Function and Characteristic Analysis of Candidate PEAR Proteins in Populus yunnanensis
Abstract
:1. Introduction
2. Results
2.1. Genome-Wide Identification of PEAR Candidates
2.2. Sequence Characteristics and Phylogenetic Relationship of PEAR Candidates
2.3. Phylogenetic Relationships and Molecular Characterization of PEAR Candidates in P. yunnanensis
2.4. Cis-Elements in Promoter Region of Candidate Proteins of AtPEAR Coding Genes
2.5. Expression Patterns of PEAR Candidates in Different Tissues and Stress Treatment
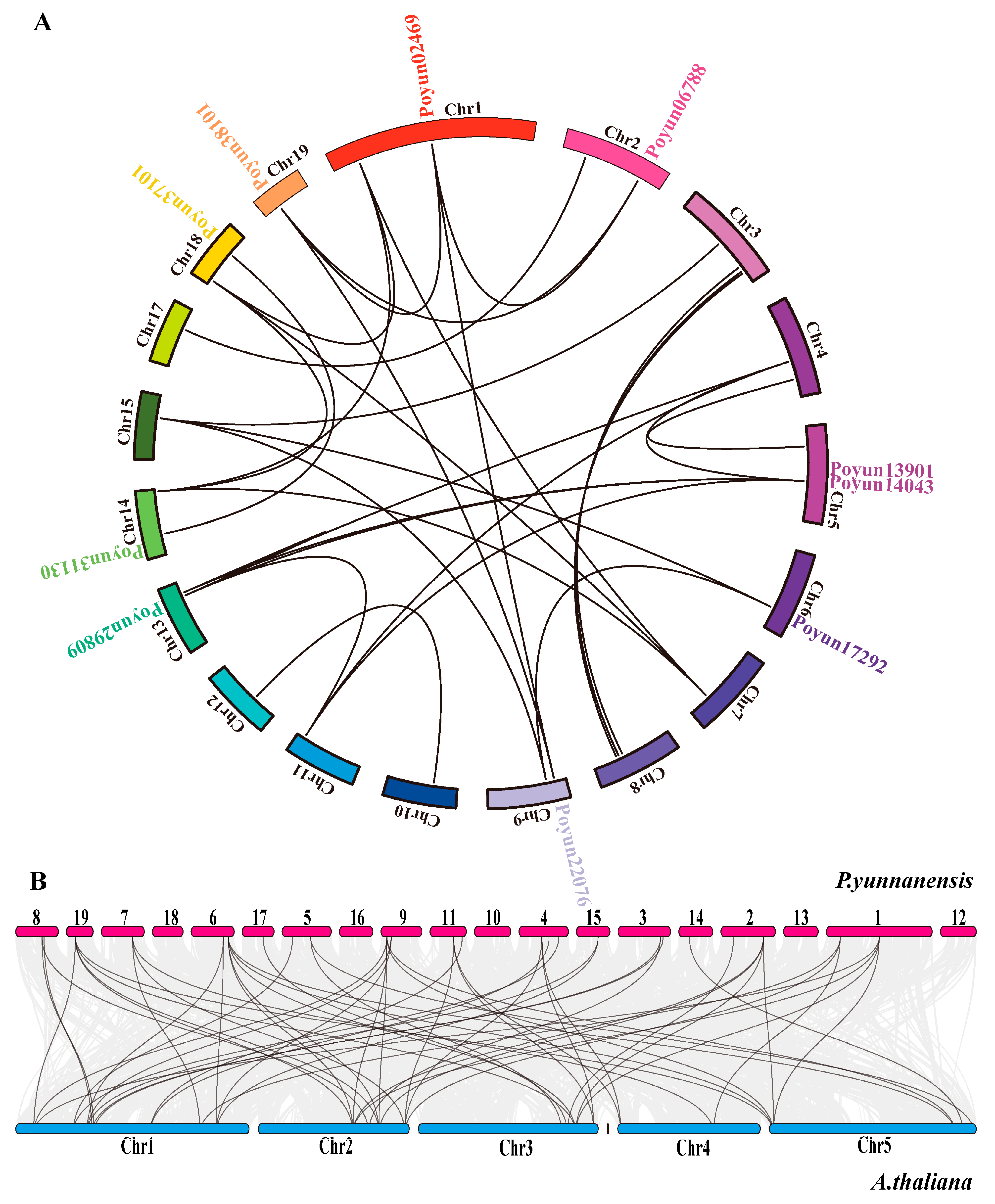
2.6. Chromosomal Localization and Collinearity Analysis of PEAR Candidates
2.7. Interaction Networks of PEAR Candidates
3. Discussion
4. Materials and Methods
4.1. Identification of Candidate PEAR Proteins
4.2. Phylogenetic Analysis of PEAR Candidates in P. yunnanensis
4.3. Gene Structure, Conserved Motifs, Domains and Cis-Elements of Candidate PEAR Genes
4.4. Expression Pattern of PEAR Candidates in P. trichocarpa
4.5. Plant Materials and qRT-PCR Assays
4.6. Location and Collinearity Analysis of PEAR Candidates Intraspecific and Interspecies
4.7. Prediction of the Interaction Proteins and Collinearity Analysis of PEAR Candidates in P. yunnanensis
4.8. Yeast One Hybrid Assay and Yeast Two Hybrid Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, A.; Samtani, H.; Sahu, K.; Sharma, A.K.; Khurana, J.P.; Khurana, P. Functions of Phytochrome-Interacting Factors (PIFs) in the regulation of plant growth and development: A comprehensive review. Int. J. Biol. Macromol. 2023, 244, 125234. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Cao, Y.; Zhao, L.; Zhang, J.; Li, S. Review: WRKY transcription factors: Understanding the functional divergence. Plant Sci. Int. J. Exp. Plant Biol. 2023, 334, 111770. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, J.; Sun, C.; Zhao, H.; Zhang, Y.; Chen, J. BTF3 promotes proliferation and glycolysis in hepatocellular carcinoma by regulating GLUT1. Cancer Biol. Ther. 2023, 24, 2225884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xia, P. The DREB transcription factor, a biomacromolecule, responds to abiotic stress by regulating the expression of stress-related genes. Int. J. Biol. Macromol. 2023, 243, 125231. [Google Scholar] [CrossRef]
- Zou, X.; Sun, H. DOF transcription factors: Specific regulators of plant biological processes. Front. Plant Sci. 2023, 14, 1044918. [Google Scholar] [CrossRef]
- Yanagisawa, S.; Izui, K. Molecular cloning of two DNA-binding proteins of maize that are structurally different but interact with the same sequence motif. J. Biol. Chem. 1993, 268, 16028–16036. [Google Scholar] [CrossRef]
- Umemura, Y.; Ishiduka, T.; Yamamoto, R.; Esaka, M. The Dof domain, a zinc finger DNA-binding domain conserved only in higher plants, truly functions as a Cys2/Cys2 Zn finger domain. Plant J. Cell Mol. Biol. 2004, 37, 741–749. [Google Scholar] [CrossRef]
- Gupta, S.; Malviya, N.; Kushwaha, H.; Nasim, J.; Bisht, N.C.; Singh, V.K.; Yadav, D. Insights into structural and functional diversity of Dof (DNA binding with one finger) transcription factor. Planta 2015, 241, 549–562. [Google Scholar] [CrossRef]
- Miyashima, S.; Roszak, P.; Sevilem, I.; Toyokura, K.; Blob, B.; Heo, J.O.; Mellor, N.; Help-Rinta-Rahko, H.; Otero, S.; Smet, W.; et al. Mobile PEAR transcription factors integrate positional cues to prime cambial growth. Nature 2019, 565, 490–494. [Google Scholar] [CrossRef]
- Chong, S.N.; Ravindran, P.; Kumar, P.P. Regulation of primary seed dormancy by MAJOR LATEX PROTEIN-LIKE PROTEIN329 in Arabidopsis is dependent on DNA-BINDING ONE ZINC FINGER6. J. Exp. Bot. 2022, 73, 6838–6852. [Google Scholar] [CrossRef]
- Rueda-Romero, P.; Barrero-Sicilia, C.; Gómez-Cadenas, A.; Carbonero, P.; Oñate-Sánchez, L. Arabidopsis thaliana DOF6 negatively affects germination in non-after-ripened seeds and interacts with TCP14. J. Exp. Bot. 2012, 63, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, P.; Verma, V.; Stamm, P.; Kumar, P.P. A Novel RGL2-DOF6 Complex Contributes to Primary Seed Dormancy in Arabidopsis thaliana by Regulating a GATA Transcription Factor. Mol. Plant 2017, 10, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Matsuoka, K.; Kareem, A.; Robert, M.; Roszak, P.; Blob, B.; Bisht, A.; De Veylder, L.; Voiniciuc, C.; Asahina, M.; et al. Cell-wall damage activates DOF transcription factors to promote wound healing and tissue regeneration in Arabidopsis thaliana. Curr. Biol. 2022, 32, 1883–1894.e1887. [Google Scholar] [CrossRef] [PubMed]
- Skirycz, A.; Reichelt, M.; Burow, M.; Birkemeyer, C.; Rolcik, J.; Kopka, J.; Zanor, M.I.; Gershenzon, J.; Strnad, M.; Szopa, J.; et al. DOF transcription factor AtDof1.1 (OBP2) is part of a regulatory network controlling glucosinolate biosynthesis in Arabidopsis. Plant J. Cell Mol. Biol. 2006, 47, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Dehshahri, S.; Afsharypuor, S.; Asghari, G.; Mohagheghzadeh, A. Determination of volatile glucosinolate degradation products in seed coat, stem and in vitro cultures of Moringa peregrina (Forssk.) Fiori. Res. Pharm. Sci. 2012, 7, 51–56. [Google Scholar] [PubMed]
- Matosevich, R.; Cohen, I.; Gil-Yarom, N.; Modrego, A.; Friedlander-Shani, L.; Verna, C.; Scarpella, E.; Efroni, I. Local auxin biosynthesis is required for root regeneration after wounding. Nat. Plants 2020, 6, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Scarpella, E.; Meijer, A.H. Pattern formation in the vascular system of monocot and dicot plant species. New Phytol. 2004, 164, 209–242. [Google Scholar] [CrossRef]
- Guo, Y.; Qin, G.; Gu, H.; Qu, L.J. Dof5.6/HCA2, a Dof transcription factor gene, regulates interfascicular cambium formation and vascular tissue development in Arabidopsis. Plant Cell 2009, 21, 3518–3534. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, N.; Deng, X.; Liu, D.; Li, M.; Cui, D.; Hu, Y.; Yan, Y. Genome-wide analysis of wheat DNA-binding with one finger (Dof) transcription factor genes: Evolutionary characteristics and diverse abiotic stress responses. BMC Genom. 2020, 21, 276. [Google Scholar] [CrossRef]
- Ma, J.; Li, M.Y.; Wang, F.; Tang, J.; Xiong, A.S. Genome-wide analysis of Dof family transcription factors and their responses to abiotic stresses in Chinese cabbage. BMC Genom. 2015, 16, 33. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, S.; Gao, Y.; Yang, J. Characterization of Dof Transcription Factors and Their Responses to Osmotic Stress in Poplar (Populus trichocarpa). PLoS ONE 2017, 12, e0170210. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Cheng, J.; Cui, J.; Xu, X.; Liang, G.; Luo, X.; Chen, X.; Tang, X.; Hu, K.; Qin, C. Genome-Wide Identification and Expression Profile of Dof Transcription Factor Gene Family in Pepper (Capsicum annuum L.). Front. Plant Sci. 2016, 7, 574. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Zhang, X. Genome-wide identification and comparative evolutionary analysis of the Dof transcription factor family in physic nut and castor bean. PeerJ 2019, 7, e6354. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.J.; Song, L.L.; Zhang, J.; Liu, Y.; Guo, C.H. Genome-wide identification and characterization of the Dof gene family in Medicago truncatula. Genet. Mol. Res. GMR 2015, 14, 10645–10657. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zhang, Y.; Zhang, C.; Zhang, T.; Hu, T.; Ye, J.; Zhang, J.; Wang, T.; Li, H.; Ye, Z. Genome-wide analysis of plant-specific Dof transcription factor family in tomato. J. Integr. Plant Biol. 2013, 55, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Lijavetzky, D.; Carbonero, P.; Vicente-Carbajosa, J. Genome-wide comparative phylogenetic analysis of the rice and Arabidopsis Dof gene families. BMC Evol. Biol. 2003, 3, 17. [Google Scholar] [CrossRef]
- Zou, Z.; Zhu, J.; Zhang, X. Genome-wide identification and characterization of the Dof gene family in cassava (Manihot esculenta). Gene 2019, 687, 298–307. [Google Scholar] [CrossRef]
- Rojas-Gracia, P.; Roque, E.; Medina, M.; López-Martín, M.J.; Cañas, L.A.; Beltrán, J.P.; Gómez-Mena, C. The DOF Transcription Factor SlDOF10 Regulates Vascular Tissue Formation During Ovary Development in Tomato. Front. Plant Sci. 2019, 10, 216. [Google Scholar] [CrossRef]
- Skirycz, A.; Radziejwoski, A.; Busch, W.; Hannah, M.A.; Czeszejko, J.; Kwaśniewski, M.; Zanor, M.I.; Lohmann, J.U.; De Veylder, L.; Witt, I.; et al. The DOF transcription factor OBP1 is involved in cell cycle regulation in Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2008, 56, 779–792. [Google Scholar] [CrossRef]
- Negi, J.; Moriwaki, K.; Konishi, M.; Yokoyama, R.; Nakano, T.; Kusumi, K.; Hashimoto-Sugimoto, M.; Schroeder, J.I.; Nishitani, K.; Yanagisawa, S.; et al. A Dof transcription factor, SCAP1, is essential for the development of functional stomata in Arabidopsis. Curr. Biol. 2013, 23, 479–484. [Google Scholar] [CrossRef]
- Sun, S.; Wang, B.; Jiang, Q.; Li, Z.; Jia, S.; Wang, Y.; Guo, H. Genome-wide analysis of BpDof genes and the tolerance to drought stress in birch (Betula platyphylla). PeerJ 2021, 9, e11938. [Google Scholar] [CrossRef]
- Liu, D.; Zheng, K.; Wang, Y.; Zhang, Y.; Lao, R.; Qin, Z.; Li, T.; Zhao, Z. Harnessing an arbuscular mycorrhizal fungus to improve the adaptability of a facultative metallophytic poplar (Populus yunnanensis) to cadmium stress: Physiological and molecular responses. J. Hazard. Mater. 2022, 424, 127430. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, C.; Hu, N.; Wang, B.; Zheng, K.; Zhao, Z.; Li, T. Contributions of ectomycorrhizal fungi in a reclaimed poplar forest (Populus yunnanensis) in an abandoned metal mine tailings pond, southwest China. J. Hazard. Mater. 2023, 448, 130962. [Google Scholar] [CrossRef]
- Sang, Y.; Long, Z.; Dan, X.; Feng, J.; Shi, T.; Jia, C.; Zhang, X.; Lai, Q.; Yang, G.; Zhang, H.; et al. Genomic insights into local adaptation and future climate-induced vulnerability of a keystone forest tree in East Asia. Nat. Commun. 2022, 13, 6541. [Google Scholar] [CrossRef]
- Tippmann, H.F. Analysis for free: Comparing programs for sequence analysis. Brief. Bioinform. 2004, 5, 82–87. [Google Scholar] [CrossRef]
- Pan, X.; Wang, C.; Liu, Z.; Gao, R.; Feng, L.; Li, A.; Yao, K.; Liao, W. Identification of ABF/AREB gene family in tomato (Solanum lycopersicum L.) and functional analysis of ABF/AREB in response to ABA and abiotic stresses. PeerJ 2023, 11, e15310. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, J.; Mao, X.; Li, C.; Li, L.; Yang, X.; Hao, C.; Chang, X.; Li, R.; Jing, R. Association Analysis Revealed That TaPYL4 Genes Are Linked to Plant Growth Related Traits in Multiple Environment. Front. Plant Sci. 2021, 12, 641087. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Yu, Q.; Zeng, J.; He, X.; Ma, W.; Ge, L.; Liu, W. Comprehensive Analysis of the INDETERMINATE DOMAIN (IDD) Gene Family and Their Response to Abiotic Stress in Zea mays. Int. J. Mol. Sci. 2023, 24, 6185. [Google Scholar] [CrossRef]
- Yang, W.; Feng, L.; Luo, J.; Zhang, H.; Jiang, F.; He, Y.; Li, X.; Du, J.; Owusu Adjei, M.; Luan, A.; et al. Genome-Wide Identification and Characterization of R2R3-MYB Provide Insight into Anthocyanin Biosynthesis Regulation Mechanism of Ananas comosus var. bracteatus. Int. J. Mol. Sci. 2023, 24, 3133. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Yan, J.; Ali, M.M.; Wang, S.; Tian, S.; Chen, F.; Lin, Z. Isolation and Functional Characterization of a Green-Tissue Promoter in Japonica Rice (Oryza sativa subsp. Japonica). Biology 2022, 11, 1092. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Y.; Zhang, H.; Xu, J.; Gao, X.; Zhang, T.; Liu, X.; Guo, L.; Zhao, D. Environmental F actors coordinate circadian clock function and rhythm to regulate plant development. Plant Signal. Behav. 2023, 18, 2231202. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef]
- Tokunaga, S.; Sanda, S.; Uraguchi, Y.; Nakagawa, S.; Sawayama, S. Overexpression of the DOF-Type Transcription Factor Enhances Lipid Synthesis in Chlorella vulgaris. Appl. Biochem. Biotechnol. 2019, 189, 116–128. [Google Scholar] [CrossRef]
- Wu, J.; Chen, L.; Chen, M.; Zhou, W.; Dong, Q.; Jiang, H.; Cheng, B. The DOF-Domain Transcription Factor ZmDOF36 Positively Regulates Starch Synthesis in Transgenic Maize. Front. Plant Sci. 2019, 10, 465. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Song, Y.; Gao, H.; Wang, M.; Cui, H.; Ji, C.; Wang, J.; Yuan, L.; Li, R. Genome-wide identification and functional analysis of Dof transcription factor family in Camelina sativa. BMC Genom. 2022, 23, 812. [Google Scholar] [CrossRef]
- Li, M.; Chen, X.; Huang, W.; Li, Y.; Liu, Q.; Yan, W.; Guo, C.; Shu, Y. Genome-wide analysis of the CDPK gene family and their important roles response to cold stress in white clover. Plant Signal. Behav. 2023, 18, 2213924. [Google Scholar] [CrossRef]
- Żyła, N.; Babula-Skowrońska, D. Evolutionary Consequences of Functional and Regulatory Divergence of HD-Zip I Transcription Factors as a Source of Diversity in Protein Interaction Networks in Plants. J. Mol. Evol. 2023. [Google Scholar] [CrossRef]
- Luu, T.B.; Carles, N.; Bouzou, L.; Gibelin-Viala, C.; Remblière, C.; Gasciolli, V.; Bono, J.J.; Lefebvre, B.; Pauly, N.; Cullimore, J. Analysis of the structure and function of the LYK cluster of Medicago truncatula A17 and R108. Plant Sci. Int. J. Exp. Plant Biol. 2023, 332, 111696. [Google Scholar] [CrossRef]
- Guo, Y.; Qiu, L.J. Genome-wide analysis of the Dof transcription factor gene family reveals soybean-specific duplicable and functional characteristics. PLoS ONE 2013, 8, e76809. [Google Scholar] [CrossRef]
- Chen, M.; He, X.; Huang, X.; Lu, T.; Zhang, Y.; Zhu, J.; Yu, H.; Luo, C. Cis-element amplified polymorphism (CEAP), a novel promoter- and gene-targeted molecular marker of plants. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2022, 28, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.; Wang, Y.; Xu, L.; Yao, S.; Wang, K.; Dong, J.; Ma, Y.; Wang, L.; Xie, Y.; Yan, K.; et al. RsGLK2.1-RsNF-YA9a module positively regulates the chlorophyll biosynthesis by activating RsHEMA2 in green taproot of radish. Plant Sci. Int. J. Exp. Plant Biol. 2023, 334, 111768. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zong, X.; Ren, P.; Qian, Y.; Fu, A. Basic Helix-Loop-Helix (bHLH) Transcription Factors Regulate a Wide Range of Functions in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 7152. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Takahashi, H.; Kunieda, T.; Fuji, K.; Shimada, T.; Hara-Nishimura, I. Arabidopsis KAM2/GRV2 is required for proper endosome formation and functions in vacuolar sorting and determination of the embryo growth axis. Plant Cell 2007, 19, 320–332. [Google Scholar] [CrossRef]
- Imaizumi, T.; Tran, H.G.; Swartz, T.E.; Briggs, W.R.; Kay, S.A. FKF1 is essential for photoperiodic-specific light signalling in Arabidopsis. Nature 2003, 426, 302–306. [Google Scholar] [CrossRef]
- Mitsui, H.; Hasezawa, S.; Nagata, T.; Takahashi, H. Cell cycle-dependent accumulation of a kinesin-like protein, KatB/C in synchronized tobacco BY-2 cells. Plant Mol. Biol. 1996, 30, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Hemerly, A.; Bergounioux, C.; Van Montagu, M.; Inzé, D.; Ferreira, P. Genes regulating the plant cell cycle: Isolation of a mitotic-like cyclin from Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1992, 89, 3295–3299. [Google Scholar] [CrossRef] [PubMed]
- Sekine, M.; Ito, M.; Uemukai, K.; Maeda, Y.; Nakagami, H.; Shinmyo, A. Isolation and characterization of the E2F-like gene in plants. FEBS Lett. 1999, 460, 117–122. [Google Scholar] [CrossRef]
- Ao, D.; Li, S.; Jiang, S.; Luo, J.; Chen, N.; Meurens, F.; Zhu, J. Inter-relation analysis of signaling adaptors of porcine innate immune pathways. Mol. Immunol. 2020, 121, 20–27. [Google Scholar] [CrossRef]
- Ayaz, A.; Saqib, S.; Huang, H.; Zaman, W.; Lü, S.; Zhao, H. Genome-wide comparative analysis of long-chain acyl-CoA synthetases (LACSs) gene family: A focus on identification, evolution and expression profiling related to lipid synthesis. Plant Physiol. Biochem. 2021, 161, 1–11. [Google Scholar] [CrossRef]
- Emms, D.M.; Kelly, S. OrthoFinder: Phylogenetic orthology inference for comparative genomics. Genome Biol. 2019, 20, 238. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Series 1999, 41, 95–98. [Google Scholar]
- Hall, B.G. Building Phylogenetic Trees from Molecular Data with MEGA. Mol. Biol. Evol. 2013, 30, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ma, D.; Hu, Z.; Zong, D.; He, C. PyunBBX18 Is Involved in the Regulation of Anthocyanins Biosynthesis under UV-B Stress. Genes 2022, 13, 1811. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yu, A.; Sun, R.; Liu, A. Function and Evolution of C1-2i Subclass of C2H2-Type Zinc Finger Transcription Factors in POPLAR. Genes 2022, 13, 1843. [Google Scholar] [CrossRef]
- Regier, N.; Frey, B. Experimental comparison of relative RT-qPCR quantification approaches for gene expression studies in poplar. BMC Mol. Biol. 2010, 11, 57. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Phizicky, E.M.; Fields, S. Protein-protein interactions: Methods for detection and analysis. Microbiol. Rev. 1995, 59, 94–123. [Google Scholar] [CrossRef]






| Gene ID a | Number of Amino Acids b | Zf-Dof domain c | Molecular Weight d | PI e | Location f | Chromosomal Location g | ||
|---|---|---|---|---|---|---|---|---|
| Start | End | Length | ||||||
| Poyun37101 | 312 | 52 | 110 | 58 | 34,201.15 | 6.43 | nucl | 18 |
| Poyun31130 | 313 | 52 | 110 | 58 | 34,667.67 | 6.19 | nucl | 14 |
| Poyun31438 | 321 | 32 | 90 | 58 | 35,219.45 | 7.7 | nucl | 14 |
| Poyun36927 | 329 | 32 | 90 | 58 | 36,234.49 | 6.36 | nucl | 18 |
| Poyun31819 | 255 | 32 | 90 | 58 | 28,121.26 | 8.78 | nucl | 14 |
| Poyun02469 | 323 | 69 | 127 | 58 | 34,513.42 | 9.58 | nucl | 1 |
| Poyun09605 | 324 | 29 | 87 | 58 | 35,482 | 9.25 | nucl | 3 |
| Poyun19219 | 279 | 38 | 96 | 58 | 30,702.25 | 8.63 | nucl | 7 |
| Poyun00813 | 281 | 38 | 96 | 58 | 30,940.44 | 8.17 | nucl | 1 |
| Poyun17292 | 338 | 74 | 132 | 58 | 35,599.59 | 9.3 | nucl | 6 |
| Poyun29809 | 344 | 33 | 91 | 58 | 37,066.87 | 8.28 | nucl | 13 |
| Poyun13901 | 342 | 33 | 91 | 58 | 37,178.24 | 8.68 | nucl | 5 |
| Poyun14043 | 316 | 52 | 110 | 58 | 33,785.64 | 9.37 | nucl | 5 |
| Poyun26623 | 261 | 21 | 79 | 58 | 28,665.65 | 9.15 | nucl | 11 |
| Poyun22076 | 345 | 71 | 129 | 58 | 36,891.26 | 9.22 | nucl | 9 |
| Poyun09599 | 344 | 56 | 114 | 58 | 37,933.06 | 8.37 | nucl | 3 |
| Poyun21042 | 325 | 30 | 88 | 58 | 35,740.27 | 9.37 | nucl | 8 |
| Poyun21037 | 354 | 58 | 116 | 58 | 38,875.33 | 8.87 | nucl | 8 |
| Poyun12085 | 300 | 28 | 86 | 58 | 24,100.95 | 4.77 | nucl | 4 |
| Poyun13505 | 301 | 28 | 86 | 58 | 34,055.84 | 4.82 | nucl | 5 |
| Poyun11000 | 278 | 36 | 94 | 58 | 30,617.93 | 9.1 | nucl | 4 |
| Poyun06788 | 326 | 71 | 129 | 58 | 34,632.6 | 9.1 | nucl | 2 |
| Poyun38101 | 327 | 71 | 129 | 58 | 34,892.83 | 9.32 | nucl | 19 |
| Poyun18110 | 235 | 18 | 76 | 58 | 25,176.39 | 8.58 | nucl | 7 |
| Poyun30052 | 248 | 33 | 91 | 58 | 25,498.43 | 8.57 | mito | 13 |
| Poyun30013 | 248 | 33 | 91 | 58 | 25,498.43 | 8.57 | mito | 13 |
| Poyun05571 | 288 | 10 | 68 | 58 | 31,891.04 | 6.25 | nucl | 2 |
| Poyun27200 | 261 | 27 | 85 | 58 | 27,456.1 | 5.95 | nucl | 11 |
| Poyun36479 | 397 | 34 | 92 | 58 | 32,723.99 | 8.19 | nucl | 18 |
| Poyun09665 | 304 | 44 | 102 | 58 | 33,883.68 | 8.73 | nucl | 3 |
| Poyun35699 | 288 | 10 | 68 | 58 | 32,217.52 | 5.93 | nucl | 17 |
| Poyun14051 | 253 | 33 | 91 | 58 | 25,892.81 | 8.66 | cyto | 5 |
| Poyun11459 | 253 | 27 | 85 | 58 | 25,892.81 | 8.66 | nucl | 4 |
| Poyun21099 | 305 | 45 | 102 | 57 | 33,822.45 | 8.12 | nucl | 8 |
| Poyun09476 | 159 | 40 | 98 | 58 | 17,696.89 | 9.23 | nucl | 3 |
| Poyun08772 | 503 | 139 | 197 | 58 | 55,181.76 | 5.46 | nucl | 3 |
| Poyun22412 | 500 | 143 | 201 | 58 | 54,146.23 | 6.29 | nucl | 9 |
| Poyun16915 | 496 | 139 | 197 | 58 | 54,103.07 | 6.91 | nucl | 6 |
| Poyun32726 | 504 | 147 | 205 | 58 | 55,096.33 | 5.63 | nucl | 15 |
| Poyun20952 | 161 | 40 | 98 | 58 | 17,857.2 | 9.15 | nucl | 8 |
| Poyun27992 | 493 | 101 | 159 | 58 | 53,516.07 | 5.5 | nucl | 12 |
| Poyun24653 | 494 | 101 | 159 | 58 | 53,742.44 | 6.48 | nucl | 10 |
| Poyun21040 | 165 | 43 | 87 | 44 | 19,267.95 | 10.46 | nucl | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Wang, J.; Jiang, D.; Yu, A.; Sun, R.; Liu, A. Function and Characteristic Analysis of Candidate PEAR Proteins in Populus yunnanensis. Int. J. Mol. Sci. 2023, 24, 13101. https://doi.org/10.3390/ijms241713101
Li P, Wang J, Jiang D, Yu A, Sun R, Liu A. Function and Characteristic Analysis of Candidate PEAR Proteins in Populus yunnanensis. International Journal of Molecular Sciences. 2023; 24(17):13101. https://doi.org/10.3390/ijms241713101
Chicago/Turabian StyleLi, Ping, Jing Wang, Derui Jiang, Anmin Yu, Rui Sun, and Aizhong Liu. 2023. "Function and Characteristic Analysis of Candidate PEAR Proteins in Populus yunnanensis" International Journal of Molecular Sciences 24, no. 17: 13101. https://doi.org/10.3390/ijms241713101





