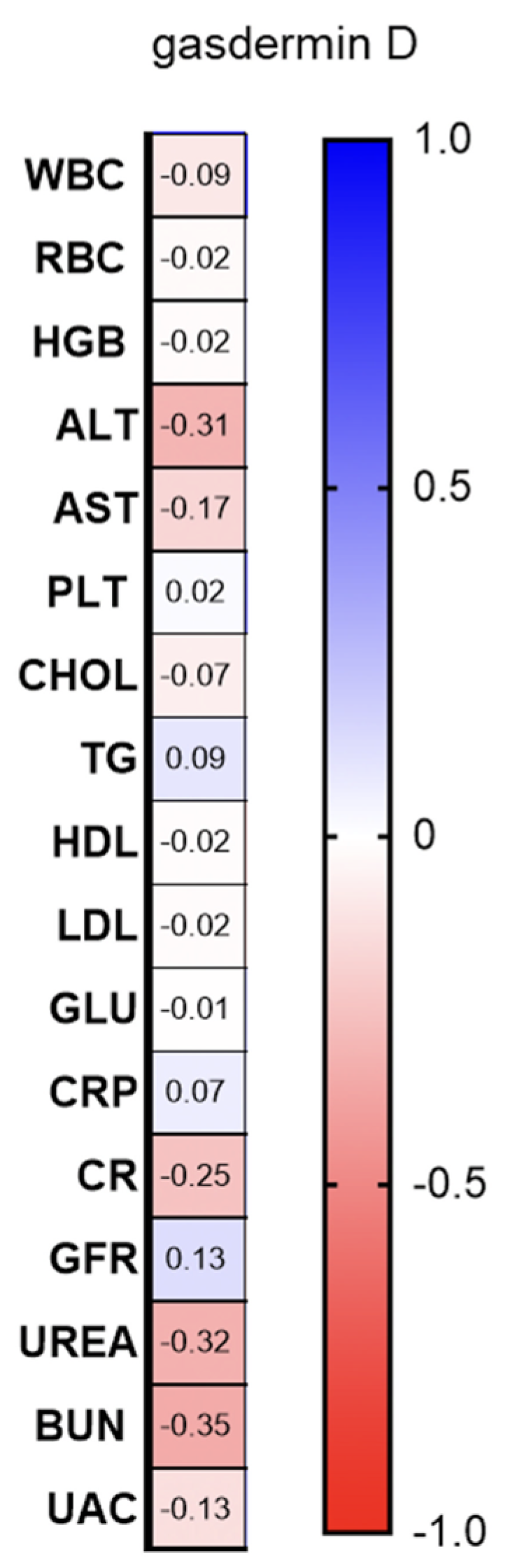
2.1. Serum and Urine
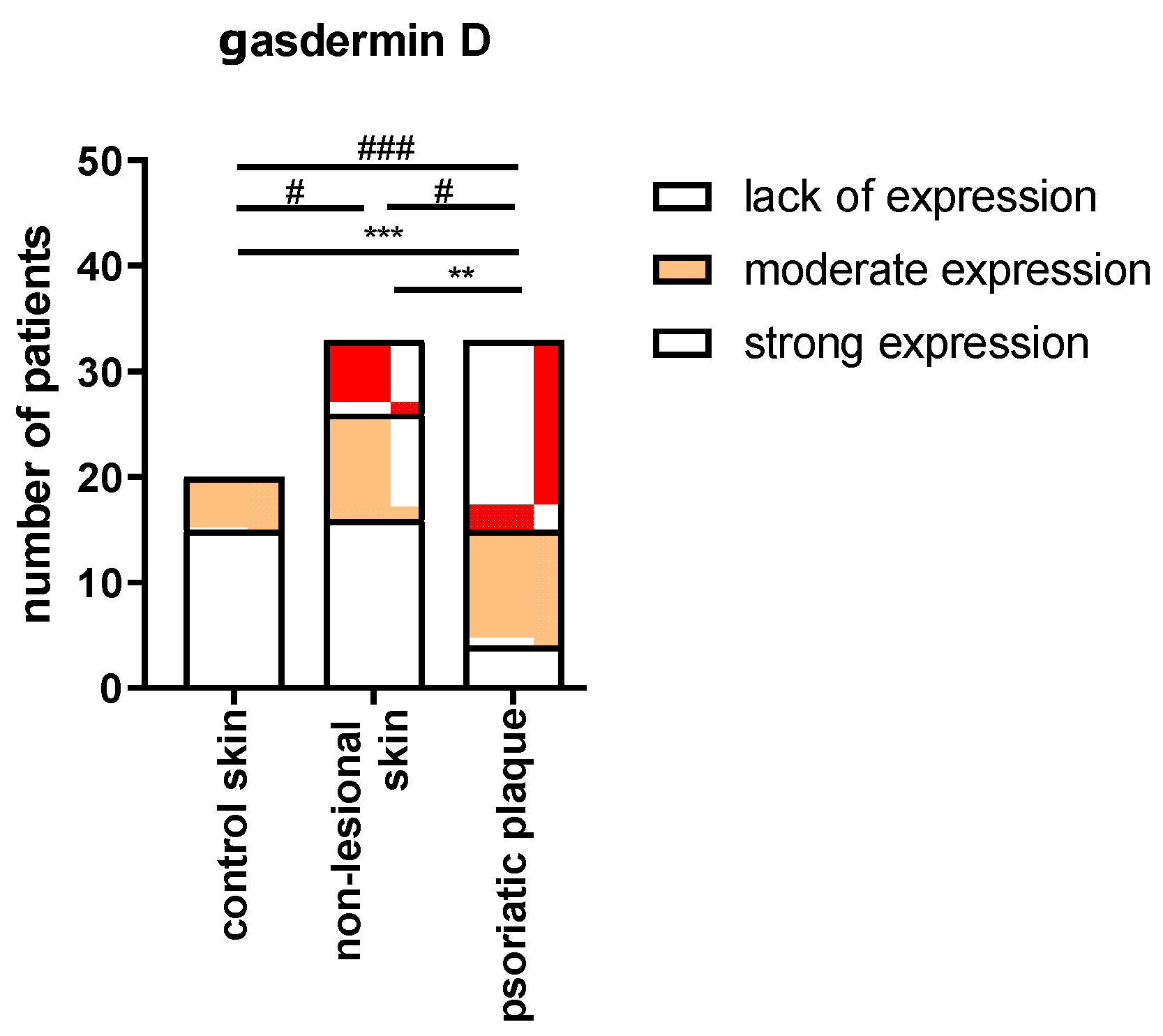
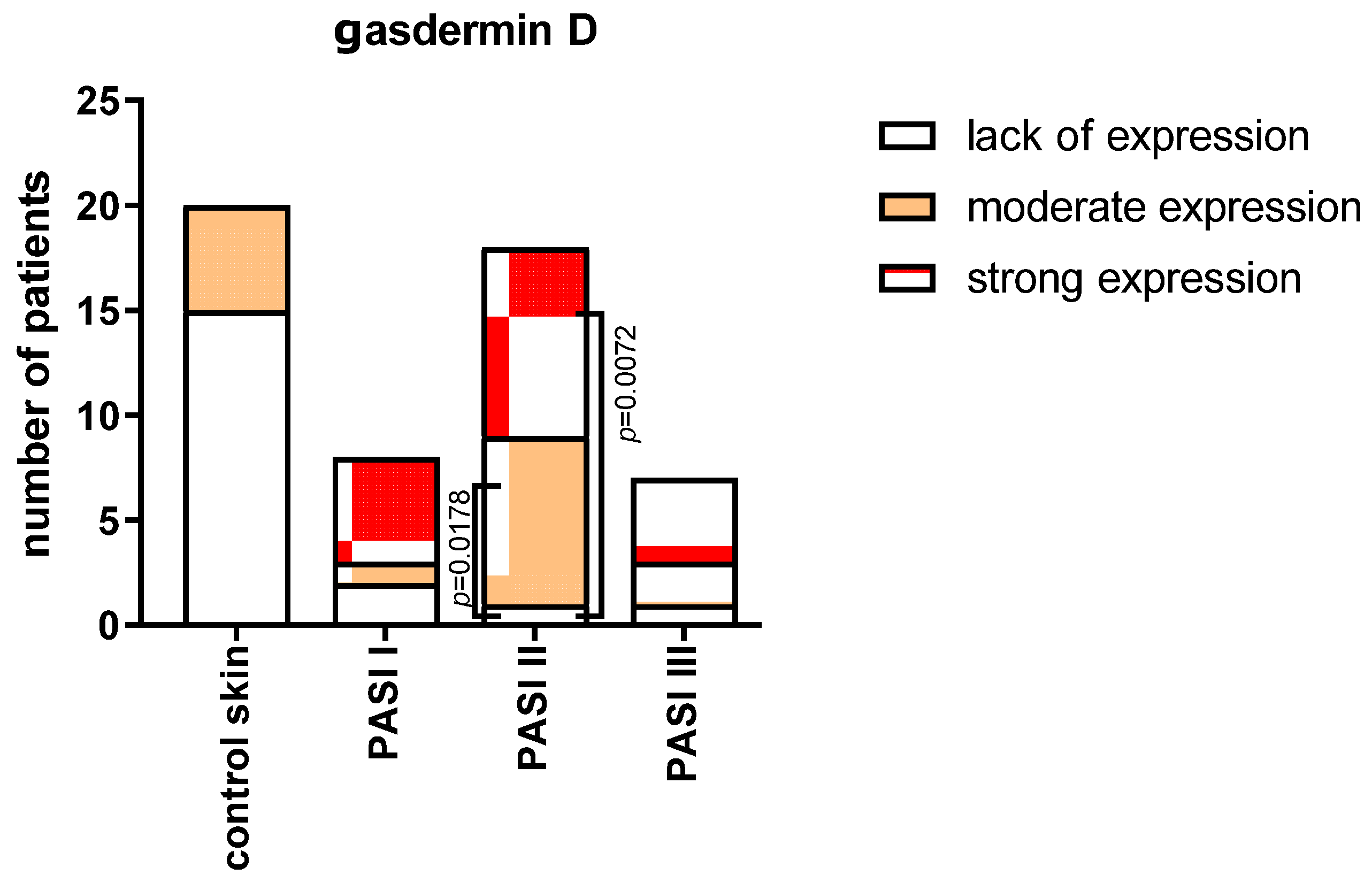
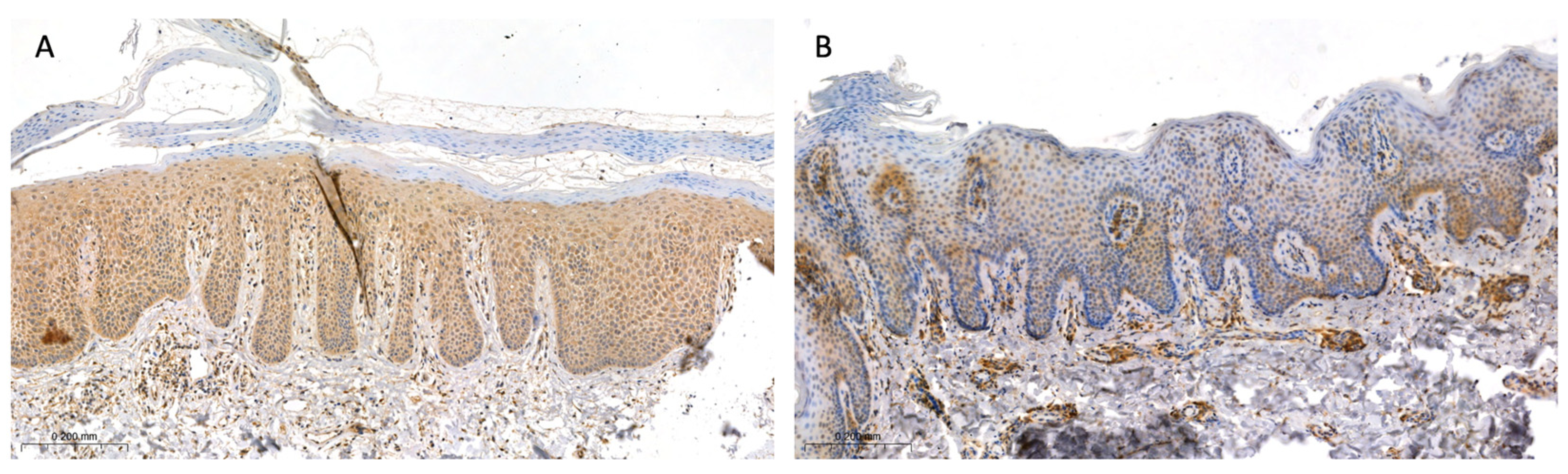
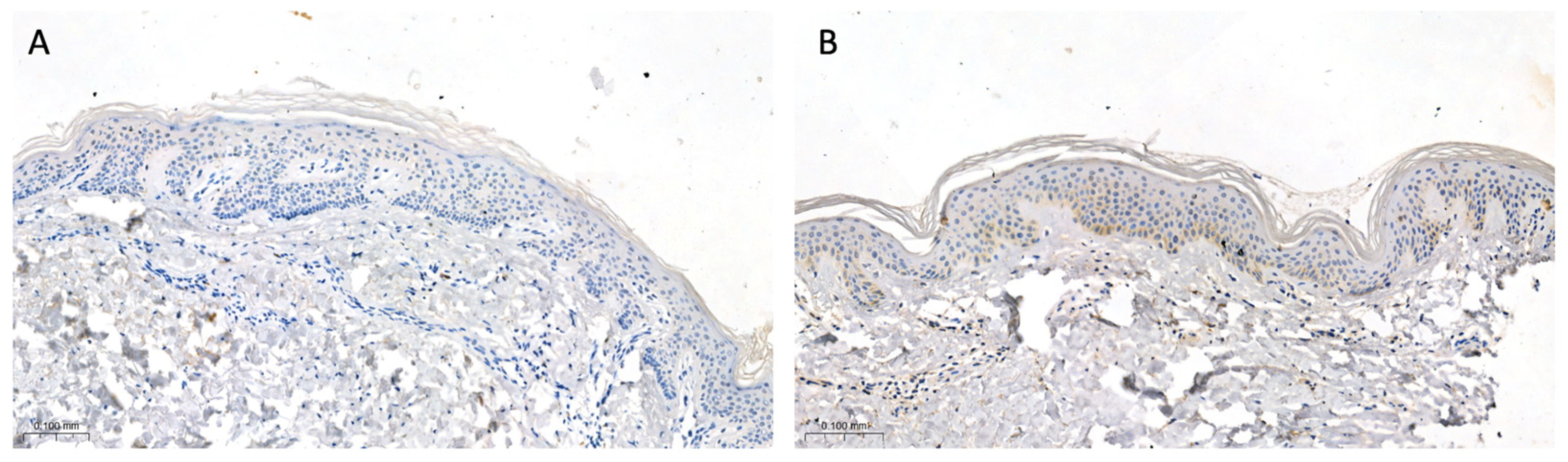
2.2. Tissue
3. Discussion
4. Materials and Methods
4.1. Serum and Urine
4.2. Tissue Samples
4.3. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Psoriasis Statistic. National Psoriasis Foundation. Available online: https://www.psoriasis.org/psoriasis-statistics/ (accessed on 25 July 2023).
- Wu, J.J.; Suryavanshi, M.; Davidson, D.; Patel, V.; Jain, A.; Seigel, L. Economic Burden of Comorbidities in Patients with Psoriasis in the USA. Dermatol. Ther. 2023, 13, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Hirotsu, C.; Rydlewski, M.; Araújo, M.S.; Tufik, S.; Andersen, M.L. Sleep loss and cytokines levels in an experimental model of psoriasis. PLoS ONE. 2012, 7, e51183. [Google Scholar] [CrossRef] [PubMed]
- Lanna, C.; Mancini, M.; Gaziano, R.; Cannizzaro, M.V.; Galluzzo, M.; Talamonti, M.; Rovella, V.; Annicchiarico-Petruzzelli, M.; Melino, G.; Wang, Y.; et al. Skin immunity and its dysregulation in psoriasis. Cell Cycle 2019, 18, 2581–2589. [Google Scholar] [CrossRef]
- Grän, F.; Kerstan, A.; Serfling, E.; Goebeler, M.; Muhammad, K. Current Developments in the Immunology of Psoriasis. Yale J. Biol. Med. 2020, 93, 97–110. [Google Scholar] [PubMed]
- De Alcantara, C.C.; Reiche, E.M.V.; Simão, A.N.C. Cytokines in psoriasis. Adv. Clin. Chem. 2021, 100, 171–204. [Google Scholar]
- Ciążyńska, M.; Olejniczak-Staruch, I.; Sobolewska-Sztychny, D.; Narbutt, J.; Skibińska, M.; Lesiak, A. The Role of NLRP1, NLRP3, and AIM2 Inflammasomes in Psoriasis: Review. Int. J. Mol. Sci. 2021, 22, 5898. [Google Scholar] [CrossRef]
- Jang, D.-i.; Lee, A.-H.; Shin, H.-Y.; Song, H.-R.; Park, J.-H.; Kang, T.-B.; Lee, S.-R.; Yang, S.-H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.; Du, C.; Sang, L.; Liu, L.; Li, Y.; Wang, F.; Fan, W.; Tang, P.; Zhang, S.; et al. Evidence of pyroptosis and ferroptosis extensively involved in autoimmune diseases at the single-cell transcriptome level. J. Transl. Med. 2022, 20, 363. [Google Scholar] [CrossRef]
- Lowes, M.A.; Suárez-Fariñas, M.; Krueger, J.G. Immunology of psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef]
- Bugaut, H.; Aractingi, S. Major Role of the IL17/23 Axis in Psoriasis Supports the Development of New Targeted Therapies. Front. Immunol. 2021, 12, 621956. [Google Scholar] [CrossRef]
- Ruggiero, A.; Picone, V.; Martora, F.; Fabbrocini, G.; Megna, M. Guselkumab, Risankizumab, and Tildrakizumab in the Management of Psoriasis: A Review of the Real-World Evidence. Clin. Cosmet. Investig. Dermatol. 2022, 15, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Vu, A.; Ulschmid, C.; Gordon, K.B. Anti-IL 23 biologics for the treatment of plaque psoriasis. Expert Opin. Biol. Ther. 2022, 22, 1489–1502. [Google Scholar] [CrossRef] [PubMed]
- Furue, K.; Ito, T.; Tsuji, G.; Kadono, T.; Furue, M. Psoriasis and the TNF/IL23/IL17 axis. G Ital. Dermatol. Venereol. 2019, 154, 418–424. [Google Scholar] [CrossRef]
- Marasca, C.; Fornaro, L.; Martora, F.; Picone, V.; Fabbrocini, G.; Megna, M. Onset of vitiligo in a psoriasis patient on ixekizumab. Dermatol. Ther. 2021, 34, e15102. [Google Scholar] [CrossRef] [PubMed]
- Pathmarajah, P.; Benjamin-Laing, Z.; Abdurrahman, M.; Grunova, A.; Sinclair, C. Generalized vitiligo in a psoriatic patient treated with ixekizumab. Dermatol. Ther. 2022, 35, e15872. [Google Scholar] [CrossRef]
- Țiburcă, L.; Bembea, M.; Zaha, D.C.; Jurca, A.D.; Vesa, C.M.; Rațiu, I.A.; Jurca, C.M. The Treatment with Interleukin 17 Inhibitors and Immune-Mediated Inflammatory Diseases. Curr. Issues Mol. Biol. 2022, 44, 1851–1866. [Google Scholar] [CrossRef]
- Kovacs, S.B.; Miao, E.A. Gasdermins: Effectors of Pyroptosis. Trends Cell Biol. 2017, 27, 673–684. [Google Scholar] [CrossRef]
- Zou, J.; Zheng, Y.; Huang, Y.; Tang, D.; Kang, R.; Chen, R. The Versatile Gasdermin Family: Their Function and Roles in Diseases. Front. Immunol. 2021, 12, 751533. [Google Scholar] [CrossRef]
- Kuc-Ciepluch, D.; Ciepluch, K.; Arabski, M. Gasdermin family proteins as a permeabilization factor of cell membrane in pyroptosis process. Postepy. Hig. Med. Dosw. 2021, 75, 337–344. [Google Scholar] [CrossRef]
- Qiu, S.; Liu, J.; Xing, F. ‘Hints’ in the killer protein gasdermin D: Unveiling the secrets of gasdermins driving cell death. Cell Death Differ. 2017, 24, 588–596. [Google Scholar] [CrossRef]
- Rogers, C.; Alnemri, E.S. Gasdermins in Apoptosis: New players in an Old Game. Yale J. Biol. Med. 2019, 92, 603–617. [Google Scholar] [PubMed]
- Pandeya, A.; Li, L.; Li, Z.; Wei, Y. Gasdermin D (GSDMD) as a new target for the treatment of infection. Medchemcomm 2019, 10, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Dai, S.; Zhong, L.; Shi, X.; Fan, X.; Zhong, X.; Lin, W.; Su, L.; Lin, S.; Han, B.; et al. GSDMD (Gasdermin D) Mediates Pathological Cardiac Hypertrophy and Generates a Feed-Forward Amplification Cascade via Mitochondria-STING (Stimulator of Interferon Genes) Axis. Hypertension 2022, 79, 2505–2518. [Google Scholar] [CrossRef]
- Jiang, M.; Sun, X.; Liu, S.; Tang, Y.; Shi, Y.; Bai, Y.; Wang, Y.; Yang, Q.; Yang, Q.; Jiang, W.; et al. Caspase-11-Gasdermin D-Mediated Pyroptosis Is Involved in the Pathogenesis of Atherosclerosis. Front. Pharmacol. 2021, 12, 657486. [Google Scholar] [CrossRef]
- Miao, N.; Yin, F.; Xie, H.; Wang, Y.; Xu, Y.; Shen, Y.; Xu, D.; Yin, J.; Wang, B.; Zhou, Z.; et al. The cleavage of gasdermin D by caspase-11 promotes tubular epithelial cell pyroptosis and urinary IL-18 excretion in acute kidney injury. Kidney Int. 2019, 96, 1105–1120. [Google Scholar] [CrossRef]
- El-Gamal, R.; Abdelrahim, M.; El-Sherbiny, M.; Enan, E.T.; El-Nablaway, M. Gasdermin D: A potential mediator and prognostic marker of bladder cancer. Front. Mol. Biosci. 2022, 9, 972087. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Jiang, M.; Chu, Y.; Wang, W.; Chen, D.; Li, X.; Zhang, Z.; Zhang, D.; Fan, D.; Nie, Y.; et al. Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice. J. Hepatol. 2018, 68, 773–782. [Google Scholar] [CrossRef]
- Kenealy, S.; Manils, J.; Raverdeau, M.; Munoz-Wolf, N.; Barber, G.; Liddicoat, A.; Lavelle, E.C.; Creagh, E.M. Caspase-11-Mediated Cell Death Contributes to the Patho-genesis of Imiquimod-Induced Psoriasis. J. Investig. Dermatol. 2019, 139, 2389–2393.e3. [Google Scholar] [CrossRef]
- Bauernfeind, F.; Niepmann, S.; Knolle, P.A.; Hornung, V. Aging-Associated TNF Production Primes Inflammasome Activation and NLRP3-Related Metabolic Disturbances. J. Immunol. 2016, 197, 2900–2908. [Google Scholar] [CrossRef]
- Hu, S.C.; Yu, H.S.; Yen, F.L.; Lin, C.L.; Chen, G.S.; Lan, C.C. Neutrophil extracellular trap formation is increased in psoriasis and induces human β-defensin-2 production in epidermal keratinocytes. Sci. Rep. 2016, 6, 31119. [Google Scholar] [CrossRef]
- Bellinato, F.; Gisondi, P.; Mantovani, A.; Girolomoni, G.; Targher, G. Risk of non-alcoholic fatty liver disease in patients with chronic plaque psoriasis: An updated systematic review and meta-analysis of observational studies. J. Endocrinol. Investig. 2022, 45, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Antonio, I.; López-Sánchez, G.N.; Uribe, M.; Chávez-Tapia, N.C.; Nuño-Lámbarri, N. Role of the inflammasome, gasdermin D, and pyroptosis in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2021, 36, 2720–2727. [Google Scholar] [CrossRef] [PubMed]
- Helsinki Declaration. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 1 December 2022).

| Parameter | Controls (n = 30) | Psoriatic Patients (n = 60) |
|---|---|---|
| Sex (M/F) | 20/10 | 39/21 NS |
| Age (years) | 48 ± 2.45 | 50 ± 2.34 NS |
| Height (cm) | 1.75 (1.5–1.9) | 1.71 (1.5–1.9) NS |
| Weight (kg) | 78.40 ± 2.9 | 85.43 ± 2.5 NS |
| BMI | 25.85 ± 0.77 | 27.85 ± 0.64 NS |
| Parameter | Controls (n = 20) | Psoriatic Patients (n = 33) |
|---|---|---|
| Sex (M/F) | 7/13 | 21/12 NS |
| Age (years) | 48 ± 3.0 | 47 ± 3.3 NS |
| Height (cm) | 1.72 ± 0.01 | 1.72 ± 0.01 NS |
| Weight (kg) | 76.0 ± 3.2 | 85.28 ± 3.1 NS |
| BMI ratio | 25.31 ± 0.7 | 27.42 ± 0.7 NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowowiejska, J.; Baran, A.; Hermanowicz, J.M.; Pryczynicz, A.; Sieklucka, B.; Pawlak, D.; Flisiak, I. Gasdermin D (GSDMD) Is Upregulated in Psoriatic Skin—A New Potential Link in the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2023, 24, 13047. https://doi.org/10.3390/ijms241713047
Nowowiejska J, Baran A, Hermanowicz JM, Pryczynicz A, Sieklucka B, Pawlak D, Flisiak I. Gasdermin D (GSDMD) Is Upregulated in Psoriatic Skin—A New Potential Link in the Pathogenesis of Psoriasis. International Journal of Molecular Sciences. 2023; 24(17):13047. https://doi.org/10.3390/ijms241713047
Chicago/Turabian StyleNowowiejska, Julia, Anna Baran, Justyna Magdalena Hermanowicz, Anna Pryczynicz, Beata Sieklucka, Dariusz Pawlak, and Iwona Flisiak. 2023. "Gasdermin D (GSDMD) Is Upregulated in Psoriatic Skin—A New Potential Link in the Pathogenesis of Psoriasis" International Journal of Molecular Sciences 24, no. 17: 13047. https://doi.org/10.3390/ijms241713047
APA StyleNowowiejska, J., Baran, A., Hermanowicz, J. M., Pryczynicz, A., Sieklucka, B., Pawlak, D., & Flisiak, I. (2023). Gasdermin D (GSDMD) Is Upregulated in Psoriatic Skin—A New Potential Link in the Pathogenesis of Psoriasis. International Journal of Molecular Sciences, 24(17), 13047. https://doi.org/10.3390/ijms241713047










