A Two-Step Transcriptome Analysis of the Human Heart Reveals Broad and Disease-Responsive Expression of Ectopic Olfactory Receptors
Abstract
:1. Introduction
2. Results
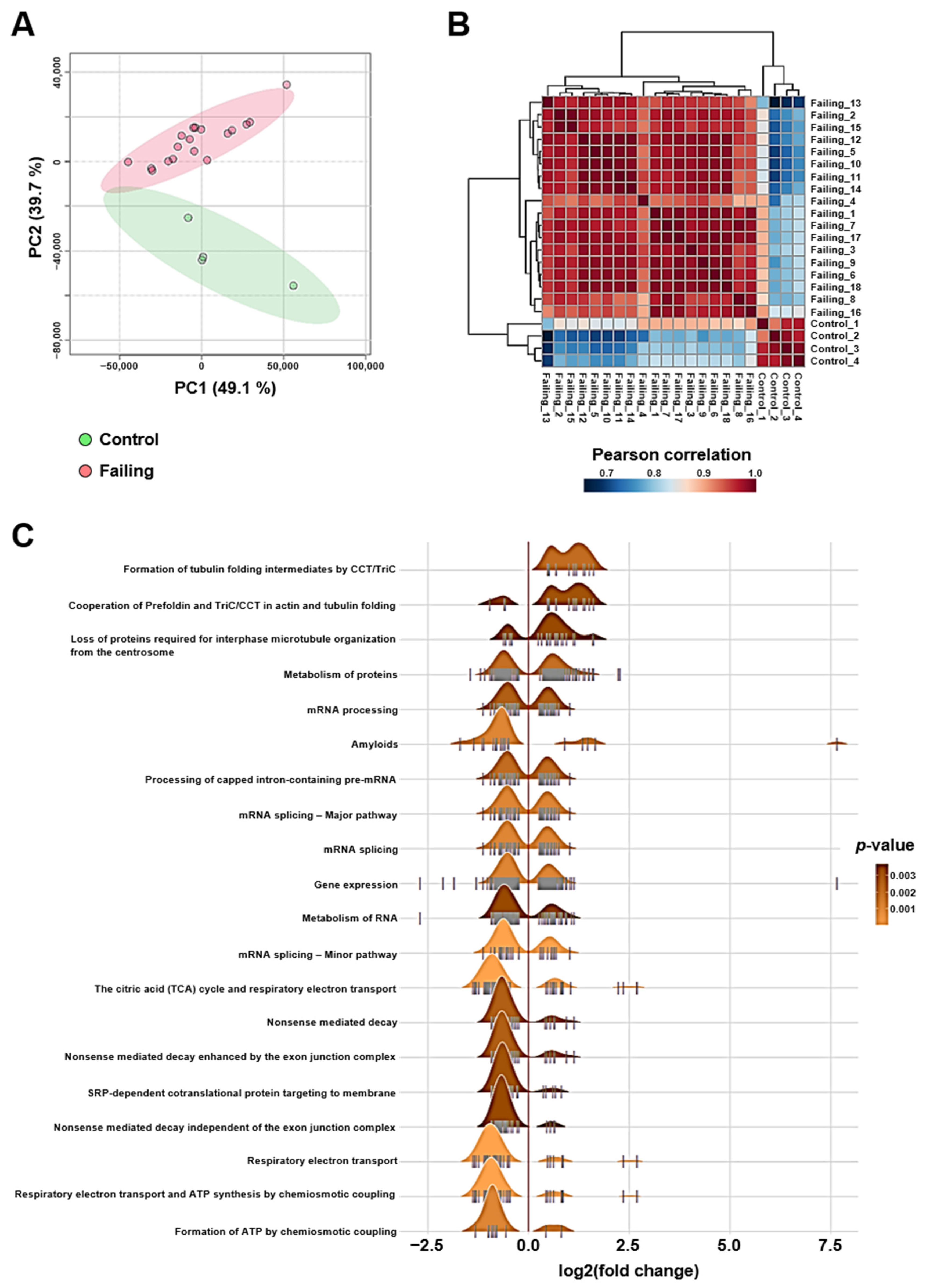
2.1. Altered mRNA Splicing, Proteostasis, and Bioenergetics Are Transcriptomic Features of Heart Failure Caused by Ischemic Cardiomyopathy
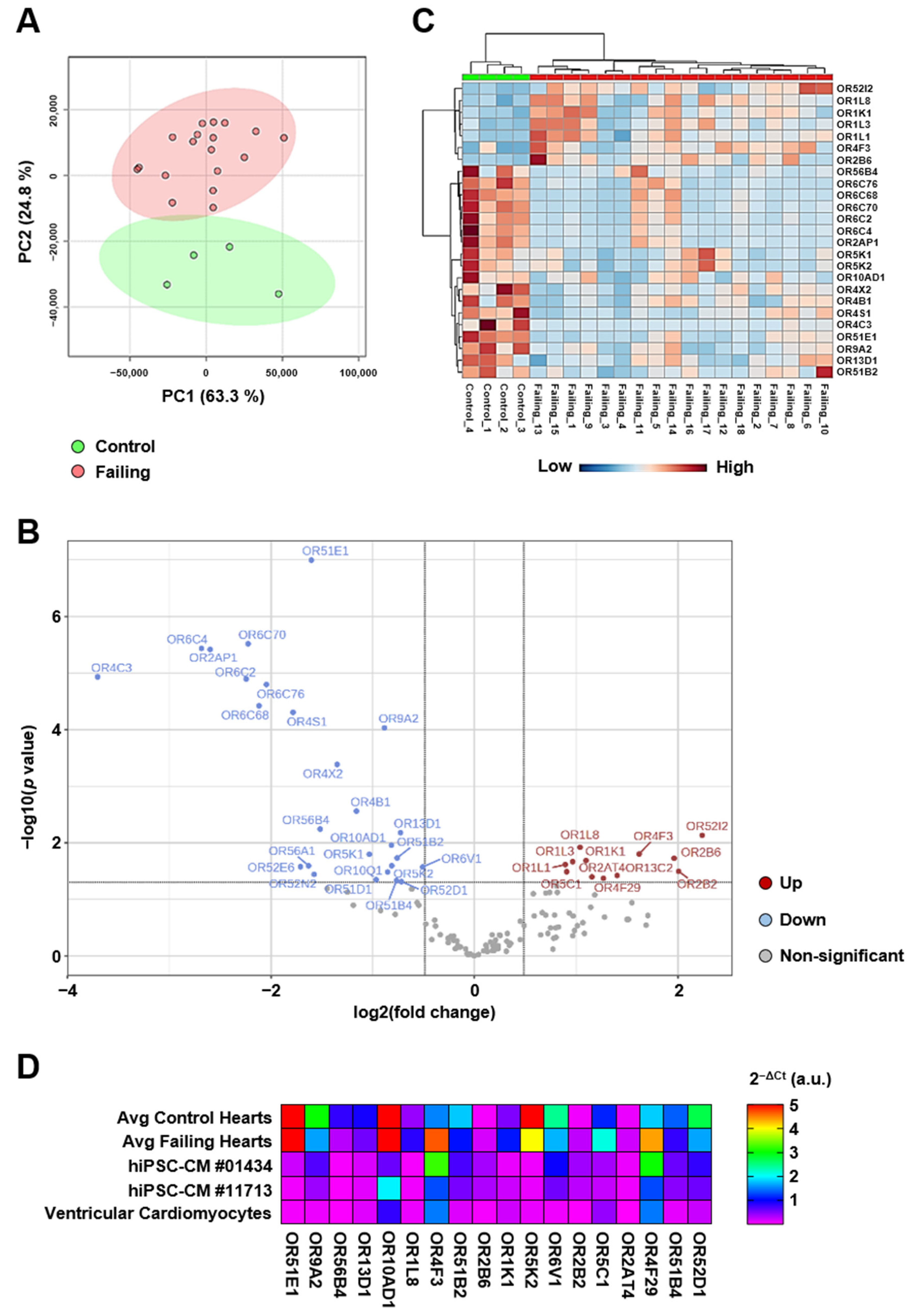
2.2. Cardiac Olfactory Receptor Signaling Is Altered with Heart Failure
2.3. Real-Time PCR Quantification Reveals Broad Expression of Olfactory Receptors in the Human Heart
2.4. Heart Failure Caused by Ischemic Cardiomyopathy Is Characterized by a Specific Ectopic Olfactory Receptor Expression Profile
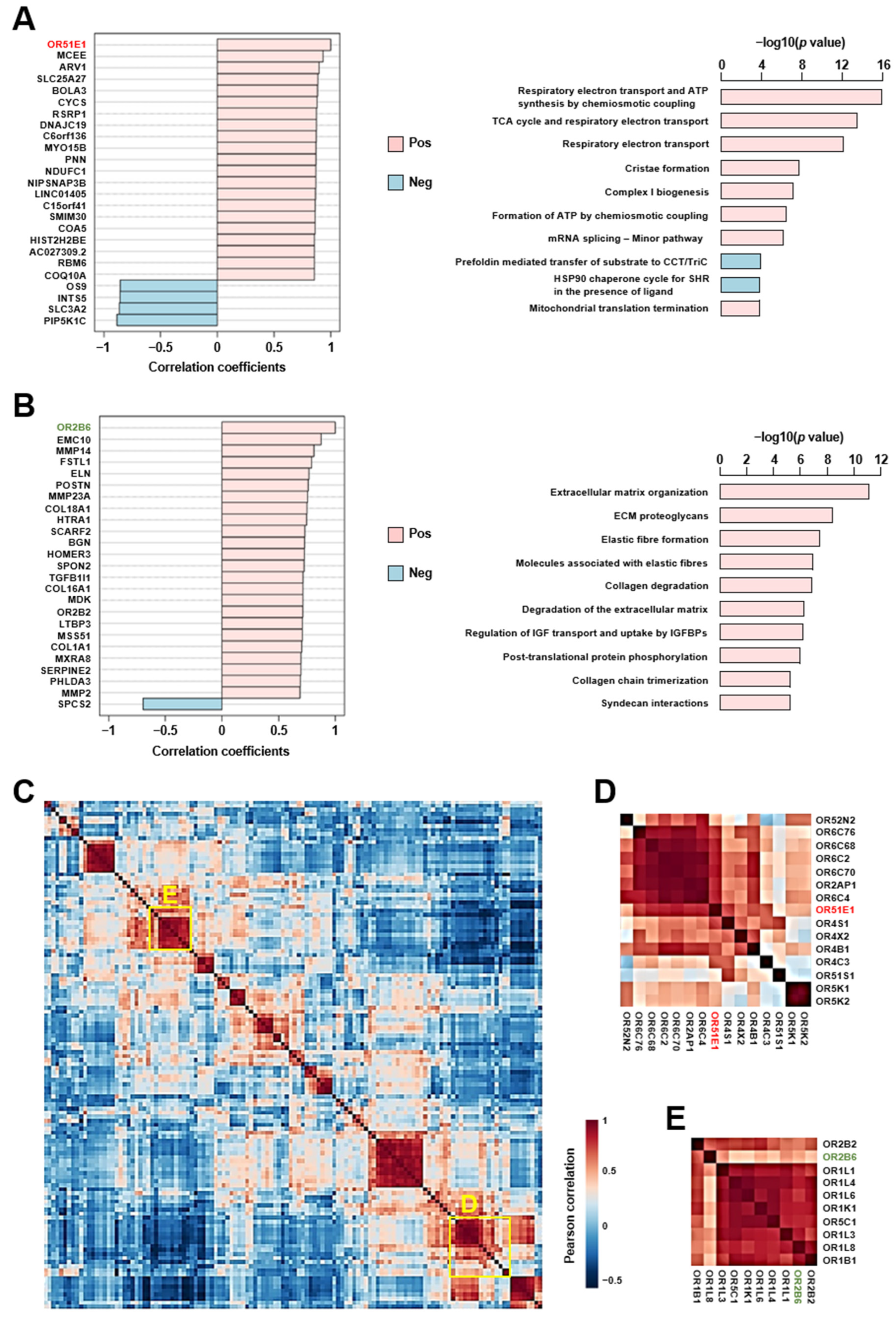
2.5. Altered Cardiac Olfactory Receptor Signaling May Play a Role in the Pathogenesis of Heart Failure
3. Discussion
3.1. A Large Number of Ectopic ORs Are Expressed in the Human Heart
3.2. Heart Failure Caused by Ischemic Cardiomyopathy Changes the Expression Pattern of Cardiac ORs
3.3. Ectopic ORs as Regulators of Cardiometabolic Health
3.4. A Potential Role for Ectopic ORs in Cardiac Remodeling
3.5. Study Limitations
4. Materials and Methods
4.1. Human Heart Samples
4.2. Human Cardiomyocyte Models
4.3. RNA Extraction and RNA Quality Control
4.4. Bulk RNAsequencing
4.5. Real-Time PCR Quantification of Olfactory Receptors
4.6. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bragazzi, N.L.; Zhong, W.; Shu, J.; Abu Much, A.; Lotan, D.; Grupper, A.; Younis, A.; Dai, H. Burden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017. Eur. J. Prev. Cardiol. 2021, 28, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [PubMed]
- Wang, J.; Gareri, C.; Rockman, H.A. G-Protein-Coupled Receptors in Heart Disease. Circ. Res. 2018, 123, 716–735. [Google Scholar] [CrossRef] [PubMed]
- Hakak, Y.; Shrestha, D.; Goegel, M.C.; Behan, D.P.; Chalmers, D.T. Global analysis of G-protein-coupled receptor signaling in human tissues. FEBS Lett. 2003, 550, 11–17. [Google Scholar] [CrossRef]
- Regard, J.B.; Sato, I.T.; Coughlin, S.R. Anatomical profiling of G protein-coupled receptor expression. Cell 2008, 135, 561–571. [Google Scholar] [CrossRef]
- Godfrey, P.A.; Malnic, B.; Buck, L.B. The mouse olfactory receptor gene family. Proc. Natl. Acad. Sci. USA 2004, 101, 2156–2161. [Google Scholar] [CrossRef]
- Trimmer, C.; Keller, A.; Murphy, N.R.; Snyder, L.L.; Willer, J.R.; Nagai, M.H.; Katsanis, N.; Vosshall, L.B.; Matsunami, H.; Mainland, J.D. Genetic variation across the human olfactory receptor repertoire alters odor perception. Proc. Natl. Acad. Sci. USA 2019, 116, 9475–9480. [Google Scholar] [CrossRef]
- Massberg, D.; Hatt, H. Human Olfactory Receptors: Novel Cellular Functions Outside of the Nose. Physiol. Rev. 2018, 98, 1739–1763. [Google Scholar] [CrossRef]
- Zhang, S.; Li, L.; Li, H. Role of ectopic olfactory receptors in glucose and lipid metabolism. Br. J. Pharmacol. 2021, 178, 4792–4807. [Google Scholar] [CrossRef]
- Shepard, B.D. The Sniffing Kidney: Roles for Renal Olfactory Receptors in Health and Disease. Kidney360 2021, 2, 1056–1062. [Google Scholar] [CrossRef]
- Jovancevic, N.; Dendorfer, A.; Matzkies, M.; Kovarova, M.; Heckmann, J.C.; Osterloh, M.; Boehm, M.; Weber, L.; Nguemo, F.; Semmler, J.; et al. Medium-chain fatty acids modulate myocardial function via a cardiac odorant receptor. Basic Res. Cardiol. 2017, 112, 13. [Google Scholar] [CrossRef] [PubMed]
- Flegel, C.; Manteniotis, S.; Osthold, S.; Hatt, H.; Gisselmann, G. Expression profile of ectopic olfactory receptors determined by deep sequencing. PLoS ONE 2013, 8, e55368. [Google Scholar] [CrossRef]
- Zhang, X.; Bedigian, A.V.; Wang, W.; Eggert, U.S. G protein-coupled receptors participate in cytokinesis. Cytoskeleton 2012, 69, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Haywood, G.A.; Gullestad, L.; Katsuya, T.; Hutchinson, H.G.; Pratt, R.E.; Horiuchi, M.; Fowler, M.B. AT1 and AT2 angiotensin receptor gene expression in human heart failure. Circulation 1997, 95, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.J.; Engelhardt, S.; Eschenhagen, T. What is the role of beta-adrenergic signaling in heart failure? Circ. Res. 2003, 93, 896–906. [Google Scholar] [CrossRef]
- Sha, Y.; Phan, J.H.; Wang, M.D. Effect of low-expression gene filtering on detection of differentially expressed genes in RNA-seq data. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2015, 2015, 6461–6464. [Google Scholar] [PubMed]
- Ramirez Flores, R.O.; Lanzer, J.D.; Holland, C.H.; Leuschner, F.; Most, P.; Schultz, J.H.; Levinson, R.T.; Saez-Rodriguez, J. Consensus Transcriptional Landscape of Human End-Stage Heart Failure. J. Am. Heart Assoc. 2021, 10, e019667. [Google Scholar] [CrossRef]
- Raka, R.N.; Wu, H.; Xiao, J.; Hossen, I.; Cao, Y.; Huang, M.; Jin, J. Human ectopic olfactory receptors and their food originated ligands: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 5424–5443. [Google Scholar] [CrossRef]
- Furudono, Y.; Sone, Y.; Takizawa, K.; Hirono, J.; Sato, T. Relationship between peripheral receptor code and perceived odor quality. Chem. Senses 2009, 34, 151–158. [Google Scholar] [CrossRef]
- Malnic, B.; Hirono, J.; Sato, T.; Buck, L.B. Combinatorial receptor codes for odors. Cell 1999, 96, 713–723. [Google Scholar] [CrossRef]
- Bushdid, C.; de March, C.A.; Fiorucci, S.; Matsunami, H.; Golebiowski, J. Agonists of G-Protein-Coupled Odorant Receptors Are Predicted from Chemical Features. J. Phys. Chem. Lett. 2018, 9, 2235–2240. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.E.; Kang, C.W.; Oh, J.H.; Park, S.H.; Ku, C.R.; Cho, Y.H.; Lee, M.K.; Lee, E.J. Olfactory Receptor OR51E1 Mediates GLP-1 Secretion in Human and Rodent Enteroendocrine L Cells. J. Endocr. Soc. 2018, 2, 1251–1258. [Google Scholar] [CrossRef]
- Halperin Kuhns, V.L.; Sanchez, J.; Sarver, D.C.; Khalil, Z.; Rajkumar, P.; Marr, K.A.; Pluznick, J.L. Characterizing novel olfactory receptors expressed in the murine renal cortex. Am. J. Physiol. Renal. Physiol. 2019, 317, F172–F186. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Moore, B.N.; Pluznick, J.L. Short-Chain Fatty Acid Receptors and Blood Pressure Regulation: Council on Hypertension Mid-Career Award for Research Excellence 2021. Hypertension 2022, 79, 2127–2137. [Google Scholar] [CrossRef] [PubMed]
- Miller, H.E.; Bishop, A.J.R. Correlation AnalyzeR: Functional predictions from gene co-expression correlations. BMC Bioinform. 2021, 22, 206. [Google Scholar] [CrossRef]
- Murphy, K.A.; Harsch, B.A.; Healy, C.L.; Joshi, S.S.; Huang, S.; Walker, R.E.; Wagner, B.M.; Ernste, K.M.; Huang, W.; Block, R.C.; et al. Free fatty acid receptor 4 responds to endogenous fatty acids to protect the heart from pressure overload. Cardiovasc. Res. 2022, 118, 1061–1073. [Google Scholar] [CrossRef]
- Zhang, N.; Harsch, B.; Zhang, M.J.; Gyberg, D.J.; Stevens, J.A.; Wagner, B.M.; Mendelson, J.; Patterson, M.T.; Orchard, D.A.; Healy, C.L.; et al. FFAR4 regulates cardiac oxylipin balance to promote inflammation resolution in HFpEF secondary to metabolic syndrome. J. Lipid Res. 2023, 64, 100374. [Google Scholar] [CrossRef]
- Michel, M.C.; Wieland, T.; Tsujimoto, G. How reliable are G-protein-coupled receptor antibodies? Naunyn Schmiedebergs Arch. Pharmacol. 2009, 379, 385–388. [Google Scholar] [CrossRef]
- Guo, M.; Guo, G.; Ji, X. Genetic polymorphisms associated with heart failure: A literature review. J. Int. Med. Res. 2016, 44, 15–29. [Google Scholar] [CrossRef]
- De la Cruz, O.; Blekhman, R.; Zhang, X.; Nicolae, D.; Firestein, S.; Gilad, Y. A signature of evolutionary constraint on a subset of ectopically expressed olfactory receptor genes. Mol. Biol. Evol. 2009, 26, 491–494. [Google Scholar] [CrossRef]
- Feldmesser, E.; Olender, T.; Khen, M.; Yanai, I.; Ophir, R.; Lancet, D. Widespread ectopic expression of olfactory receptor genes. BMC Genom. 2006, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.T.; Toy, P.; McClary, J.A.; Lin, R.J.; Miyamoto, N.G.; Kretschmer, P.J. Cloning and genetic characterization of an evolutionarily conserved human olfactory receptor that is differentially expressed across species. Gene 2001, 278, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Liu, P.; Ewald, J.; Pang, Z.; Legrand, E.; Jeon, Y.S.; Sangiovanni, J.; Hacariz, O.; Zhou, G.; Head, J.A.; Basu, N.; et al. ExpressAnalyst: A unified platform for RNA-sequencing analysis in non-model species. Nat. Commun. 2023, 14, 2995. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.E.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashraf, S.; Frazier, O.H.; Carranza, S.; McPherson, D.D.; Taegtmeyer, H.; Harmancey, R. A Two-Step Transcriptome Analysis of the Human Heart Reveals Broad and Disease-Responsive Expression of Ectopic Olfactory Receptors. Int. J. Mol. Sci. 2023, 24, 13709. https://doi.org/10.3390/ijms241813709
Ashraf S, Frazier OH, Carranza S, McPherson DD, Taegtmeyer H, Harmancey R. A Two-Step Transcriptome Analysis of the Human Heart Reveals Broad and Disease-Responsive Expression of Ectopic Olfactory Receptors. International Journal of Molecular Sciences. 2023; 24(18):13709. https://doi.org/10.3390/ijms241813709
Chicago/Turabian StyleAshraf, Sadia, O. Howard Frazier, Sylvia Carranza, David D. McPherson, Heinrich Taegtmeyer, and Romain Harmancey. 2023. "A Two-Step Transcriptome Analysis of the Human Heart Reveals Broad and Disease-Responsive Expression of Ectopic Olfactory Receptors" International Journal of Molecular Sciences 24, no. 18: 13709. https://doi.org/10.3390/ijms241813709
APA StyleAshraf, S., Frazier, O. H., Carranza, S., McPherson, D. D., Taegtmeyer, H., & Harmancey, R. (2023). A Two-Step Transcriptome Analysis of the Human Heart Reveals Broad and Disease-Responsive Expression of Ectopic Olfactory Receptors. International Journal of Molecular Sciences, 24(18), 13709. https://doi.org/10.3390/ijms241813709






