Antiproliferative and Antimetastatic Properties of 16-Azidomethyl Substituted 3-O-Benzyl Estrone Analogs †
Abstract
:1. Introduction
2. Results
2.1. Chemistry
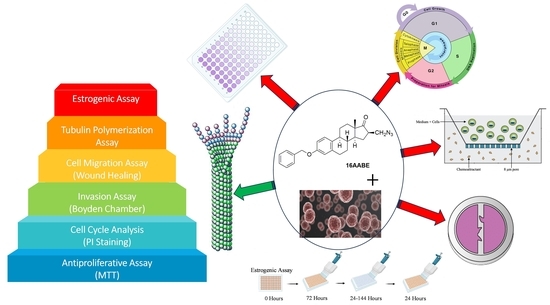
2.2. Antiproliferative Assay
2.3. Propidium Iodide-Based Cell Cycle Analysis
2.4. Tubulin Polymerization Assay
2.5. Wound Healing Assay
2.6. Boyden Chamber Assay
2.7. Estrogenic Activities of the Test Compounds
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.2. Cell CUlture and CHemicals
4.3. Determination of Antiproliferative Activity (MTT Assay)
4.4. Propidium Iodide-Based Cell Cycle Analysis
4.5. Tubulin Polymerization Assay
4.6. Migration Assay
4.7. Invasion Assay
4.8. Determination of Estrogenic Activity
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Azadnajafabad, S.; Saeedi Moghaddam, S.; Mohammadi, E.; Delazar, S.; Rashedi, S.; Baradaran, H.R.; Mansourian, M. Patterns of better breast cancer care in countries with higher human development index and healthcare expenditure: Insights from GLOBOCAN 2020. Front. Public Health 2023, 11, 1137286. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- AlQathama, A.; Shao, L.; Bader, A.; Khondkar, P.; Gibbons, S.; Prieto, J.M. Differential Antiproliferative and Anti-Migratory Activities of Ursolic Acid, 3-O-Acetylursolic Acid and Their Combination Treatments with Quercetin on Melanoma Cells. Biomolecules 2020, 10, 894. [Google Scholar] [CrossRef]
- Bednarczyk-Cwynar, B.; Ruszkowski, P.; Bobkiewicz-Kozlowska, T.; Zaprutko, L. Oleanolic Acid A-lactams Inhibit the Growth of HeLa, KB, MCF-7 and Hep-G2 Cancer Cell Lines at Micromolar Concentrations. Anticancer Agents Med. Chem. 2016, 16, 579–592. [Google Scholar] [CrossRef]
- Gheorgheosu, D.; Duicu, O.; Dehelean, C.; Soica, C.; Muntean, D. Betulinic acid as a potent and complex antitumor phytochemical: A minireview. Anticancer Agents Med. Chem. 2014, 14, 936–945. [Google Scholar] [CrossRef]
- Liu, J.; Wu, N.; Ma, L.N.; Zhong, J.T.; Liu, G.; Zheng, L.H.; Lin, X.K. p38 MAPK signaling mediates mitochondrial apoptosis in cancer cells induced by oleanolic acid. Asian Pac. J. Cancer Prev. 2014, 15, 4519–4525. [Google Scholar] [CrossRef]
- Sun, H.; Lv, C.; Yang, L.; Wang, Y.; Zhang, Q.; Yu, S.; Kong, H.; Wang, M.; Xie, J.; Zhang, C.; et al. Solanine induces mitochondria-mediated apoptosis in human pancreatic cancer cells. BioMed Res. Int. 2014, 2014, 805926. [Google Scholar] [CrossRef]
- Wu, J.; Yang, C.; Guo, C.; Li, X.; Yang, N.; Zhao, L.; Hang, H.; Liu, S.; Chu, P.; Sun, Z.; et al. SZC015, a synthetic oleanolic acid derivative, induces both apoptosis and autophagy in MCF-7 breast cancer cells. Chem. Biol. Interact. 2016, 244, 94–104. [Google Scholar] [CrossRef]
- Yang, S.J.; Liu, M.C.; Xiang, H.M.; Zhao, Q.; Xue, W.; Yang, S. Synthesis and in vitro antitumor evaluation of betulin acid ester derivatives as novel apoptosis inducers. Eur. J. Med. Chem. 2015, 102, 249–255. [Google Scholar] [CrossRef]
- Mernyák, E.; Kovács, I.; Minorics, R.; Sere, P.; Czégány, D.; Sinka, I.; Wölfling, J.; Schneider, G.; Újfaludi, Z.; Boros, I.; et al. Synthesis of trans-16-triazolyl-13α-methyl-17-estradiol diastereomers and the effects of structural modifications on their in vitro antiproliferative activities. J. Steroid Biochem. Mol. Biol. 2015, 150, 123–134. [Google Scholar] [CrossRef]
- Agarwal, D.S.; Sakhuja, R.; Beteck, R.M.; Legoabe, L.J. Steroid-triazole conjugates: A brief overview of synthesis and their application as anticancer agents. Steroids 2023, 197, 109258. [Google Scholar] [CrossRef]
- Gomes, A.R.; Pires, A.S.; Roleira, F.M.F.; Tavares-da-Silva, E.J. The structural diversity and biological activity of steroid oximes. Molecules 2023, 28, 1690. [Google Scholar] [CrossRef] [PubMed]
- Jurášek, M.; Černohorská, M.; Řehulka, J.; Spiwok, V.; Sulimenko, T.; Dráberová, E.; Darmostuk, M.; Gurská, S.; Frydrych, I.; Buriánová, R.; et al. Estradiol dimer inhibits tubulin polymerization and microtubule dynamics. J. Steroid Biochem. Mol. Biol. 2018, 183, 68–79. [Google Scholar] [CrossRef]
- Jójárt, R.; Senobar Tahaei, S.A.; Trungel-Nagy, P.; Kele, Z.; Minorics, R.; Paragi, G.; Zupkó, I.; Mernyák, E. Synthesis and evaluation of anticancer activities of 2- or 4-substituted 3-(N-benzyltriazolylmethyl)-13α-oestrone derivatives. J. Enzym. Inhib. Med. Chem. 2021, 36, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.; Mernyak, E.; Wolfling, J.; Sinka, I.; Zupko, I.; Schneider, G. Stereoselective synthesis of the four 16-hydroxymethyl-3-methoxy- and 16-hydroxymethyl-3-benzyloxy-13α-estra-1,3,5(10)-trien-17-ol isomers and their antiproliferative activities. Steroids 2018, 134, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.; Wölfling, J.; Mernyák, E.; Frank, É.; Benke, Z.; Senobar Tahaei, S.A.; Zupkó, I.; Mahó, S.; Schneider, G. Stereocontrolled synthesis of the four possible 3-methoxy and 3-benzyloxy-16-triazolyl-methyl-estra-17-ol hybrids and their antiproliferative activities. Steroids 2019, 152, 108500. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.D.; Khanna, S.; Ramchandani, S.; Kakoti, L.M.; Baruah, A.; Mamidala, V. Prevalence of Molecular Subtypes of Breast Carcinoma and Its Comparison between Two Different Age Groups: A Retrospective Study from a Tertiary Care Center of Northeast India. South Asian J. Cancer 2021, 10, 220–224. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Merkher, Y.; Chen, L.; Liu, N.; Leonov, S.; Chen, Y. Recent advances in therapeutic strategies for triple-negative breast cancer. J. Hematol. Oncol. 2022, 15, 121. [Google Scholar] [CrossRef]
- Derakhshan, F.; Reis-Filho, J.S. Pathogenesis of Triple-Negative Breast Cancer. Annu. Rev. Pathol. 2022, 17, 181–204. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C.; Reutelingsperger, C. Flow cytometry of apoptotic cell death. J. Immunol. Methods 2000, 243, 167–190. [Google Scholar] [CrossRef] [PubMed]
- Dumontet, C.; Jordan, M.A. Microtubule-binding agents: A dynamic field of cancer therapeutics. Nat. Rev. Drug Discov. 2010, 9, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Mehlen, P.; Puisieux, A. Metastasis: A question of life or death. Nat. Rev. Cancer 2006, 6, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Eger, A.; Mikulits, W. Models of epithelial–mesenchymal transition. Drug Discov. Today Dis. Model. 2005, 2, 57–63. [Google Scholar] [CrossRef]
- Bhatla, N.; Berek, J.S.; Cuello Fredes, M.; Denny, L.A.; Grenman, S.; Karunaratne, K.; Kehoe, S.T.; Konishi, I.; Olawaiye, A.B.; Prat, J.; et al. Revised FIGO staging for carcinoma of the cervix uteri. Int. J. Gynaecol. Obstet. 2019, 145, 129–135. [Google Scholar] [CrossRef]
- Franco, E.L.; Schlecht, N.F.; Saslow, D. The epidemiology of cervical cancer. Cancer J. 2003, 9, 348–359. [Google Scholar] [CrossRef]
- Stolnicu, S.; Hoang, L.; Soslow, R.A. Recent advances in invasive adenocarcinoma of the cervix. Virchows Arch. 2019, 475, 537–549. [Google Scholar] [CrossRef]
- Vandeperre, A.; Van Limbergen, E.; Leunen, K.; Moerman, P.; Amant, F.; Vergote, I. Para-aortic lymph node metastases in locally advanced cervical cancer: Comparison between surgical staging and imaging. Gynecol. Oncol. 2015, 138, 299–303. [Google Scholar] [CrossRef]
- Ali, H.; Traj, P.; Szebeni, G.J.; Gémes, N.; Resch, V.; Paragi, G.; Mernyák, E.; Minorics, R.; Zupkó, I. Investigation of the antineoplastic effects of 2-(4-Chlorophenyl)-13α-estrone sulfamate against the HPV16-positive human invasive cervical carcinoma cell line SiHa. Int. J. Mol. Sci. 2023, 24, 6625. [Google Scholar] [CrossRef]
- Cermak, V.; Dostal, V.; Jelinek, M.; Libusova, L.; Kovar, J.; Rosel, D.; Brabek, J. Microtubule-targeting agents and their impact on cancer treatment. Eur. J. Cell Biol. 2020, 99, 151075. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Wilson, V.S.; Bobseine, K.; Gray, L.E., Jr. Development and characterization of a cell line that stably expresses an estrogen-responsive luciferase reporter for the detection of estrogen receptor agonist and antagonists. Toxicol. Sci. 2004, 81, 69–77. [Google Scholar] [CrossRef] [PubMed]












| Cancer Cell Line | ||
|---|---|---|
| 16AABE | 16BABE | |
| HeLa | 0.369 | 0.243 |
| SiHa | 0.302 | 0.203 |
| MCF-7 | 0.230 | 0.166 |
| MDA-MB-231 | 0.347 | 0.429 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senobar Tahaei, S.A.; Kulmány, Á.; Minorics, R.; Kiss, A.; Szabó, Z.; Germán, P.; Szebeni, G.J.; Gémes, N.; Mernyák, E.; Zupkó, I. Antiproliferative and Antimetastatic Properties of 16-Azidomethyl Substituted 3-O-Benzyl Estrone Analogs. Int. J. Mol. Sci. 2023, 24, 13749. https://doi.org/10.3390/ijms241813749
Senobar Tahaei SA, Kulmány Á, Minorics R, Kiss A, Szabó Z, Germán P, Szebeni GJ, Gémes N, Mernyák E, Zupkó I. Antiproliferative and Antimetastatic Properties of 16-Azidomethyl Substituted 3-O-Benzyl Estrone Analogs. International Journal of Molecular Sciences. 2023; 24(18):13749. https://doi.org/10.3390/ijms241813749
Chicago/Turabian StyleSenobar Tahaei, Seyyed Ashkan, Ágnes Kulmány, Renáta Minorics, Anita Kiss, Zoltán Szabó, Péter Germán, Gábor J. Szebeni, Nikolett Gémes, Erzsébet Mernyák, and István Zupkó. 2023. "Antiproliferative and Antimetastatic Properties of 16-Azidomethyl Substituted 3-O-Benzyl Estrone Analogs" International Journal of Molecular Sciences 24, no. 18: 13749. https://doi.org/10.3390/ijms241813749
APA StyleSenobar Tahaei, S. A., Kulmány, Á., Minorics, R., Kiss, A., Szabó, Z., Germán, P., Szebeni, G. J., Gémes, N., Mernyák, E., & Zupkó, I. (2023). Antiproliferative and Antimetastatic Properties of 16-Azidomethyl Substituted 3-O-Benzyl Estrone Analogs. International Journal of Molecular Sciences, 24(18), 13749. https://doi.org/10.3390/ijms241813749