Changes in Expression and Function of Placental and Intestinal P-gp and BCRP Transporters during Pregnancy
Abstract
:1. Introduction
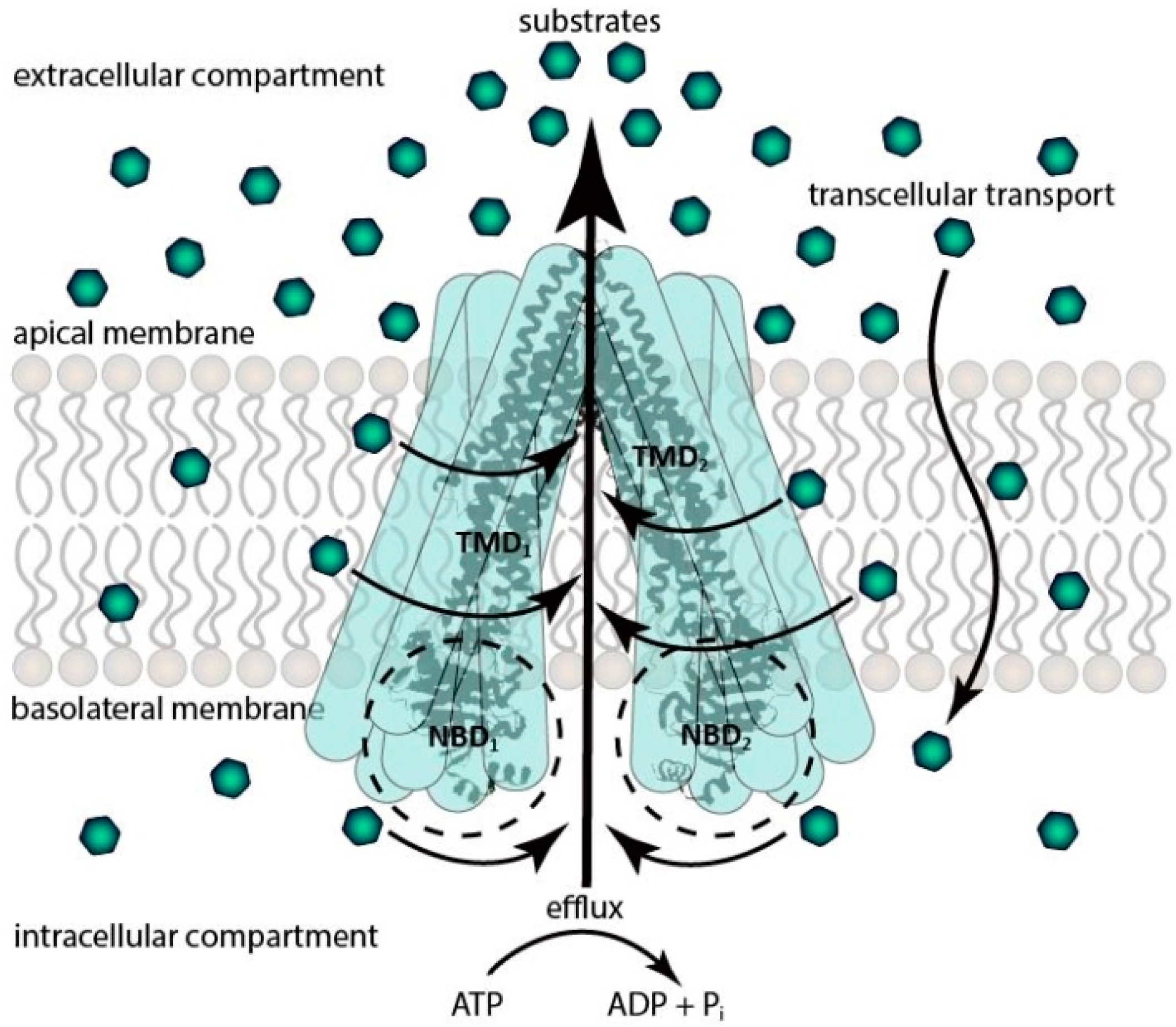
1.1. ATP-Binding Cassette (ABC) Transporters
1.1.1. ABCB Subfamily
1.1.2. ABCG Subfamily
| P-gp | BCRP | References | ||
|---|---|---|---|---|
| Names | classification | ABCB1 | ABCG2 | [7] |
| other | MDR1 | MXR, ABCP | [13,31] | |
| Molecular weight | monomer formation | 170 kDa | 70–75 kDa | [21,32] |
| dimer formation | - | 140–150 kDa | [32] | |
| Encoding genes | human | ABCB1 | ABCG2 | [20,33] |
| rodent | Abcb1a (Mdr1a), Abcb1b (Mdr1b) | Abcg2 | [20,33] | |
| Substrates | analgetics | morphine, loperamide | - | [36] |
| antibiotics | cefoperazone, ceftriazone, erythromycin | ciprofloxacin, nitrofurantoin | [37,38,39] | |
| antidiabetics | metformin | glyburide | [37,39,40] | |
| antihistamines | cetirizine, fexofenadine, desloratadine, bilastine | - | [41,42,43] | |
| antineoplastics | doxorubicin, paclitaxel | mitoxantron, methotrexate, imatinib, paclitaxel | [37] | |
| antivirals | abacavir, lopinavir, ritonavir, tenofovir | abacavir, lamivudin, zidovudin, lopinavir | [37,44] | |
| Ca-channel blockers | diltiazem, nifedipine, verapamil | nicardipine | [26] | |
| H2-antagonists | cimetidine, ranitidine | cimetidine | [39,45,46] | |
| others | rhodamine 123, quercetin | folic acid, quercetin | [26] | |
2. Expression of ABC Transporters during Pregnancy
2.1. Placental ABC Transporters
2.1.1. Expression of P-gp during Pregnancy
2.1.2. Expression of BCRP during Pregnancy
2.2. Intestinal ABC Transporters
2.2.1. Expression of P-gp in the Small Intestine during Pregnancy
2.2.2. Expression of BCRP in the Small Intestine during Pregnancy
| Transporter | In Vivo mRNA and Protein Expression Alterations with Gestational Age in the Intestine | Reference | ||
|---|---|---|---|---|
| Species | mRNA Expression | Protein Expression | ||
| P-gp (ABCB1) | mouse | Abcb1a mRNA decreases on days 14 and 17 of gestation and increases on day 19 | protein level decreases on gd 17 by 70% | [78] |
| mouse | no significant differences between the pregnant and non-pregnant groups | [79] | ||
| BCRP (ABCG2) | mouse | higher mRNA level on gd 14 and gd 19, but it is not statistically significant significantly lower mRNA and protein levels on gd 17 | [78] | |
| mouse | increases toward term, but it is not significant | [3] | ||
2.3. Effect of Pathological Conditions
3. Pharmacokinetic and Pharmacological Relevance of Expression Alterations during Pregnancy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hee Choi, Y.; Yu, A.M. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr. Pharm. Des. 2014, 20, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Feghali, M.; Venkataramanan, R.; Caritis, S. Pharmacokinetics of drugs in pregnancy. Semin. Perinatol. 2015, 39, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, X.; Hudkins, K.; Mikheev, A.; Zhang, H.; Gupta, A.; Unadkat, J.D.; Mao, Q. Expression of the breast cancer resistance protein (Bcrp1/Abcg2) in tissues from pregnant mice: Effects of pregnancy and correlations with nuclear receptors. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E1295–E1304. [Google Scholar] [CrossRef] [PubMed]
- Staud, F.; Ceckova, M.; Micuda, S.; Pavek, P. Expression and function of p-glycoprotein in normal tissues: Effect on pharmacokinetics. Methods Mol. Biol. 2010, 596, 199–222. [Google Scholar] [CrossRef]
- Dano, K. Active outward transport of daunomycin in resistant Ehrlich ascites tumor cells. Biochim. Biophys. Acta 1973, 323, 466–483. [Google Scholar] [CrossRef]
- Juliano, R.L.; Ling, V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Biophys. Acta 1976, 455, 152–162. [Google Scholar] [CrossRef]
- Liu, X. ABC Family Transporters. Adv. Exp. Med. Biol. 2019, 1141, 13–100. [Google Scholar] [CrossRef]
- Liu, X. Overview: Role of Drug Transporters in Drug Disposition and Its Clinical Significance. In Advances in Experimental Medicine and Biology; Springer: Singapore, 2019; Volume 1141, pp. 1–12. [Google Scholar] [CrossRef]
- Vasiliou, V.; Vasiliou, K.; Nebert, D.W. Human ATP-binding cassette (ABC) transporter family. Hum. Genomics. 2009, 3, 281–290. [Google Scholar] [CrossRef]
- Juan-Carlos, P.D.; Perla-Lidia, P.P.; Stephanie-Talia, M.M.; Mónica-Griselda, A.M.; Luz-María, T.E. ABC transporter superfamily. An updated overview, relevance in cancer multidrug resistance and perspectives with personalized medicine. Mol. Biol. Rep. 2021, 48, 1883–1901. [Google Scholar] [CrossRef]
- Kotlyarov, S.; Kotlyarova, A. The Role of ABC Transporters in Lipid Metabolism and the Comorbid Course of Chronic Obstructive Pulmonary Disease and Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 6711. [Google Scholar] [CrossRef]
- Hlaváč, V.; Souček, P. Role of family D ATP-binding cassette transporters (ABCD) in cancer. Biochem. Soc. Trans. 2015, 43, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Walker, N.; Filis, P.; Soffientini, U.; Bellingham, M.; O’Shaughnessy, P.J.; Fowler, P.A. Placental transporter localization and expression in the Human: The importance of species, sex, and gestational age differences. Biol. Reprod. 2017, 96, 733–742. [Google Scholar] [CrossRef]
- Brouwer, K.L.; Evers, R.; Hayden, E.; Hu, S.; Li, C.Y.; Meyer zu Schwabedissen, H.E.; Neuhoff, S.; Oswald, S.; Piquette-Miller, M.; Saran, C.; et al. Regulation of Drug Transport Proteins-From Mechanisms to Clinical Impact: A White Paper on Behalf of the International Transporter Consortium. Clin. Pharmacol. Ther. 2022, 112, 461–484. [Google Scholar] [CrossRef]
- Moitra, K.; Dean, M. Evolution of ABC transporters by gene duplication and their role in human disease. Biol. Chem. 2011, 392, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Aberuyi, N.; Rahgozar, S.; Khosravi Dehaghi, Z.; Moafi, A.; Masotti, A.; Paolini, A. The translational expression of ABCA2 and ABCA3 is a strong prognostic biomarker for multidrug resistance in pediatric acute lymphoblastic leukemia. Onco. Targets. Ther. 2017, 10, 3373–3380. [Google Scholar] [CrossRef]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. ICH Harmonised Guideline, Drug Interaction Studies M12. 2022. Available online: https://www.ema.europa.eu/en/ich-m12-drug-interaction-studies-scientific-guideline (accessed on 20 November 2022).
- Theodoulou, F.L.; Kerr, I.D. ABC transporter research: Going strong 40 years on. Biochem. Soc. Trans. 2015, 43, 1033–1040. [Google Scholar] [CrossRef]
- Juvale, I.I.; Hamid, A.A.; Abd Halim, K.B.; Has, A.T. P-glycoprotein: New insights into structure, physiological function, regulation and alterations in disease. Heliyon 2022, 8, e09777. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Mruk, D.D.; Cheng, C.Y. Drug transporters, the blood–testis barrier, and spermatogenesis. J. Endocrinol. 2011, 208, 207–223. [Google Scholar] [CrossRef]
- Sababi, M.; Borgå, O.; Hultkvist-Bengtsson, U. The role of P-glycoprotein in limiting intestinal regional absorption of digoxin in rats. Eur. J. Pharm. Sci. 2001, 14, 21–27. [Google Scholar] [CrossRef]
- Aller, S.G.; Yu, J.; Ward, A.; Weng, Y.; Chittaboina, S.; Zhuo, R.; Harrell, P.M.; Trinh, Y.T.; Zhang, Q.; Urbatsch, I.L.; et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 2009, 323, 1718–1722. [Google Scholar] [CrossRef]
- Dewanjee, S.; Dua, T.K.; Bhattacharjee, N.; Das, A.; Gangopadhyay, M.; Khanra, R.; Joardar, S.; Riaz, M.; De Feo, V.; Zia-Ul-Haq, M. Natural Products as Alternative Choices for P-Glycoprotein (P-gp) Inhibition. Molecules 2017, 22, 871. [Google Scholar] [CrossRef]
- Sauna, Z.E.; Ambudkar, S.V. About a switch: How P-glycoprotein (ABCB1) harnesses the energy of ATP binding and hydrolysis to do mechanical work. Mol. Cancer. Ther. 2007, 6, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Keogh, J.P. Membrane transporters in drug development. Adv. Pharmacol. 2012, 63, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Szakács, G.; Váradi, A.; Ozvegy-Laczka, C.; Sarkadi, B. The role of ABC transporters in drug absorption, distribution, metabolism, excretion and toxicity (ADME-Tox). Drug Discov. Today 2008, 13, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.E.; Soffir, R.; Noonan, K.E.; Choi, K.; Roninson, I.B. Structure and Expression of the Human MDR (P-Glycoprotein) Gene Family. Mol. Cell. Biol. 1989, 9, 3808–3820. [Google Scholar] [CrossRef]
- Kerr, I.D.; Hutchison, E.; Gerard, L.; Aleidi, S.M.; Gelissen, I.C. Mammalian ABCG-transporters, sterols and lipids: To bind perchance to transport? Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158860. [Google Scholar] [CrossRef]
- Austin Doyle, L.; Ross, D.D. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene 2003, 22, 7340–7358. [Google Scholar] [CrossRef]
- Doyle, L.A.; Yang, W.; Abruzzo, L.V.; Krogmann, T.; Gao, Y.; Rishi, A.K.; Ross, D.D. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. USA 1998, 95, 15665–15670. [Google Scholar] [CrossRef]
- Horsey, A.J.; Cox, M.H.; Sarwat, S.; Kerr, I.D. The multidrug transporter ABCG2: Still more questions than answers. Biochem. Soc. Trans. 2016, 44, 824–830. [Google Scholar] [CrossRef]
- Shigeta, J.; Katayama, K.; Mitsuhashi, J.; Noguchi, K.; Sugimoto, Y. BCRP/ABCG2 confers anticancer drug resistance without covalent dimerization. Cancer Sci. 2010, 101, 1813–1821. [Google Scholar] [CrossRef]
- Ni, Z.; Bikadi, Z.; FRosenberg, M.; Mao, Q. Structure and function of the human breast cancer resistance protein (BCRP/ABCG2). Curr. Drug Metab. 2010, 11, 603–617. [Google Scholar] [CrossRef]
- Kage, K.; Tsukahara, S.; Sugiyama, T.; Asada, S.; Ishikawa, E.; Tsuruo, T.; Sugimoto, Y. Dominant-negative inhibition of breast cancer resistance protein as drug efflux pump through the inhibition of S-S dependent homodimerization. Int. J. Cancer 2002, 97, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, I.; Nishikawa, S.; Yamamoto, A.; Kono, Y.; Fujita, T. Assessment of contribution of BCRP to intestinal absorption of various drugs using portal-systemic blood concentration difference model in mice. Pharmacol. Res. Perspect. 2020, 8, e00544. [Google Scholar] [CrossRef] [PubMed]
- Susan, L. Mercer, Andrew Coop, Opioid analgesics and P-glycoprotein efflux transporters: A potential systems-level contribution to analgesic tolerance. Curr. Top. Med. Chem. 2011, 11, 1157–1164. [Google Scholar] [CrossRef]
- Han, L.W.; Gao, C.; Mao, Q. An update on expression and function of P-gp/ABCB1 and BCRP/ABCG2 in the placenta and fetus. Expert Opin. Drug Metab. Toxicol. 2018, 14, 817–829. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Unadkat, J.D.; Mao, Q. Breast cancer resistance protein 1 limits fetal distribution of nitrofurantoin in the pregnant mouse. Drug Metab. Dispos. 2007, 35, 2154–2158. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q. BCRP/ABCG2 in the placenta: Expression, function and regulation. Pharm. Res. 2008, 25, 1244–1255. [Google Scholar] [CrossRef] [PubMed]
- Ellfolk, M.; Tornio, A.; Niemi, M.; Leinonen, M.K.; Lahesmaa-Korpinen, A.M.; Malm, H. Placental transporter-mediated drug interactions and offspring congenital anomalies. Br. J. Clin. Pharmacol. 2020, 86, 868–879. [Google Scholar] [CrossRef]
- Wessler, J.D.; Grip, L.T.; Mendell, J.; Giugliano, R.P. The P-glycoprotein transport system and cardiovascular drugs. J. Am. Coll. Cardiol. 2013, 61, 2495–2502. [Google Scholar] [CrossRef]
- Tahara, H.; Kusuhara, H.; Fuse, E.; Sugiyama, Y. P-glycoprotein plays a major role in the efflux of fexofenadine in the small intestine and blood-brain barrier, but only a limited role in its biliary excretion. Drug Metab. Dispos. 2005, 33, 963–968. [Google Scholar] [CrossRef]
- Chen, C.; Hanson, E.; Watson, J.W.; Lee, J.S. P-glycoprotein limits the brain penetration of nonsedating but not sedating H1-antagonists. Drug Metab. Dispos. 2003, 31, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Tomi, M.; Nishimura, T.; Nakashima, E. Mother-to-fetus transfer of antiviral drugs and the involvement of transporters at the placental barrier. J. Pharm. Sci. 2011, 100, 3708–3718. [Google Scholar] [CrossRef] [PubMed]
- Collett, A.; Higgs, N.B.; Sims, E.; Rowland, M.; Warhurst, G. Modulation of the permeability of H2 receptor antagonists cimetidine and ranitidine by P-glycoprotein in rat intestine and the human colonic cell line Caco-2. J. Pharmacol. Exp. Ther. 1999, 288, 171–178. [Google Scholar]
- Staud, F.; Vackova, Z.; Pospechova, K.; Pavek, P.; Ceckova, M.; Libra, A.; Cygalova, L.; Nachtigal, P.; Fendrich, Z. Expression and transport activity of breast cancer resistance protein (Bcrp/Abcg2) in dually perfused rat placenta and HRP-1 cell line. J. Pharmacol. Exp. Ther. 2006, 319, 53–62. [Google Scholar] [CrossRef]
- Unadkat, J.D.; Dahlin, A.; Vijay, S. Placental drug transporters. Curr. Drug Metab. 2004, 5, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Staud, F.; Cerveny, L.; Ceckova, M. Pharmacotherapy in pregnancy; effect of ABC and SLC transporters on drug transport across the placenta and fetal drug exposure. J. Drug Target. 2012, 20, 736–763. [Google Scholar] [CrossRef]
- Taggi, V.; Riera Romo, M.; Piquette-Miller, M.; Meyer zu Schwabedissen, H.E.; Neuhoff, S. Transporter Regulation in Critical Protective Barriers: Focus on Brain and Placenta. Pharmaceutics 2022, 14, 1376. [Google Scholar] [CrossRef]
- Yoshizawa, R.S.; Hird, M.J. Schrödinger’s placenta: Determining placentas as not/waste. Nat. Space 2020, 3, 246–262. [Google Scholar] [CrossRef]
- Ceckova-Novotna, M.; Pavek, P.; Staud, F. P-glycoprotein in the placenta: Expression, localization, regulation and function. Reprod. Toxicol. 2006, 22, 400–410. [Google Scholar] [CrossRef]
- Mathias, A.A.; Hitti, J.; Unadkat, J.D. P-glycoprotein and breast cancer resistance protein expression in human placentae of various gestational ages. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R963–R969. [Google Scholar] [CrossRef]
- Gil, S.; Saura, R.; Forestier, F.; Farinotti, R. P-glycoprotein expression of the human placenta during pregnancy. Placenta 2005, 26, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Kingdom, J.; Baczyk, D.; Lye, S.J.; Matthews, S.G.; Gibb, W. Expression of the multidrug resistance P-glycoprotein, (ABCB1 glycoprotein) in the human placenta decreases with advancing gestation. Placenta 2006, 27, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, V.; Kojovic, D.; Cressman, A.; Piquette-Miller, M. Maternal bacterial infections impact expression of drug transporters in human placenta. Int. Immunopharmacol. 2015, 26, 349–356. [Google Scholar] [CrossRef]
- Novotna, M.; Libra, A.; Kopecky, M.; Pavek, P.; Fendrich, Z.; Semecky, V.; Staud, F. P-glycoprotein expression and distribution in the rat placenta during pregnancy. Reprod. Toxicol. 2004, 18, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Kalabis, G.M.; Kostaki, A.; Andrews, M.H.; Petropoulos, S.; Gibb, W.; Matthews, S.G. Multidrug resistance phosphoglycoprotein (ABCB1) in the mouse placenta: Fetal protection. Biol. Reprod. 2005, 73, 591–597. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, X.; Wang, H.; Mikheev, A.M.; Mao, Q.; Unadkat, J.D. Effect of pregnancy on cytochrome P450 3a and P-glycoprotein expression and activity in the mouse: Mechanisms, tissue specificity, and time course. Mol. Pharmacol. 2008, 74, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Coles, L.D.; Lee, I.J.; Hassan, H.E.; Eddington, N.D. Distribution of saquinavir, methadone, and buprenorphine in maternal brain, placenta, and fetus during two different gestational stages of pregnancy in mice. J. Pharm. Sci. 2009, 98, 2832–2846. [Google Scholar] [CrossRef]
- Akashi, T.; Nishimura, T.; Takaki, Y.; Takahashi, M.; Shin, B.-C.; Tomi, M.; Nakashima, E. Layer II of placental syncytiotrophoblasts expresses MDR1 and BCRP at the apical membrane in rodents. Reprod. Toxicol. 2016, 65, 375–381. [Google Scholar] [CrossRef]
- Yeboah, D.; Sun, M.; Kingdom, J.; Baczyk, D.; Lye, S.J.; Matthews, S.G.; Gibb, W. Expression of breast cancer resistance protein (BCRP/ABCG2) in human placenta throughout gestation and at term before and after labor. Can. J. Physiol. Pharmacol. 2006, 84, 1251–1258. [Google Scholar] [CrossRef]
- zu Schwabedissen, H.E.; Grube, M.; Dreisbach, A.; Jedlitschky, G.; Meissner, K.; Linnemann, K.; Fusch, C.; Ritter, C.A.; Völker, U.; Kroemer, H.K. Epidermal growth factor-mediated activation of the map kinase cascade results in altered expression and function of ABCG2 (BCRP). Drug Metab. Dispos. 2006, 34, 524–533. [Google Scholar] [CrossRef]
- Cygalova, L.; Ceckova, M.; Pavek, P.; Staud, F. Role of breast cancer resistance protein (Bcrp/Abcg2) in fetal protection during gestation in rat. Toxicol. Lett. 2008, 178, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Itagaki, S.; Hirano, T.; Iseki, K. Expression level of ABCG2 in the placenta decreases from the mid stage to the end of gestation. Biosci. Biotechnol. Biochem. 2005, 69, 1871–1876. [Google Scholar] [CrossRef]
- Kalabis, G.M.; Petropoulos, S.; Gibb, W.; Matthews, S.G. Breast cancer resistance protein (Bcrp1/Abcg2) in mouse placenta and yolk sac: Ontogeny and its regulation by progesterone. Placenta 2007, 28, 1073–1081. [Google Scholar] [CrossRef]
- Bircsak, K.M.; Moscovitz, J.E.; Wen, X.; Archer, F.; Yuen, P.Y.; Mohammed, M.; Memon, N.; Weinberger, B.I.; Saba, L.M.; Vetrano, A.M.; et al. Interindividual Regulation of the Breast Cancer Resistance Protein/ABCG2 Transporter in Term Human Placentas. Drug Metab. Dispos. 2018, 46, 619–627. [Google Scholar] [CrossRef]
- Hua, S. Advances in Oral Drug Delivery for Regional Targeting in the Gastrointestinal Tract—Influence of Physiological, Pathophysiological and Pharmaceutical Factors. Front. Pharmacol. 2020, 11, 524. [Google Scholar] [CrossRef]
- Keita, A.V.; Söderholm, J.D. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol. Motil. 2010, 22, 718–733. [Google Scholar] [CrossRef] [PubMed]
- Van Asperen, J.; Van Tellingen, O.; Beijnen, J.H. The pharmacological role of P-glycoprotein in the intestinal epithelium. Pharmacol. Res. 1998, 37, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Khalil, M.; Angelis, M.D.; Calabrese, F.M.; D’amato, M.; Wang, D.Q.; Di Ciaula, A. Intestinal Barrier and Permeability in Health, Obesity and NAFLD. Biomedicines 2021, 10, 83. [Google Scholar] [CrossRef]
- Müller, J.; Keiser, M.; Drozdzik, M.; Oswald, S. Expression, regulation and function of intestinal drug transporters: An update. Biol. Chem. 2017, 398, 175–192. [Google Scholar] [CrossRef]
- Takano, M.; Yumoto, R.; Murakami, T. Expression and function of efflux drug transporters in the intestine. Pharmacol. Ther. 2006, 109, 137–161. [Google Scholar] [CrossRef]
- Dietrich, C.G.; Geier, A.; Elferink, R.P.J.O. ABC of oral bioavailability: Transporters as gatekeepers in the gut. Gut 2003, 52, 1788–1795. [Google Scholar] [CrossRef]
- Gutmann, H.; Hruz, P.; Zimmermann, C.; Beglinger, C.; Drewe, J. Distribution of breast cancer resistance protein (BCRP/ABCG2) mRNA expression along the human GI tract. Biochem. Pharmacol. 2005, 70, 695–699. [Google Scholar] [CrossRef]
- Mai, Y.; Dou, L.; Yao, Z.; Madla, C.M.; Gavins, F.K.H.; Taherali, F.; Yin, H.; Orlu, M.; Murdan, S.; Basit, A.W. Quantification of P-Glycoprotein in the Gastrointestinal Tract of Humans and Rodents: Methodology, Gut Region, Sex, and Species Matter. Mol. Pharm. 2021, 18, 1895–1904. [Google Scholar] [CrossRef]
- Rost, D.; Mahner, S.; Sugiyama, Y.; Stremmel, W. Expression and localization of the multidrug resistance-associated protein 3 in rat small and large intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G720–G726. [Google Scholar] [CrossRef] [PubMed]
- Mouly, S.; Paine, M.F. P-glycoprotein increases from proximal to distal regions of human small intestine. Pharm. Res. 2003, 20, 1595–1599. [Google Scholar] [CrossRef] [PubMed]
- Moscovitz, J.E.; Yarmush, G.; Herrera-Garcia, G.; Guo, G.L.; Aleksunes, L.M. Differential Regulation of Intestinal Efflux Transporters by Pregnancy in Mice. Xenobiotica 2017, 47, 989–997. [Google Scholar] [CrossRef]
- Mathias, A.A.; Maggio-Price, L.; Lai, Y.; Gupta, A.; Unadkat, J.D. Changes in pharmacokinetics of anti-HIV protease inhibitors during pregnancy: The role of CYP3A and P-glycoprotein. J. Pharmacol. Exp. Ther. 2006, 316, 1202–1209. [Google Scholar] [CrossRef]
- Tanaka, Y.; Slitt, A.L.; Leazer, T.M.; Maher, J.M.; Klaassen, C.D. Tissue distribution and hormonal regulation of the breast cancer resistance protein (Bcrp/Abcg2) in rats and mice. Biochem. Biophys. Res. Commun. 2005, 326, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Staud, F.; Ceckova, M. Regulation of drug transporter expression and function in the placenta. Expert Opin. Drug Metab. Toxicol. 2015, 11, 533–555. [Google Scholar] [CrossRef] [PubMed]
- Kozlosky, D.; Barrett, E.; Aleksunes, L.M. Regulation of Placental Efflux Transporters during Pregnancy Complications. Drug Metab. Dispos. 2022, 50, 1364–1375. [Google Scholar] [CrossRef]
- Murakami, T.; Bodor, E.; Bodor, N. Modulation of expression/function of intestinal P-glycoprotein under disease states. Expert Opin. Drug Metab. Toxicol. 2020, 16, 59–78. [Google Scholar] [CrossRef] [PubMed]
- Mina, T.H.; Räikkönen, K.; Riley, S.C.; Norman, J.E.; Reynolds, R.M. Maternal distress associates with placental genes regulating fetal glucocorticoid exposure and IGF2: Role of obesity and sex. Psychoneuroendocrinology 2015, 59, 112–122. [Google Scholar] [CrossRef]
- Nawa, A.; Fujita-Hamabe, W.; Kishioka, S.; Tokuyama, S. Decreased expression of intestinal P-glycoprotein increases the analgesic effects of oral morphine in a streptozotocin-induced diabetic mouse model. Drug Metab. Pharmacokinet. 2011, 26, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Daud, A.N.; Bergman, J.E.; Bakker, M.K.; Wang, H.; Kerstjens-Frederikse, W.S.; de Walle, H.E.; Groen, H.; Bos, J.H.; Hak, E.; Wilffert, B. P-Glycoprotein-Mediated Drug Interactions in Pregnancy and Changes in the Risk of Congenital Anomalies: A Case-Reference Study. Drug Saf. 2015, 38, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Daw, J.R.; Hanley, G.E.; Greyson, D.L.; Morgan, S.G. Prescription drug use during pregnancy in developed countries: A systematic review. Pharmacoepidemiol. Drug Saf. 2011, 20, 895–902. [Google Scholar] [CrossRef]
- Brown, B.; Wright, C. Safety and efficacy of supplements in pregnancy. Nutr. Rev. 2020, 78, 813–826. [Google Scholar] [CrossRef]
- Hui, L.; Bianchi, D.W. Prenatal pharmacotherapy for fetal anomalies: A 2011 update. Prenat. Diagn. 2011, 31, 735–743. [Google Scholar] [CrossRef]
- Pottier, G.; Marie, S.; Goutal, S.; Auvity, S.; Peyronneau, M.-A.; Stute, S.; Boisgard, R.; Dollé, F.; Buvat, I.; Caillé, F.; et al. Imaging the Impact of the P-Glycoprotein (ABCB1) Function on the Brain Kinetics of Metoclopramide. J. Nucl. Med. 2016, 57, 309–314. [Google Scholar] [CrossRef]
- Smit, J.W.; Huisman, M.T.; van Tellingen, O.; Wiltshire, H.R.; Schinkel, A.H. Absence or pharmacological blocking of placental P-glycoprotein profoundly increases fetal drug exposure. J. Clin. Investig. 1999, 104, 1441–1447. [Google Scholar] [CrossRef]
- Dallas, S.; Salphati, L.; Gomez-Zepeda, D.; Wanek, T.; Chen, L.; Chu, X.; Kunta, J.; Mezler, M.; Menet, M.C.; Chasseigneaux, S.; et al. Generation and Characterization of a Breast Cancer Resistance Protein Humanized Mouse Model. Mol. Pharmacol. 2016, 89, 492–504. [Google Scholar] [CrossRef]
- Murakami, T.; Takano, M. Intestinal efflux transporters and drug absorption. Expert Opin. Drug Metab. Toxicol. 2008, 4, 923–939. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Chen, X. An update on placental drug transport and its relevance to fetal drug exposure. Med. Rev. 2022, 2, 501–511. [Google Scholar] [CrossRef]
- Zhou, L.; Naraharisetti, S.B.; Wang, H.; Unadkat, J.D.; Hebert, M.F.; Mao, Q. The Breast Cancer Resistance Protein (Bcrp1/Abcg2) Limits Fetal Distribution of Glyburide in the Pregnant Mouse: An Obstetric-Fetal Pharmacology Research Unit Network and University of Washington Specialized Center of Research Study. Mol. Pharmacol. 2008, 73, 949–959. [Google Scholar] [CrossRef]
- Lankas, G.; Wise, L.; Cartwright, M.; Pippert, T.; Umbenhauer, D. Placental Pglycoprotein deficiency enhances susceptibility to chemically induced birth defects in mice. Reprod. Toxicol. 1998, 12, 457–463. [Google Scholar] [CrossRef]
- Popova, N.M.; Shchulkin, A.V.; Chernykh, I.V.; Mylnikov, P.Y.; Yakusheva, E.N. Functioning of P-Glycoprotein during Pregnancy in Rabbits. Bull. Exp. Biol. Med. 2023, 174, 431–434. [Google Scholar] [CrossRef]
- Elmeliegy, M.; Vourvahis, M.; Guo, C.; Wang, D.D. Effect of P-glycoprotein (P-gp) Inducers on Exposure of P-gp Substrates: Review of Clinical Drug–Drug Interaction Studies. Clin. Pharmacokinet. 2020, 59, 699–714. [Google Scholar] [CrossRef] [PubMed]
| In Vivo mRNA and Protein Expression Alterations with Gestational Age in the Placenta | Reference | |||
|---|---|---|---|---|
| Species | mRNA Expression | Protein Expression | ||
| P-gp (ABCB1) | human | high mRNA and protein levels in the early stages, then significant decrease until term | [52] | |
| human | no mRNA data | high protein level in the early stages, then decrease until term | [53] | |
| human | mRNA and protein levels decrease with the progression of gestational age no significant differences between cesarean section and vaginal delivery samples | [54] | ||
| human | no mRNA data | significantly lower protein expression in term pregnancies compared to the preterm pregnancies | [55] | |
| rat | Abcb1a mRNA level increases from gd 11 and reaches its maximum on gd 19 Abcb1b mRNA level increases from gd 11 until gd 22 | protein expression reaches its peak on gd 18 | [56] | |
| mouse | Abcb1a/1b levels reach their peak on gd 12.5 then decrease until gd 19 | protein level reaches its peak on gd 12.5 then decreases until gd 19 | [57] | |
| mouse | mRNA levels have no significant changes | protein level decreases from gd 10 until gd 19 | [58] | |
| mouse | mRNA levels have no significant changes | protein level decreases from gd 13 until gd 18 | [59] | |
| Transporter | In Vivo mRNA and Protein Expression Alterations with Gestational Age in the Placenta | Reference | ||
|---|---|---|---|---|
| Species | mRNA Expression | Protein Expression | ||
| BCRP (ABCG2) | human | mRNA and protein expression levels are similar in the early stages and at term, while in the middle stage of pregnancy, they decrease, but not significantly | [52] | |
| human | no significant differences between mRNA levels | protein level at 38–41 weeks is higher than at 6–13 weeks no significant differences between cesarean section and vaginal delivery samples | [61] | |
| human | mRNA and protein levels decrease significantly from early preterm to term | [62] | ||
| human | no mRNA data | significantly lower protein expression in term pregnancies compared to the preterm pregnancies | [55] | |
| rat | mRNA is detected on gd 12, reaches its peak on gd 15 and then decreases until gd 21 | no data | [63] | |
| rat | mRNA and protein levels decrease from gd 14 to gd 20 | [64] | ||
| mouse | mRNA and protein levels are detected on gd 10, reach their peak on gd 15 and then decrease on gd 19 | [3] | ||
| mouse | mRNA levels decrease from day 9.5 to day 18.5 | protein levels are higher on gd 12.5 than on gd 15.5 and gd 18.5, but not significantly | [65] | |
| Drug | Drug Class | Transporter | Reference |
|---|---|---|---|
| cimetidine | H2 antagonist | P-gp and BCRP | [45] |
| nitrofurantoin | antibiotic | BCRP | [38] |
| pantoprazole | proton pump inhibitor | P-gp | [40] |
| fexofenadinedesloratadine | antihistamine | P-gp | [42] |
| metformin | antidiabetic | P-gp | [40] |
| metoklopramid | antiemetic | P-gp | [90] |
| digoxin * | cardiac glycoside | P-gp | [91] |
| zidovudine * | antiviral | BCRP | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szatmári, P.; Ducza, E. Changes in Expression and Function of Placental and Intestinal P-gp and BCRP Transporters during Pregnancy. Int. J. Mol. Sci. 2023, 24, 13089. https://doi.org/10.3390/ijms241713089
Szatmári P, Ducza E. Changes in Expression and Function of Placental and Intestinal P-gp and BCRP Transporters during Pregnancy. International Journal of Molecular Sciences. 2023; 24(17):13089. https://doi.org/10.3390/ijms241713089
Chicago/Turabian StyleSzatmári, Péter, and Eszter Ducza. 2023. "Changes in Expression and Function of Placental and Intestinal P-gp and BCRP Transporters during Pregnancy" International Journal of Molecular Sciences 24, no. 17: 13089. https://doi.org/10.3390/ijms241713089
APA StyleSzatmári, P., & Ducza, E. (2023). Changes in Expression and Function of Placental and Intestinal P-gp and BCRP Transporters during Pregnancy. International Journal of Molecular Sciences, 24(17), 13089. https://doi.org/10.3390/ijms241713089






