Emerging Roles of Phospholipase C Beta Isozymes as Potential Biomarkers in Cardiac Disorders
Abstract
1. Introduction
2. Myocardial Phospholipases C Isozymes
Phospholipases C β Isozymes: Structural Organization and Expression in Myocardium
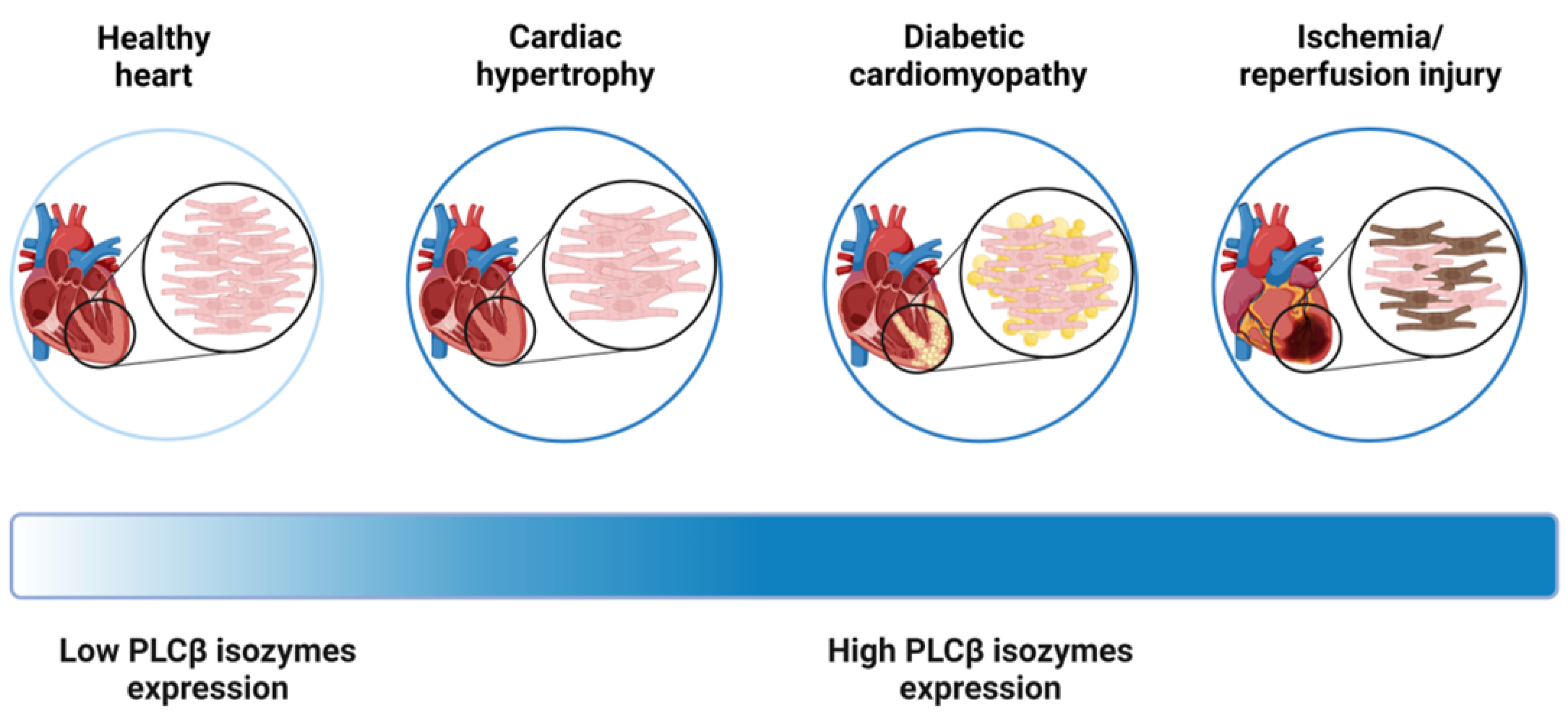
3. Phospholipases C β Isozymes and Their Impact on the Cardiovascular System
3.1. Phospholipases C β Isozymes in Cardiac Hypertrophy
3.2. Phospholipases C β Isozymes in Diabetic Cardiomyopathy
3.3. Phospholipases C β in Myocardial Ischemia/Reperfusion Injury
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kitmitto, A.; Baudoin, F.; Cartwright, E.J. Cardiomyocyte damage control in heart failure and the role of the sarcolemma. J. Muscle Res. Cell Motil. 2019, 40, 319–333. [Google Scholar] [CrossRef] [PubMed]
- See Hoe, L.E.; May, L.T.; Headrick, J.P.; Peart, J.N. Sarcolemmal dependence of cardiac protection and stress-resistance: Roles in aged or diseased hearts. Br. J. Pharmacol. 2016, 173, 2966–2991. [Google Scholar] [CrossRef] [PubMed]
- Pistritu, D.V.; Vasiliniuc, A.C.; Vasiliu, A.; Visinescu, E.F.; Visoiu, I.E.; Vizdei, S.; Martínez Anghel, P.; Tanca, A.; Bucur, O.; Liehn, E.A. Phospholipids, the Masters in the Shadows during Healing after Acute Myocardial Infarction. Int. J. Mol. Sci. 2023, 24, 8360. [Google Scholar] [CrossRef] [PubMed]
- Houang, E.M.; Bartos, J.; Hackel, B.J.; Lodge, T.P.; Yannopoulos, D.; Bates, F.S.; Metzger, J.M. Cardiac Muscle Membrane Stabilization in Myocardial Reperfusion Injury. JACC Basic Transl. Sci. 2019, 4, 275–287. [Google Scholar] [CrossRef]
- Maulik, N.; Kagan, V.E.; Tyurin, V.A.; Das, D.K. Redistribution of phosphatidylethanolamine and phosphatidylserine precedes reperfusion-induced apoptosis. Am. J. Physiol. 1998, 274, H242–H248. [Google Scholar] [CrossRef]
- Fazio, A.; Owusu Obeng, E.; Rusciano, I.; Marvi, M.V.; Zoli, M.; Mongiorgi, S.; Ramazzotti, G.; Follo, M.Y.; McCubrey, J.A.; Cocco, L.; et al. Subcellular Localization Relevance and Cancer-Associated Mechanisms of Diacylglycerol Kinases. Int. J. Mol. Sci. 2020, 21, 5297. [Google Scholar] [CrossRef]
- Owusu Obeng, E.; Rusciano, I.; Marvi, M.V.; Fazio, A.; Ratti, S.; Follo, M.Y.; Xian, J.; Manzoli, L.; Billi, A.M.; Mongiorgi, S.; et al. Phosphoinositide-Dependent Signaling in Cancer: A Focus on Phospholipase C Isozymes. Int. J. Mol. Sci. 2020, 21, 2581. [Google Scholar] [CrossRef]
- Janmey, P.A.; Bucki, R.; Radhakrishnan, R. Regulation of actin assembly by PI(4,5)P2 and other inositol phospholipids: An update on possible mechanisms. Biochem. Biophys. Res. Commun. 2018, 506, 307–314. [Google Scholar] [CrossRef]
- Cocco, L.; Follo, M.Y.; Manzoli, L.; Suh, P.G. Phosphoinositide-specific phospholipase C in health and disease. J. Lipid Res. 2015, 56, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- Katan, M.; Cockcroft, S. Phosphatidylinositol(4,5)bisphosphate: Diverse functions at the plasma membrane. Essays Biochem. 2020, 64, 513–531. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, E.A.; Matkovich, S.J. Ins(1,4,5)P3 receptors and inositol phosphates in the heart-evolutionary artefacts or active signal transducers? Pharmacol. Ther. 2005, 107, 240–251. [Google Scholar] [CrossRef]
- Hohendanner, F.; McCulloch, A.D.; Blatter, L.A.; Michailova, A.P. Calcium and IP3 dynamics in cardiac myocytes: Experimental and computational perspectives and approaches. Front. Pharmacol. 2014, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Kondo, J.; Yamada, Y.; Okumura, K.; Hashimoto, H.; Ito, T.; Satake, T. 1,2-diacylglycerol content in myocardium from spontaneously hypertensive rats during the development of hypertension. Basic Res. Cardiol. 1990, 85, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, E.A.; Matkovich, S.J.; Binah, O. Ins(1,4,5)P3 and cardiac dysfunction. Cardiovasc. Res. 1998, 40, 251–256. [Google Scholar] [CrossRef][Green Version]
- Zhou, J.; Singh, N.; Monnier, C.; Marszalec, W.; Gao, L.; Jin, J.; Frisk, M.; Louch, W.E.; Verma, S.; Krishnamurthy, P.; et al. Phosphatidylinositol-4,5-Bisphosphate Binding to Amphiphysin-II Modulates T-Tubule Remodeling: Implications for Heart Failure. Front. Physiol. 2021, 12, 782767. [Google Scholar] [CrossRef]
- Shoki, M.; Kawaguchi, H.; Okamoto, H.; Sano, H.; Sawa, H.; Kudo, T.; Hirao, N.; Sakata, Y.; Yasuda, H. Phosphatidylinositol and inositolphosphatide metabolism in hypertrophied rat heart. Jpn. Circ. J. 1992, 56, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Prakoso, D.; De Blasio, M.J.; Tate, M.; Kiriazis, H.; Donner, D.G.; Qian, H.; Nash, D.; Deo, M.; Weeks, K.L.; Parry, L.J.; et al. Gene therapy targeting cardiac phosphoinositide 3-kinase (p110α) attenuates cardiac remodeling in type 2 diabetes. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H840–H852. [Google Scholar] [CrossRef] [PubMed]
- Filtz, T.M.; Grubb, D.R.; McLeod-Dryden, T.J.; Luo, J.; Woodcock, E.A. Gq-initiated cardiomyocyte hypertrophy is mediated by phospholipase Cbeta1b. FASEB J. 2009, 23, 3564–3570. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.I.; McGrath, M.F.; de Bold, A.J. Phospholipase C signaling tonically represses basal atrial natriuretic factor secretion from the atria of the heart. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1328–H1336. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grubb, D.R.; Vasilevski, O.; Huynh, H.; Woodcock, E.A. The extreme C-terminal region of phospholipase Cbeta1 determines subcellular localization and function; the “b” splice variant mediates alpha1-adrenergic receptor responses in cardiomyocytes. FASEB J. 2008, 22, 2768–2774. [Google Scholar] [CrossRef]
- Singal, T.; Dhalla, N.S.; Tappia, P.S. Reciprocal regulation of transcription factors and PLC isozyme gene expression in adult cardiomyocytes. J. Cell Mol. Med. 2010, 14, 1824–1835. [Google Scholar] [CrossRef]
- Sawicki, K.T.; Sala, V.; Prever, L.; Hirsch, E.; Ardehali, H.; Ghigo, A. Preventing and Treating Anthracycline Cardiotoxicity: New Insights. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 309–332. [Google Scholar] [CrossRef]
- Dent, M.R.; Aroutiounova, N.; Dhalla, N.S.; Tappia, P.S. Losartan attenuates phospholipase C isozyme gene expression in hypertrophied hearts due to volume overload. J. Cell Mol. Med. 2006, 10, 470–479. [Google Scholar] [CrossRef]
- Saadane, N.; Alpert, L.; Chalifour, L.E. Expression of immediate early genes, GATA-4, and Nkx-2.5 in adrenergic-induced cardiac hypertrophy and during regression in adult mice. Br. J. Pharmacol. 1999, 127, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Otaegui, D.; Querejeta, R.; Arrieta, A.; Lazkano, A.; Bidaurrazaga, A.; Arriandiaga, J.R.; Aldazabal, P.; Garro, M.A. Phospholipase Cbeta4 isozyme is expressed in human, rat, and murine heart left ventricles and in HL-1 cardiomyocytes. Mol. Cell Biochem. 2010, 337, 167–173. [Google Scholar] [CrossRef]
- Tappia, P.S.; Dhalla, N.S. Upregulation of Phospholipase C Gene Expression Due to Norepinephrine-Induced Hypertrophic Response. Cells 2022, 11, 2488. [Google Scholar] [CrossRef]
- Dent, M.R.; Dhalla, N.S.; Tappia, P.S. Phospholipase C gene expression, protein content, and activities in cardiac hypertrophy and heart failure due to volume overload. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H719–H727. [Google Scholar] [CrossRef] [PubMed]
- Kamp, T.J.; Hell, J.W. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ. Res. 2000, 87, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Singh, M. Possible mechanism of cardioprotective effect of ischaemic preconditioning in isolated rat heart. Pharmacol. Res. 2000, 41, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Albert, C.J.; Ford, D.A. Protein kinase C translocation and PKC-dependent protein phosphorylation during myocardial ischemia. Am. J. Physiol. 1999, 276, H642–H650. [Google Scholar] [CrossRef]
- Aloulou, A.; Rahier, R.; Arhab, Y.; Noiriel, A.; Abousalham, A. Phospholipases: An Overview. Methods Mol. Biol. 2018, 1835, 69–105. [Google Scholar] [CrossRef]
- Kadamur, G.; Ross, E.M. Mammalian phospholipase C. Annu. Rev. Physiol. 2013, 75, 127–154. [Google Scholar] [CrossRef]
- Gellatly, S.A.; Kalujnaia, S.; Cramb, G. Cloning, tissue distribution and sub-cellular localisation of phospholipase C X-domain containing protein (PLCXD) isoforms. Biochem. Biophys. Res. Commun. 2012, 424, 651–656. [Google Scholar] [CrossRef]
- Cockcroft, S.; Thomas, G.M. Inositol-lipid-specific phospholipase C isoenzymes and their differential regulation by receptors. Biochem. J. 1992, 288 Pt 1, 1–14. [Google Scholar] [CrossRef]
- Yagisawa, H.; Sakuma, K.; Paterson, H.F.; Cheung, R.; Allen, V.; Hirata, H.; Watanabe, Y.; Hirata, M.; Williams, R.L.; Katan, M. Replacements of single basic amino acids in the pleckstrin homology domain of phospholipase C-delta1 alter the ligand binding, phospholipase activity, and interaction with the plasma membrane. J. Biol. Chem. 1998, 273, 417–424. [Google Scholar] [CrossRef]
- Katan, M.; Cockcroft, S. Phospholipase C families: Common themes and versatility in physiology and pathology. Prog. Lipid Res. 2020, 80, 101065. [Google Scholar] [CrossRef]
- Bill, C.A.; Vines, C.M. Phospholipase C. Adv. Exp. Med. Biol. 2020, 1131, 215–242. [Google Scholar] [CrossRef]
- Lyon, A.M.; Tesmer, J.J. Structural insights into phospholipase C-β function. Mol. Pharmacol. 2013, 84, 488–500. [Google Scholar] [CrossRef]
- Wang, C.K.; Pan, L.; Chen, J.; Zhang, M. Extensions of PDZ domains as important structural and functional elements. Protein Cell 2010, 1, 737–751. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, C.; Liu, C.; Zhang, A.; Li, A.; Zhang, J.; Zhang, Y. Aortic constriction induces hypertension and cardiac hypertrophy via (pro)renin receptor activation and the PLC-β3 signaling pathway. Mol. Med. Rep. 2019, 19, 573–580. [Google Scholar] [CrossRef]
- Harden, T.K.; Waldo, G.L.; Hicks, S.N.; Sondek, J. Mechanism of activation and inactivation of Gq/phospholipase C-β signaling nodes. Chem. Rev. 2011, 111, 6120–6129. [Google Scholar] [CrossRef]
- Grubb, D.R.; Crook, B.; Ma, Y.; Luo, J.; Qian, H.W.; Gao, X.M.; Kiriazis, H.; Du, X.J.; Gregorevic, P.; Woodcock, E.A. The atypical ‘b’ splice variant of phospholipase Cβ1 promotes cardiac contractile dysfunction. J. Mol. Cell Cardiol. 2015, 84, 95–103. [Google Scholar] [CrossRef]
- Woodcock, E.A.; Grubb, D.R.; Filtz, T.M.; Marasco, S.; Luo, J.; McLeod-Dryden, T.J.; Kaye, D.M.; Sadoshima, J.; Du, X.J.; Wong, C.; et al. Selective activation of the “b” splice variant of phospholipase Cbeta1 in chronically dilated human and mouse atria. J. Mol. Cell Cardiol. 2009, 47, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Dewing, J.M.; Saunders, V.; O’Kelly, I.; Wilson, D.I. Defining cardiac cell populations and relative cellular composition of the early fetal human heart. PLoS ONE 2022, 17, e0259477. [Google Scholar] [CrossRef] [PubMed]
- Rainbow, R.D.; Brennan, S.; Jackson, R.; Beech, A.J.; Bengreed, A.; Waldschmidt, H.V.; Tesmer, J.J.G.; Challiss, R.A.J.; Willets, J.M. Small-Molecule G Protein-Coupled Receptor Kinase Inhibitors Attenuate G Protein-Coupled Receptor Kinase 2-Mediated Desensitization of Vasoconstrictor-Induced Arterial Contractions. Mol. Pharmacol. 2018, 94, 1079–1091. [Google Scholar] [CrossRef]
- Su, Y. Regulation of endothelial nitric oxide synthase activity by protein-protein interaction. Curr. Pharm. Des. 2014, 20, 3514–3520. [Google Scholar] [CrossRef]
- Ju, H.; Zhao, S.; Tappia, P.S.; Panagia, V.; Dixon, I.M. Expression of Gq alpha and PLC-beta in scar and border tissue in heart failure due to myocardial infarction. Circulation 1998, 97, 892–899. [Google Scholar] [CrossRef]
- Lian, L.; Wang, Y.; Draznin, J.; Eslin, D.; Bennett, J.S.; Poncz, M.; Wu, D.; Abrams, C.S. The relative role of PLCbeta and PI3Kgamma in platelet activation. Blood 2005, 106, 110–117. [Google Scholar] [CrossRef]
- Koupenova, M.; Kehrel, B.E.; Corkrey, H.A.; Freedman, J.E. Thrombosis and platelets: An update. Eur. Heart J. 2017, 38, 785–791. [Google Scholar] [CrossRef]
- Lee, S.B.; Rao, A.K.; Lee, K.H.; Yang, X.; Bae, Y.S.; Rhee, S.G. Decreased expression of phospholipase C-beta 2 isozyme in human platelets with impaired function. Blood 1996, 88, 1684–1691. [Google Scholar] [CrossRef]
- Yang, X.; Sun, L.; Ghosh, S.; Rao, A.K. Human platelet signaling defect characterized by impaired production of inositol-1,4,5-triphosphate and phosphatidic acid and diminished Pleckstrin phosphorylation: Evidence for defective phospholipase C activation. Blood 1996, 88, 1676–1683. [Google Scholar] [CrossRef]
- Ziaeian, B.; Fonarow, G.C. Epidemiology and aetiology of heart failure. Nat. Rev. Cardiol. 2016, 13, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Dorn, G.W.; Force, T. Protein kinase cascades in the regulation of cardiac hypertrophy. J. Clin. Investig. 2005, 115, 527–537. [Google Scholar] [CrossRef]
- Arthur, J.F.; Matkovich, S.J.; Mitchell, C.J.; Biden, T.J.; Woodcock, E.A. Evidence for selective coupling of alpha 1-adrenergic receptors to phospholipase C-beta 1 in rat neonatal cardiomyocytes. J. Biol. Chem. 2001, 276, 37341–37346. [Google Scholar] [CrossRef] [PubMed]
- Sayed-Ahmed, M.M.; Khattab, M.M.; Gad, M.Z.; Osman, A.M. Increased plasma endothelin-1 and cardiac nitric oxide during doxorubicin-induced cardiomyopathy. Pharmacol. Toxicol. 2001, 89, 140–144. [Google Scholar] [CrossRef]
- Picard, P.; Smith, P.J.; Monge, J.C.; Rouleau, J.L.; Nguyen, Q.T.; Calderone, A.; Stewart, D.J. Coordinated upregulation of the cardiac endothelin system in a rat model of heart failure. J. Cardiovasc. Pharmacol. 1998, 31 (Suppl. S1), S294–S297. [Google Scholar] [CrossRef]
- Schwebe, M.; Ameling, S.; Hammer, E.; Monzel, J.V.; Bonitz, K.; Budde, S.; Schult, K.; Oswald, S.; Scheuch, E.; Grube, M.; et al. Protective effects of endothelin receptor A and B inhibitors against doxorubicin-induced cardiomyopathy. Biochem. Pharmacol. 2015, 94, 109–129. [Google Scholar] [CrossRef] [PubMed]
- Singal, T.; Dhalla, N.S.; Tappia, P.S. Regulation of c-Fos and c-Jun gene expression by phospholipase C activity in adult cardiomyocytes. Mol. Cell Biochem. 2009, 327, 229–239. [Google Scholar] [CrossRef]
- Schnabel, P.; Mies, F.; Nohr, T.; Geisler, M.; Böhm, M. Differential regulation of phospholipase C-beta isozymes in cardiomyocyte hypertrophy. Biochem. Biophys. Res. Commun. 2000, 275, 1–6. [Google Scholar] [CrossRef]
- Park, J.J. Epidemiology, Pathophysiology, Diagnosis and Treatment of Heart Failure in Diabetes. Diabetes Metab. J. 2021, 45, 146–157. [Google Scholar] [CrossRef]
- Ghosh, N.; Chacko, L.; Bhattacharya, H.; Vallamkondu, J.; Nag, S.; Dey, A.; Karmakar, T.; Reddy, P.H.; Kandimalla, R.; Dewanjee, S. Exploring the Complex Relationship between Diabetes and Cardiovascular Complications: Understanding Diabetic Cardiomyopathy and Promising Therapies. Biomedicines 2023, 11, 1126. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.A.; Tappia, P.S.; Yu, C.H.; Bibeau, M.; Panagia, V. Impairment of the sarcolemmal phospholipase D-phosphatidate phosphohydrolase pathway in diabetic cardiomyopathy. J. Mol. Cell Cardiol. 1998, 30, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Heyliger, C.E.; Pierce, G.N.; Singal, P.K.; Beamish, R.E.; Dhalla, N.S. Cardiac alpha- and beta-adrenergic receptor alterations in diabetic cardiomyopathy. Basic. Res. Cardiol. 1982, 77, 610–618. [Google Scholar] [CrossRef]
- Rhee, S.G. Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 2001, 70, 281–312. [Google Scholar] [CrossRef] [PubMed]
- Tappia, P.S.; Asemu, G.; Aroutiounova, N.; Dhalla, N.S. Defective sarcolemmal phospholipase C signaling in diabetic cardiomyopathy. Mol. Cell Biochem. 2004, 261, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yu, M.; Zhou, T.; Zhang, S.; He, G.; Wang, G.; Gang, X. Current advances in the study of diabetic cardiomyopathy: From clinicopathological features to molecular therapeutics (Review). Mol. Med. Rep. 2019, 20, 2051–2062. [Google Scholar] [CrossRef] [PubMed]
- Connelly, K.A.; Kelly, D.J.; Zhang, Y.; Prior, D.L.; Advani, A.; Cox, A.J.; Thai, K.; Krum, H.; Gilbert, R.E. Inhibition of protein kinase C-beta by ruboxistaurin preserves cardiac function and reduces extracellular matrix production in diabetic cardiomyopathy. Circ. Heart Fail. 2009, 2, 129–137. [Google Scholar] [CrossRef]
- Tappia, P.S.; Asemu, G.; Rodriguez-Leyva, D. Phospholipase C as a potential target for cardioprotection during oxidative stress. Can. J. Physiol. Pharmacol. 2010, 88, 249–263. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion—From mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef]
- He, J.; Liu, D.; Zhao, L.; Zhou, D.; Rong, J.; Zhang, L.; Xia, Z. Myocardial ischemia/reperfusion injury: Mechanisms of injury and implications for management (Review). Exp. Ther. Med. 2022, 23, 430. [Google Scholar] [CrossRef]
- Wang, R.; Wang, M.; He, S.; Sun, G.; Sun, X. Targeting Calcium Homeostasis in Myocardial Ischemia/Reperfusion Injury: An Overview of Regulatory Mechanisms and Therapeutic Reagents. Front. Pharmacol. 2020, 11, 872. [Google Scholar] [CrossRef] [PubMed]
- Lefort, C.; Benoist, L.; Chadet, S.; Piollet, M.; Heraud, A.; Babuty, D.; Baron, C.; Ivanes, F.; Angoulvant, D. Stimulation of P2Y11 receptor modulates cardiac fibroblasts secretome toward immunomodulatory and protective roles after Hypoxia/Reoxygenation injury. J. Mol. Cell Cardiol. 2018, 121, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.C. Protein kinase activation and myocardial ischemia/reperfusion injury. Cardiovasc. Res. 2004, 61, 427–436. [Google Scholar] [CrossRef]
- Anderson, K.E.; Dart, A.M.; Woodcock, E.A. Inositol phosphate release and metabolism during myocardial ischemia and reperfusion in rat heart. Circ. Res. 1995, 76, 261–268. [Google Scholar] [CrossRef]
- Prasad, M.R.; Popescu, L.M.; Moraru, I.I.; Liu, X.K.; Maity, S.; Engelman, R.M.; Das, D.K. Role of phospholipases A2 and C in myocardial ischemic reperfusion injury. Am. J. Physiol. 1991, 260, H877–H883. [Google Scholar] [CrossRef]
- Mouton, R.; Huisamen, B.; Lochner, A. Increased myocardial inositol trisphosphate levels during alpha 1-adrenergic stimulation and reperfusion of ischaemic rat heart. J. Mol. Cell Cardiol. 1991, 23, 841–850. [Google Scholar] [CrossRef]
- Asemu, G.; Tappia, P.S.; Dhalla, N.S. Identification of the changes in phospholipase C isozymes in ischemic-reperfused rat heart. Arch. Biochem. Biophys. 2003, 411, 174–182. [Google Scholar] [CrossRef]
- Asemu, G.; Dhalla, N.S.; Tappia, P.S. Inhibition of PLC improves postischemic recovery in isolated rat heart. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2598–H2605. [Google Scholar] [CrossRef]
- Ping, P.; Song, C.; Zhang, J.; Guo, Y.; Cao, X.; Li, R.C.; Wu, W.; Vondriska, T.M.; Pass, J.M.; Tang, X.L.; et al. Formation of protein kinase C(epsilon)-Lck signaling modules confers cardioprotection. J. Clin. Investig. 2002, 109, 499–507. [Google Scholar] [CrossRef]
- Stamm, C.; del Nido, P.J. Protein kinase C and myocardial calcium handling during ischemia and reperfusion: Lessons learned using Rhod-2 spectrofluorometry. Thorac. Cardiovasc. Surg. 2004, 52, 127–134. [Google Scholar] [CrossRef]
- Chen, L.; Shi, D.; Guo, M. The roles of PKC-δ and PKC-ε in myocardial ischemia/reperfusion injury. Pharmacol. Res. 2021, 170, 105716. [Google Scholar] [CrossRef] [PubMed]

| Pathologic Condition | PLCβ Isoform | Model |
|---|---|---|
| Hypertrophy | PLCβ1 | Rat neonatal cardiomyocytes [18,19], Sprague-Dawley rats [20,21,22], neonatal rat ventricular myocytes [23] |
| PLCβ2 | C57BL/6N mice [24], HL-1 murine cardiomyocytes [24] | |
| PLCβ3 | Sprague-Dawley rats [25], neonatal rat cardiomyocytes [26] | |
| PLCβ4 | Human left ventricle biopsy [27], Wistar-Kyoto rats [27], BALB/c mice [27], HL-1 murine cardiomyocytes [27] | |
| Diabetic cardiomyopathy | PLCβ3 | Sprague–Dawley rats [28] |
| Ischemia/reperfusion injury | PLCβ1 | Sprague–Dawley rats [29,30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fazio, A.; Evangelisti, C.; Cappellini, A.; Mongiorgi, S.; Koufi, F.-D.; Neri, I.; Marvi, M.V.; Russo, M.; Ghigo, A.; Manzoli, L.; et al. Emerging Roles of Phospholipase C Beta Isozymes as Potential Biomarkers in Cardiac Disorders. Int. J. Mol. Sci. 2023, 24, 13096. https://doi.org/10.3390/ijms241713096
Fazio A, Evangelisti C, Cappellini A, Mongiorgi S, Koufi F-D, Neri I, Marvi MV, Russo M, Ghigo A, Manzoli L, et al. Emerging Roles of Phospholipase C Beta Isozymes as Potential Biomarkers in Cardiac Disorders. International Journal of Molecular Sciences. 2023; 24(17):13096. https://doi.org/10.3390/ijms241713096
Chicago/Turabian StyleFazio, Antonietta, Camilla Evangelisti, Alessandra Cappellini, Sara Mongiorgi, Foteini-Dionysia Koufi, Irene Neri, Maria Vittoria Marvi, Michele Russo, Alessandra Ghigo, Lucia Manzoli, and et al. 2023. "Emerging Roles of Phospholipase C Beta Isozymes as Potential Biomarkers in Cardiac Disorders" International Journal of Molecular Sciences 24, no. 17: 13096. https://doi.org/10.3390/ijms241713096
APA StyleFazio, A., Evangelisti, C., Cappellini, A., Mongiorgi, S., Koufi, F.-D., Neri, I., Marvi, M. V., Russo, M., Ghigo, A., Manzoli, L., Fiume, R., & Ratti, S. (2023). Emerging Roles of Phospholipase C Beta Isozymes as Potential Biomarkers in Cardiac Disorders. International Journal of Molecular Sciences, 24(17), 13096. https://doi.org/10.3390/ijms241713096







