Macrophage-Derived Chemokine MDC/CCL22: An Ambiguous Finding in COVID-19
Abstract
:1. Introduction
2. Macrophage-Derived Chemokine (MDC/CCL22) and Its Functions
2.1. Characteristics of MDC/CCL22
2.2. Changes in MDC/CCL22 Concentrations in COVID-19 In Vitro and In Vivo
| Study | MDC (Me) in COVID-19 Patients | MDC (Me) in COVID-19 Convalescents | MDC (Me) in Healthy Donors | COVID-19 vs. Healthy Donors (p-Value) | COVID-19 vs. Convalescents (p-Value) | Convalescents vs. Healthy Donors (p-Value) |
|---|---|---|---|---|---|---|
| Arsentieva et al. [21] | 872.7 pg/mL | 653.5 pg/mL | 1155.0 pg/mL | N.A. | p < 0.05 | p < 0.0001 |
| Arsentieva et al. [22] | 254.0 pg/mL for survivors 230.7 pg/mL for non-survivors | 557.3 pg/mL | N.A. | p < 0.05 for survivors p < 0.01 for non survivors | N.A. | N.A. |
| Korobova et al. [23] | 629.8 pg/mL for Wuhan strain 474.1 pg/mL for Alpha variant 344.1 pg/mL for Delta variant 306.1 pg/mL for Omicron variant | N.A. | N.A. | p = 0.0005 for Wuhan strain p = 0.0067 for Alpha variant p < 0.0001 for Delta variant p < 0.0001 for Omicron variant | N.A. | N.A. |
| Tufa et al. [28] | 438.9 ng/L | N.A. | 725.7 ng/L | p < 0.0001 | N.A. | N.A. |
| Ling et al. [29] | Mild—724.9; Moderate—495.44; Critical—399.2 pg/mL | Mild—644.8; Moderate—6483; Critical—220.3 pg/mL | N.A. | N.A. | p ≤ 0.001 (for critical) | N.A. |
2.3. Macrophage-Derived Chemokine MDC/CCL22 in Various Pathologies
2.3.1. MDC/CCL22 in Oncology of Non-Respiratory Organs
2.3.2. Macrophage-Derived Chemokine MDC/CCL22 in Autoimmunity and Atopic Diseases
2.3.3. Macrophage-Derived Chemokine MDC/CCL22 in Respiratory Disease
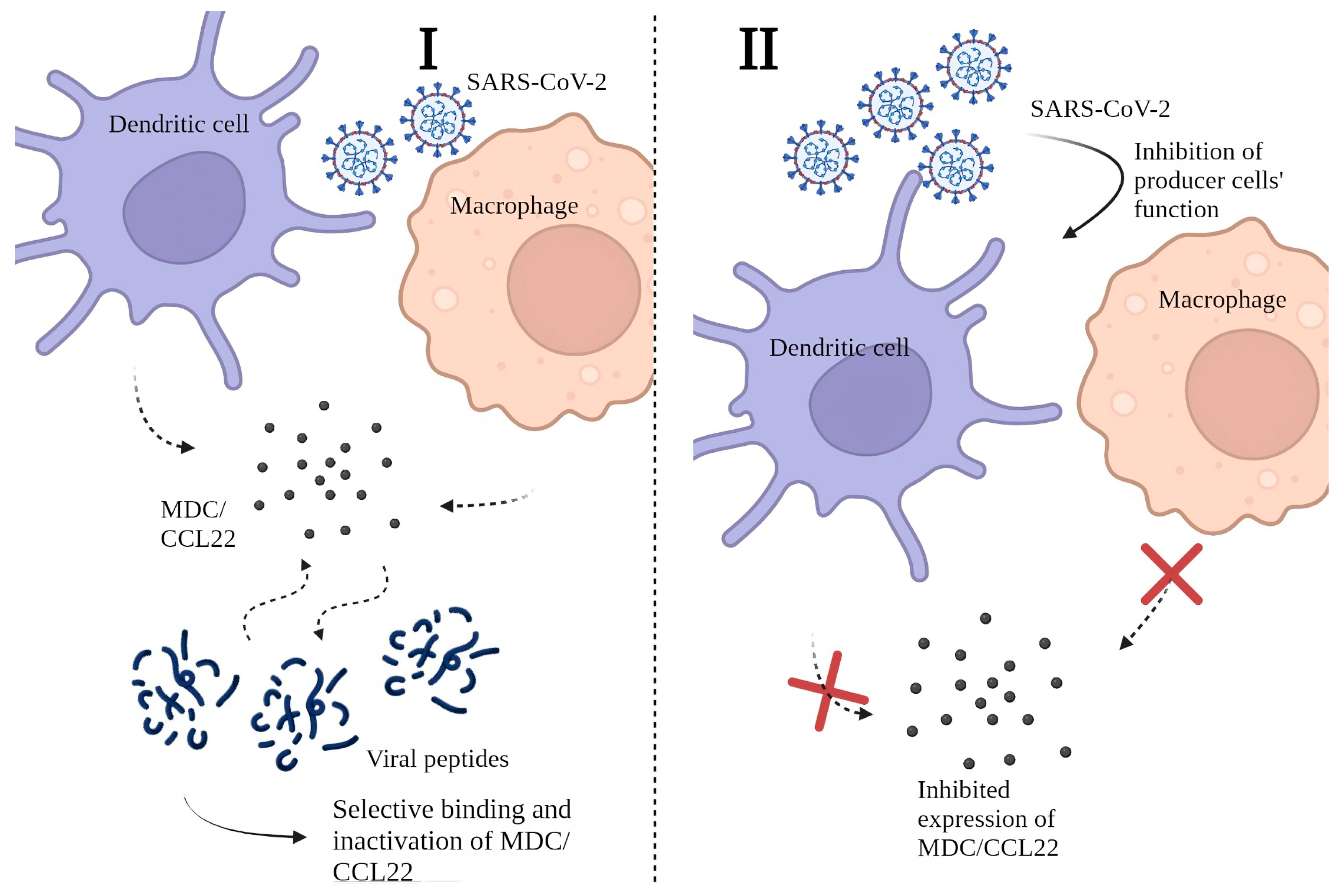
2.4. Possible Mechanisms behind the Decrease in MDC/CCL22 Concentrations in COVID-19
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.-C.; Kuo, R.-L.; Shih, S.-R. COVID-19: The first documented coronavirus pandemic in history. Biomed. J. 2020, 43, 328–333. [Google Scholar] [CrossRef]
- Borczuk, A.C.; Yantiss, R.K. The pathogenesis of coronavirus-19 disease. J. Biomed. Sci. 2022, 29, 87. [Google Scholar] [CrossRef] [PubMed]
- Borges do Nascimento, I.J.; O’Mathúna, D.P.; von Groote, T.C.; Abdulazeem, H.M.; Weerasekara, I.; Marusic, A.; Puljak, L.; Civile, V.T.; Zakarija-Grkovic, I.; Pericic, T.P.; et al. Coronavirus disease (COVID-19) pandemic: An overview of Systematic Reviews. BMC Infect. Dis. 2021, 21, 525. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Blish, C.A.; Sallusto, F.; Iwasaki, A. The immunology and immunopathology of COVID-19. Science 2022, 375, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Salamanna, F.; Maglio, M.; Landini, M.P.; Fini, M. Body Localization of ACE-2: On the Trail of the Keyhole of SARS-CoV-2. Front. Med. 2020, 7, 594495. [Google Scholar] [CrossRef]
- Shirbhate, E.; Pandey, J.; Patel, V.K.; Kamal, M.; Jawaid, T.; Gorain, B.; Kesharwani, P.; Rajak, H. Understanding the role of ACE-2 receptor in pathogenesis of COVID-19 disease: A potential approach for therapeutic intervention. Pharmacol. Rep. 2021, 73, 1539–1550. [Google Scholar] [CrossRef]
- Xu, J.; Lazartigues, E. Expression of ACE2 in Human Neurons Supports the Neuro-Invasive Potential of COVID-19 Virus. Cell. Mol. Neurobiol. 2022, 42, 305–309. [Google Scholar] [CrossRef]
- Boechat, J.; Chora, I.; Morais, A.; Delgado, L. The immune response to SARS-CoV-2 and COVID-19 immunopathology—Current perspectives. Pulmonology 2021, 27, 423–437. [Google Scholar] [CrossRef]
- Rodriguez, L.; Brodin, P. Unraveling the Immune Response in Severe COVID-19. J. Clin. Immunol. 2020, 40, 958–959. [Google Scholar] [CrossRef]
- Stanevich, O.V.; Alekseeva, E.I.; Sergeeva, M.; Fadeev, A.V.; Komissarova, K.S.; Ivanova, A.A.; Simakova, T.S.; Vasilyev, K.A.; Shurygina, A.-P.; Stukova, M.A.; et al. SARS-COV-2 escape from cytotoxic T cells during long-term COVID-19. Nat. Commun. 2023, 14, 149. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2021, 50, D439–D444. [Google Scholar] [CrossRef] [PubMed]
- Godiska, R.; Chantry, D.; Raport, C.J.; Sozzani, S.; Allavena, P.; Leviten, D.; Mantovani, A.; Gray, P.W. Human macrophage-derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J. Exp. Med. 1997, 185, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Chantry, D.; Romagnani, P.; Raport, C.J.; Wood, C.L.; Epp, A.; Gray, P.W. Macrophage-derived chemokine is localized to thymic medullary epithelial cells and is a chemoattractant for CD3+, CD4+, CD8low thymocytes. Blood 1999, 94, 1890–1898. [Google Scholar] [CrossRef] [PubMed]
- Andrew, D.P.; Chang, M.S.; McNinch, J.; Wathen, S.T.; Rihanek, M.; Tseng, J.; Spellberg, J.P.; Elias, C.G., III. STCP-1 (MDC) CC chemokine acts specifically on chronically activated Th2 lymphocytes and is produced by monocytes on stimulation with Th2 cytokines IL-4 and IL-13. J. Immunol. 1998, 161, 5027–5038. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Liu, C.; Su, L.; Zhang, D.; Fan, J.; Yang, Y.; Xiao, M.; Xie, J.; Xu, Y.; et al. IP-10 and MCP-1 as biomarkers associated with disease severity of COVID-19. Mol. Med. 2020, 26, 97. [Google Scholar] [CrossRef]
- Grishaeva, A.; Ponezheva, Z.; Chanyshev, M.; Ploskireva, A.; Usenko, D.; Tsvetkova, N.; Omarova, K.; Pshenichnaya, N. MIP-1a and MIP-1b in serum as potential markers of the severe course COVID-19. Int. J. Infect. Dis. 2022, 116, S44. [Google Scholar] [CrossRef]
- Pérez-García, F.; Martin-Vicente, M.; Rojas-García, R.L.; Castilla-García, L.; Muñoz-Gomez, M.J.; Fernández, I.H.; Ventosa, V.G.; Vidal-Alcántara, E.J.; Cuadros-González, J.; Bermejo-Martin, J.F.; et al. High SARS-CoV-2 Viral Load and Low CCL5 Expression Levels in the Upper Respiratory Tract Are Associated With COVID-19 Severity. J. Infect. Dis. 2022, 225, 977–982. [Google Scholar] [CrossRef]
- Imai, T.; Chantry, D.; Raport, C.J.; Wood, C.L.; Nishimura, M.; Godiska, R.; Yoshie, O.; Gray, P.W. Macrophage-derived chemokine is a functional ligand for the CC chemokine receptor 4. J. Biol. Chem. 1998, 273, 1764–1768. [Google Scholar] [CrossRef]
- Yamashita, U.; Kuroda, E. Regulation of macrophage-derived chemokine (MDC, CCL22) production. Crit. Rev. Immunol. 2002, 22, 105–114. [Google Scholar] [CrossRef]
- Arsentieva, N.A.; Liubimova, N.E.; Batsunov, O.K.; Korobova, Z.R.; Stanevich, O.V.; Lebedeva, A.A.; Vorobyov, E.A.; Vorobyova, S.V.; Kulikov, A.N.; Lioznov, D.A.; et al. Plasma cytokines in patients with COVID-19 during acute phase of the disease and following complete recovery. Med. Immunol. (Russ.) 2021, 23, 311–326. [Google Scholar] [CrossRef]
- Arsentieva, N.A.; Liubimova, N.E.; Batsunov, O.K.; Korobova, Z.R.; Kuznetsova, R.N.; Rubinstein, A.A.; Stanevich, O.V.; Lebedeva, A.A.; Vorobyov, E.A.; Vorobyova, S.V.; et al. Predictive value of specific cytokines for lethal COVID-19 outcome. Russ. J. Infect. Immun. 2022, 12, 859–868. [Google Scholar] [CrossRef]
- Korobova, Z.R.; Arsentieva, N.A.; Liubimova, N.E.; Batsunov, O.K.; Dedkov, V.G.; Gladkikh, A.S.; Sharova, A.A.; Adish, Z.; Chernykh, E.I.; Kaschenko, V.A.; et al. Cytokine Profiling in Different SARS-CoV-2 Genetic Variants. Int. J. Mol. Sci. 2022, 23, 14146. [Google Scholar] [CrossRef] [PubMed]
- Fergie, J.; Srivastava, A. Immunity to SARS-CoV-2: Lessons Learned. Front. Immunol. 2021, 12, 654165. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yang, J.; Zhou, L.; Xue, C.; Li, H.; Hu, W.; Liu, N. Inflammatory cytokine depletion in severe coronavirus disease 2019 infectious pneumonia: A case report. Medicine 2020, 99, e23449. [Google Scholar] [CrossRef]
- Kudryavtsev, I.V.; Arsentieva, N.A.; Korobova, Z.R.; Isakov, D.V.; Rubinstein, A.A.; Batsunov, O.K.; Khamitova, I.V.; Kuznetsova, R.N.; Savin, T.V.; Akisheva, T.V.; et al. Heterogenous CD8+ T Cell Maturation and ‘Polarization’ in Acute and Convalescent COVID-19 Patients. Viruses 2022, 14, 1906. [Google Scholar] [CrossRef] [PubMed]
- Wiech, M.; Chroscicki, P.; Swatler, J.; Stepnik, D.; De Biasi, S.; Hampel, M.; Brewinska-Olchowik, M.; Maliszewska, A.; Sklinda, K.; Durlik, M.; et al. Remodeling of T Cell Dynamics During Long COVID Is Dependent on Severity of SARS-CoV-2 Infection. Front. Immunol. 2022, 13, 886431. [Google Scholar] [CrossRef]
- Tufa, A.; Gebremariam, T.H.; Manyazewal, T.; Getinet, T.; Webb, D.-L.; Hellström, P.M.; Genet, S. Inflammatory mediators profile in patients hospitalized with COVID-19: A comparative study. Front. Immunol. 2022, 13, 964179. [Google Scholar] [CrossRef]
- Ling, L.; Chen, Z.; Lui, G.; Wong, C.K.; Wong, W.T.; Ng, R.W.Y.; Tso, E.Y.K.; Fung, K.S.C.; Chan, V.; Yeung, A.C.M.; et al. Longitudinal Cytokine Profile in Patients with Mild to Critical COVID-19. Front. Immunol. 2021, 12, 763292. [Google Scholar] [CrossRef]
- Varghese, J.; Sandmann, S.; Ochs, K.; Schrempf, I.-M.; Frömmel, C.; Dugas, M.; Schmidt, H.H.; Vollenberg, R.; Tepasse, P.-R. Persistent symptoms and lab abnormalities in patients who recovered from COVID-19. Sci. Rep. 2021, 11, 12775. [Google Scholar] [CrossRef]
- Vulcano, M.; Albanesi, C.; Stoppacciaro, A.; Bagnati, R.; D’Amico, G.; Struyf, S.; Transidico, P.; Bonecchi, R.; Del Prete, A.; Allavena, P.; et al. Dendritic cells as a major source of macrophage-derived chemokine/CCL22 in vitro and in vivo. Eur. J. Immunol. 2001, 31, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Larson, C.; Hammond, T.C.; Oronsky, A.; Kesari, S.; Lybeck, M.; Reid, T.R. A Review of Persistent Post-COVID Syndrome (PPCS). Clin. Rev. Allergy Immunol. 2021, 64, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.L.; Cyster, G.J. Chemokine up-regulation and activated T cell attraction by maturing dendritic cells. Science 1999, 284, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M.; et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004, 10, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Klarquist, J.; Tobin, K.; Oskuei, P.F.; Henning, S.W.; Fernandez, M.F.; Dellacecca, E.R.; Navarro, F.C.; Eby, J.M.; Chatterjee, S.; Mehrotra, S.; et al. Ccl22 Diverts T Regulatory Cells and Controls the Growth of Melanoma. Cancer Res. 2016, 76, 6230–6240. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Bracci, P.M.; McCoy, L.S.; Hsuang, G.; Wiemels, J.L.; Rice, T.; Zheng, S.; Kelsey, K.T.; Wrensch, M.R.; Wiencke, J.K. Serum macrophage-derived chemokine/CCL22 levels are associated with glioma risk, CD4 T cell lymphopenia and survival time. Int. J. Cancer 2015, 137, 826–836. [Google Scholar] [CrossRef]
- Gobert, M.; Treilleux, I.; Bendriss-Vermare, N.; Bachelot, T.; Goddard-Leon, S.; Arfi, V.; Biota, C.; Doffin, A.C.; Durand, I.; Olive, D.; et al. Regulatory T Cells Recruited through CCL22/CCR4 Are Selectively Activated in Lymphoid Infiltrates Surrounding Primary Breast Tumors and Lead to an Adverse Clinical Outcome. Cancer Res. 2009, 69, 2000–2009. [Google Scholar] [CrossRef]
- Anz, D.; Rapp, M.; Eiber, S.; Koelzer, V.H.; Thaler, R.; Haubner, S.; Knott, M.; Nagel, S.; Golic, M.; Wiedemann, G.M.; et al. Suppression of Intratumoral CCL22 by Type I Interferon Inhibits Migration of Regulatory T Cells and Blocks Cancer Progression. Cancer Res. 2015, 75, 4483–4493. [Google Scholar] [CrossRef]
- Furue, M.; Ulzii, D.; Vu, Y.H.; Tsuji, G.; Kido-Nakahara, M.; Nakahara, T. Pathogenesis of Atopic Dermatitis: Current Paradigm. Iran. J. Immunol. 2019, 16, 97–107. [Google Scholar] [CrossRef]
- Ushio, A.; Arakaki, R.; Otsuka, K.; Yamada, A.; Tsunematsu, T.; Kudo, Y.; Aota, K.; Azuma, M.; Ishimaru, N. CCL22-Producing Resident Macrophages Enhance T Cell Response in Sjögren’s Syndrome. Front. Immunol. 2018, 9, 2594. [Google Scholar] [CrossRef]
- Columba-Cabezas, S.; Serafini, B.; Ambrosini, E.; Sanchez, M.; Penna, G.; Adorini, L.; Aloisi, F. Induction of macrophage-derived chemokine/CCL22 expression in experimental autoimmune encephalomyelitis and cultured microglia: Implications for disease regulation. J. Neuroimmunol. 2002, 130, 10–21. [Google Scholar] [CrossRef]
- Fox, K.A.; Kirwan, D.E.; Whittington, A.M.; Krishnan, N.; Robertson, B.D.; Gilman, R.H.; López, J.W.; Singh, S.; Porter, J.C.; Friedland, J.S. Platelets Regulate Pulmonary Inflammation and Tissue Destruction in Tuberculosis. Am. J. Respir. Crit. Care Med. 2018, 198, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Imaizumi, K.; Hasegawa, Y.; Kawabe, T.; Hashimoto, N.; Okamoto, M.; Shimokata, K. Expression of macrophage-derived chemokine (MDC)/CCL22 in human lung cancer. Cancer Immunol. Immunother. 2006, 55, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.R.; Sutton, J.M.; Belizaire, R.M.; Friend, L.A.; Schuster, R.M.; Johannigman, T.A.; Miller, S.G.; Lentsch, A.B.; Pritts, T.A. Macrophage-derived chemokine (CCL22) is a novel mediator of lung inflammation following hemorrhage and resuscitation. Shock 2014, 42, 525–531. [Google Scholar] [CrossRef]
- Berin, M.C.; Dwinell, M.B.; Eckmann, L.; Kagnoff, M.F.; Yang, C.C.; Ogawa, H.; McCole, D.F.; Iimura, M. Production of MDC/CCL22 by human intestinal epithelial cells. Am. J. Physiol. Liver Physiol. 2001, 280, G1217–G1226. [Google Scholar] [CrossRef]
- Mantovani, A.; Gray, P.A.; Van Damme, J.; Sozzani, S. Macrophage-derived chemokine (MDC). J. Leukoc. Biol. 2000, 68, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Alcami, A.; Saraiva, M. Chemokine Binding Proteins Encoded by Pathogens. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Austin, TX, USA, 2009. Available online: https://www.ncbi.nlm.nih.gov/books/NBK5966/ (accessed on 21 July 2023).
- Banu, S.; Nagaraj, R.; Idris, M.M. A proteomic perspective and involvement of cytokines in SARS-CoV-2 infection. PLoS ONE 2023, 18, e0279998. [Google Scholar] [CrossRef]
- Hamdorf, M.; Imhof, T.; Bailey-Elkin, B.; Betz, J.; Theobald, S.J.; Simonis, A.; Cristanziano, V.D.; Gieselmann, L.; Dewald, F.; Lehmann, C.; et al. The unique ORF8 protein from SARS-CoV-2 binds to human dendritic cells and induces a hyper-inflammatory cytokine storm. bioRxiv 2022. [Google Scholar] [CrossRef]
- Khan, S.; Shafiei, M.S.; Longoria, C.; Schoggins, J.W.; Savani, R.C.; Zaki, H. SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway. eLife 2021, 10, e68563. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, Y.; Zhou, H.; Sun, C.; Zhang, S. The SARS-CoV-2 nucleocapsid protein: Its role in the viral life cycle, structure and functions, and use as a potential target in the development of vaccines and diagnostics. Virol. J. 2023, 20, 6. [Google Scholar] [CrossRef]
- López-Muñoz, A.D.; Kosik, I.; Holly, J.; Yewdell, J.W. Cell surface SARS-CoV-2 nucleocapsid protein modulates innate and adaptive immunity. Sci. Adv. 2022, 8, eabp9770. [Google Scholar] [CrossRef] [PubMed]
- González-Motos, V.; Kropp, K.A.; Viejo-Borbolla, A. Chemokine binding proteins: An immunomodulatory strategy going viral. Cytokine Growth Factor Rev. 2016, 30, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Tavakolpour, S.; Rakhshandehroo, T.; Wei, E.X.; Rashidian, M. Lymphopenia during the COVID-19 infection: What it shows and what can be learned. Immunol. Lett. 2020, 225, 31–32. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.Y.; Wong, C.K.; Li, E.K.; Tam, L.S.; Lam, C.W.K. Suppressive effect of combination treatment of leflunomide and methotrexate on chemokine expression in patients with rheumatoid arthritis. Clin. Exp. Immunol. 2003, 133, 132–138. [Google Scholar] [CrossRef]
- Gasca-Capote, C.; Gutierrez-Valencia, A.; Serna-Gallego, A.; Benhnia, M.R.-E.; Rivas-Jeremias, I.; Sotomayor, C.; Roca-Oporto, C.; Espinosa, N.; Crespo-Rivas, J.C.; López-Cortés, L.F.; et al. Dendritic cell deficiencies persist seven months after SARS-CoV-2 infection. Cell. Mol. Immunol. 2021, 18, 2128–2139. [Google Scholar] [CrossRef]
- Winheim, E.; Rinke, L.; Lutz, K.; Reischer, A.; Leutbecher, A.; Wolfram, L.; Rausch, L.; Kranich, J.; Wratil, P.R.; Huber, J.E.; et al. Impaired function and delayed regeneration of dendritic cells in COVID-19. PLoS Pathog. 2021, 17, e1009742. [Google Scholar] [CrossRef]
- Chang, T.; Yang, J.; Deng, H.; Chen, D.; Yang, X.; Tang, Z.-H. Depletion and Dysfunction of Dendritic Cells: Understanding SARS-CoV-2 Infection. Front. Immunol. 2022, 13, 843342. [Google Scholar] [CrossRef]
- Montazersaheb, S.; Khatibi, S.M.H.; Hejazi, M.S.; Tarhriz, V.; Farjami, A.; Sorbeni, F.G.; Farahzadi, R.; Ghasemnejad, T. COVID-19 infection: An overview on cytokine storm and related interventions. Virol. J. 2022, 19, 92. [Google Scholar] [CrossRef]
- Alamri, A.; Fisk, D.; Upreti, D.; Kung, S.K.P. A Missing Link: Engagements of Dendritic Cells in the Pathogenesis of SARS-CoV-2 Infections. Int. J. Mol. Sci. 2021, 22, 1118. [Google Scholar] [CrossRef]
- Chu, H.; Zhou, J.; Wong, B.H.-Y.; Li, C.; Cheng, Z.-S.; Lin, X.; Poon, V.K.-M.; Sun, T.; Lau, C.C.-Y.; Chan, J.F.-W.; et al. Productive replication of Middle East respiratory syndrome coronavirus in monocyte-derived dendritic cells modulates innate immune response. Virology 2014, 454–455, 197–205. [Google Scholar] [CrossRef]
- Onodi, F.; Bonnet-Madin, L.; Meertens, L.; Karpf, L.; Poirot, J.; Zhang, S.Y.; Picard, C.; Puel, A.; Jouanguy, E.; Zhang, Q.; et al. SARS-CoV-2 induces human plasmacytoid pre-dendritic cell diversification via UNC93B and IRAK4. bioRxiv 2021. Erratum in J. Exp. Med. 2021, 218, e20201387. [Google Scholar] [CrossRef]
- Cai, G.; Du, M.; Bossé, Y.; Albrecht, H.; Qin, F.; Luo, X.; Androulakis, X.M.; Cheng, C.; Nagarkatti, M.; Nagarkatti, P.; et al. SARS-CoV-2 Impairs Dendritic Cells and Regulates DC-SIGN Gene Expression in Tissues. Int. J. Mol. Sci. 2021, 22, 9228. [Google Scholar] [CrossRef] [PubMed]
- Galati, D.; Zanotta, S.; Capitelli, L.; Bocchino, M. A bird’s eye view on the role of dendritic cells in SARS-CoV-2 infection: Perspectives for immune-based vaccines. Allergy 2022, 77, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Venet, M.; Ribeiro, M.S.; Décembre, E.; Bellomo, A.; Joshi, G.; Nuovo, C.; Villard, M.; Cluet, D.; Perret, M.; Pescamona, R.; et al. Severe COVID-19 patients have impaired plasmacytoid dendritic cell-mediated control of SARS-CoV-2. Nat. Commun. 2023, 14, 694. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korobova, Z.R.; Arsentieva, N.A.; Totolian, A.A. Macrophage-Derived Chemokine MDC/CCL22: An Ambiguous Finding in COVID-19. Int. J. Mol. Sci. 2023, 24, 13083. https://doi.org/10.3390/ijms241713083
Korobova ZR, Arsentieva NA, Totolian AA. Macrophage-Derived Chemokine MDC/CCL22: An Ambiguous Finding in COVID-19. International Journal of Molecular Sciences. 2023; 24(17):13083. https://doi.org/10.3390/ijms241713083
Chicago/Turabian StyleKorobova, Zoia R., Natalia A. Arsentieva, and Areg A. Totolian. 2023. "Macrophage-Derived Chemokine MDC/CCL22: An Ambiguous Finding in COVID-19" International Journal of Molecular Sciences 24, no. 17: 13083. https://doi.org/10.3390/ijms241713083
APA StyleKorobova, Z. R., Arsentieva, N. A., & Totolian, A. A. (2023). Macrophage-Derived Chemokine MDC/CCL22: An Ambiguous Finding in COVID-19. International Journal of Molecular Sciences, 24(17), 13083. https://doi.org/10.3390/ijms241713083







