Overexpression of RAB27A in Oral Squamous Cell Carcinoma Promotes Tumor Migration and Invasion via Modulation of EGFR Membrane Stability
Abstract
:1. Introduction
2. Results
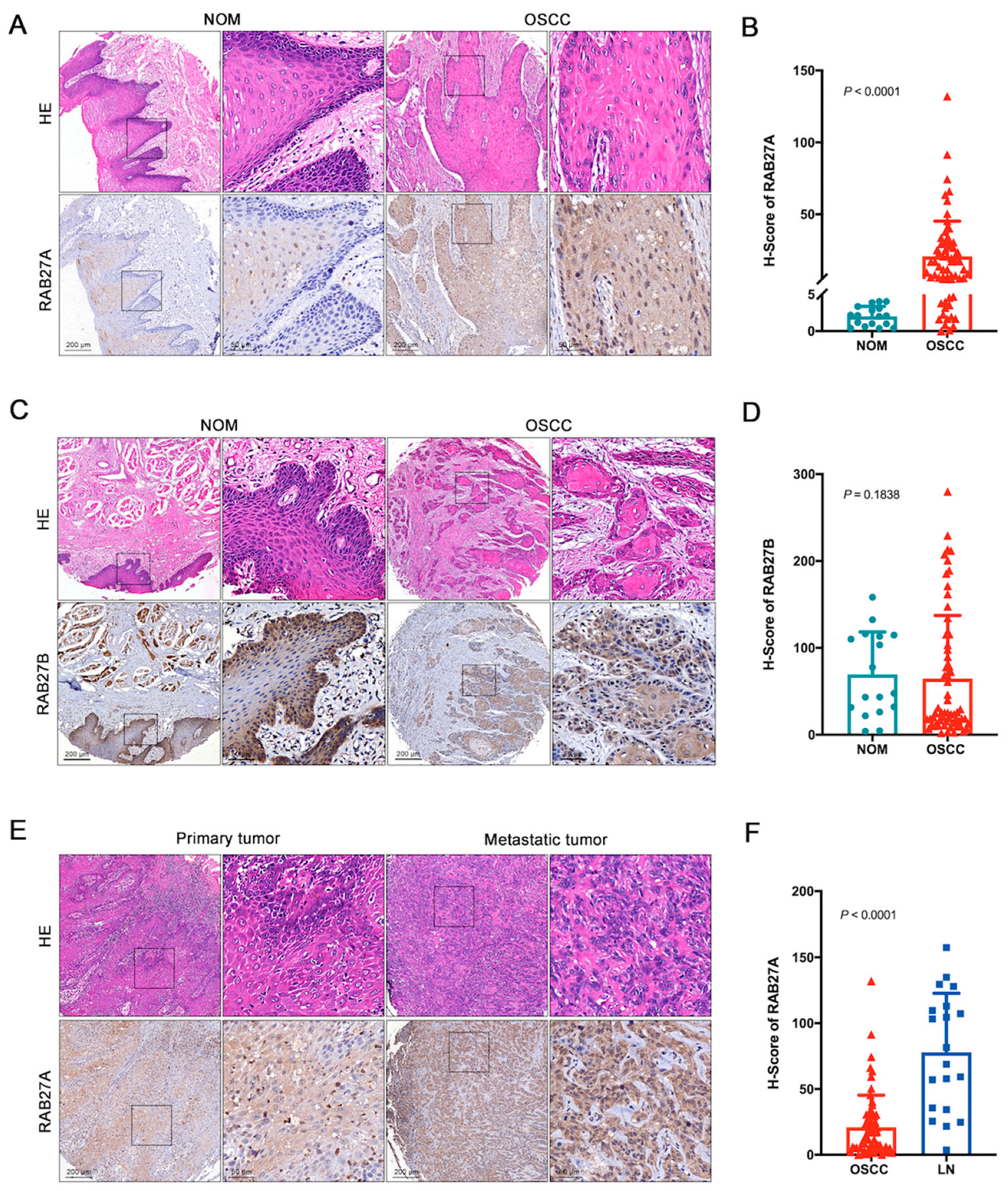
2.1. The Overexpression of RAB27A in OSCC
2.2. The Overexpression of RAB27A Is Associated with Enhanced Lymph Node Metastasis and Poor Prognosis of OSCC Patients
2.3. Knockdown of RAB27A Reduces Proliferation and Increases Apoptosis of OSCC Cells
2.4. Knockdown of RAB27A Attenuates Migration and Invasion Abilities in OSCC Cells
2.5. RAB27A Contributes to the Membrane Retention of EGFR in OSCC Cells
2.6. RAB27A-Mediated Palmitoylation of EGFR Sustains the Membrane Stability of the EGFR in OSCC
3. Discussion
4. Materials and Methods
4.1. Clinical Samples and OSCC Microarrays
4.2. Immunohistochemistry (IHC) Staining
4.3. Cell Culture
4.4. Construction of RAB27A-Knockdown Cells
4.5. Western Blot Analysis
4.6. Cell Proliferation Assay
4.7. Real-Time Quantitative PCR (RT-qPCR)
4.8. Wound Healing Assay
4.9. Cell Migration Assay
4.10. Cell Invasion Assay
4.11. Flow Cytometry
4.12. Bioinformatic Analysis
4.13. Detection of Palmitoylation of Protein
4.14. Co-Immunoprecipitation
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chi, A.C.; Day, T.A.; Neville, B.W. Oral cavity and oropharyngeal squamous cell carcinoma-an update. CA Cancer J. Clin. 2015, 65, 401–421. [Google Scholar] [CrossRef]
- Chen, Y.; Shao, Z.; Jiang, E.; Zhou, X.; Wang, L.; Wang, H.; Luo, X.; Chen, Q.; Liu, K.; Shang, Z. CCL21/CCR7 interaction promotes EMT and enhances the stemness of OSCC via a JAK2/STAT3 signaling pathway. J. Cell Physiol. 2020, 235, 5995–6009. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, T.; Wu, Y.; Xu, H.; Xie, C.; Dong, Y.; Zhong, L.; Wang, Z.; Zhao, H.; Zhou, Y.; et al. GPR39 Overexpression in OSCC Promotes YAP-Sustained Malignant Progression. J. Dent. Res. 2020, 99, 949–958. [Google Scholar] [CrossRef]
- Li, X.; Bu, W.; Meng, L.; Liu, X.; Wang, S.; Jiang, L.; Ren, M.; Fan, Y.; Sun, H. CXCL12/CXCR4 pathway orchestrates CSC-like properties by CAF recruited tumor associated macrophage in OSCC. Exp. Cell Res. 2019, 378, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Blatt, S.; Krüger, M.; Sagheb, K.; Barth, M.; Kämmerer, P.W.; Al-Nawas, B.; Sagheb, K. Tumor Recurrence and Follow-Up Intervals in Oral Squamous Cell Carcinoma. J. Clin. Med. 2022, 11, 7061. [Google Scholar] [CrossRef]
- Cheng, W.-L.; Feng, P.-H.; Lee, K.-Y.; Chen, K.-Y.; Sun, W.-L.; Van Hiep, N.; Luo, C.-S.; Wu, S.-M. The Role of EREG/EGFR Pathway in Tumor Progression. Int. J. Mol. Sci. 2021, 22, 12828. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Bonner, J.A.; Bredel, M. EGFR Mutations in Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 3818. [Google Scholar] [CrossRef] [PubMed]
- Appert-Collin, A.; Hubert, P.; Crémel, G.; Bennasroune, A. Role of ErbB Receptors in Cancer Cell Migration and Invasion. Front. Pharmacol. 2015, 6, 283. [Google Scholar] [CrossRef] [PubMed]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef]
- Gudi, R.R.; Janakiraman, H.; Howe, P.H.; Palanisamy, V.; Vasu, C. Loss of CPAP causes sustained EGFR signaling and epithelial-mesenchymal transition in oral cancer. Oncotarget 2021, 12, 807–822. [Google Scholar] [CrossRef]
- Laimer, K.; Spizzo, G.; Gastl, G.; Obrist, P.; Brunhuber, T.; Fong, D.; Barbieri, V.; Jank, S.; Doppler, W.; Rasse, M.; et al. High EGFR expression predicts poor prognosis in patients with squamous cell carcinoma of the oral cavity and oropharynx: A TMA-based immunohistochemical analysis. Oral Oncol. 2007, 43, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Homma, Y.; Hiragi, S.; Fukuda, M. Rab family of small GTPases: An updated view on their regulation and functions. FEBS J. 2021, 288, 36–55. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, F.; Liu, Z.; Tang, X.; Han, Y.; Jiang, J.; Ma, C.; He, Y. High expression of Rab31 confers a poor prognosis and enhances cell proliferation and invasion in oral squamous cell carcinoma. Oncol. Rep. 2021, 45, 1182–1192. [Google Scholar] [CrossRef]
- da Silva, S.D.; Marchi, F.A.; Xu, B.; Bijian, K.; Alobaid, F.; Mlynarek, A.; Rogatto, S.R.; Hier, M.; Kowalski, L.P.; Alaoui-Jamali, M.A. Predominant Rab-GTPase amplicons contributing to oral squamous cell carcinoma progression to metastasis. Oncotarget 2015, 6, 21950–21963. [Google Scholar] [CrossRef]
- Park, J.-I.; Song, K.-H.; Kang, S.-M.; Lee, J.; Cho, S.-J.; Choi, H.K.; Ahn, J.; Park, J.-K.; Kim, J.; Hwang, S.-G.; et al. BHMPS Inhibits Breast Cancer Migration and Invasion by Disrupting Rab27a-Mediated EGFR and Fibronectin Secretion. Cancers 2022, 14, 373. [Google Scholar] [CrossRef]
- Bollu, L.R.; Katreddy, R.R.; Blessing, A.M.; Pham, N.; Zheng, B.; Wu, X.; Weihua, Z. Intracellular activation of EGFR by fatty acid synthase dependent palmitoylation. Oncotarget 2015, 6, 34992–35003. [Google Scholar] [CrossRef]
- Jansen, M.; Beaumelle, B. How palmitoylation affects trafficking and signaling of membrane receptors. Biol. Cell 2022, 114, 61–72. [Google Scholar] [CrossRef]
- Guo, H.; Wang, J.; Ren, S.; Zheng, L.-F.; Zhuang, Y.-X.; Li, D.-L.; Sun, H.-H.; Liu, L.-Y.; Xie, C.; Wu, Y.-Y.; et al. Targeting EGFR-dependent tumors by disrupting an ARF6-mediated sorting system. Nat. Commun. 2022, 13, 6004. [Google Scholar] [CrossRef]
- Uribe, M.L.; Marrocco, I.; Yarden, Y. EGFR in Cancer: Signaling Mechanisms, Drugs, and Acquired Resistance. Cancers 2021, 13, 2748. [Google Scholar] [CrossRef]
- Sarkis, S.A.; Abdullah, B.H.; Majeed, B.A.A.; Talabani, N.G. Immunohistochemical expression of epidermal growth factor receptor (EGFR) in oral squamous cell carcinoma in relation to proliferation, apoptosis, angiogenesis and lymphangiogenesis. Head Neck Oncol. 2010, 2, 13. [Google Scholar] [CrossRef]
- Liu, L.; Chen, J.; Cai, X.; Yao, Z.; Huang, J. Progress in targeted therapeutic drugs for oral squamous cell carcinoma. Surg. Oncol. 2019, 31, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Uzawa, K.; Amelio, A.L.; Kasamatsu, A.; Saito, T.; Kita, A.; Fukamachi, M.; Sawai, Y.; Toeda, Y.; Eizuka, K.; Hayashi, F.; et al. Resveratrol Targets Urokinase-Type Plasminogen Activator Receptor Expression to Overcome Cetuximab-Resistance in Oral Squamous Cell Carcinoma. Sci. Rep. 2019, 9, 12179. [Google Scholar] [CrossRef] [PubMed]
- Tolmachova, T.; Anders, R.; Stinchcombe, J.; Bossi, G.; Griffiths, G.M.; Huxley, C.; Seabra, M.C. A General Role for Rab27a in Secretory Cells. Mol. Biol. Cell 2004, 15, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.M.; Yip, R.; Swing, D.A.; O’Sullivan, T.N.; Zhang, Y.; Novak, E.K.; Swank, R.T.; Russell, L.B.; Copeland, N.G.; Jenkins, N.A. A mutation in Rab27a causes the vesicle transport defects observed in ashen mice. Proc. Natl. Acad. Sci. USA 2000, 97, 7933–7938. [Google Scholar] [CrossRef]
- Bobrie, A.; Krumeich, S.; Reyal, F.; Recchi, C.; Moita, L.F.; Seabra, M.C.; Ostrowski, M.; Théry, C. Rab27a Supports Exosome-Dependent and -Independent Mechanisms That Modify the Tumor Microenvironment and Can Promote Tumor Progression. Cancer Res 2012, 72, 4920–4930. [Google Scholar] [CrossRef]
- Huang, H.; Hou, J.; Liu, K.; Liu, Q.; Shen, L.; Liu, B.; Lu, Q.; Zhang, N.; Che, L.; Li, J.; et al. RAB27A-dependent release of exosomes by liver cancer stem cells induces Nanog expression in their differentiated progenies and confers regorafenib resistance. J. Gastroenterol. Hepatol. 2021, 36, 3429–3437. [Google Scholar] [CrossRef]
- Guo, D.; Lui, G.Y.L.; Lai, S.L.; Wilmott, J.S.; Tikoo, S.; Jackett, L.A.; Quek, C.; Brown, D.L.; Sharp, D.M.; Kwan, R.Y.Q.; et al. RAB27A promotes melanoma cell invasion and metastasis via regulation of pro-invasive exosomes. Int. J. Cancer 2019, 144, 3070–3085. [Google Scholar] [CrossRef]
- Kadry, Y.A.; Lee, J.Y.; Witze, E.S. Regulation of EGFR signalling by palmitoylation and its role in tumorigenesis. Open Biol. 2021, 11, 210033. [Google Scholar] [CrossRef]
- Li, R.-F.; Man, Q.-W.; Liu, J.-Y.; Zheng, Y.-Y.; Gao, X.; Liu, H.-M. Overexpression of T-type calcium channel Cav3.1 in oral squamous cell carcinoma: Association with proliferation and anti-apoptotic activity. J. Mol. Histol. 2021, 52, 511–520. [Google Scholar] [CrossRef]
- Percher, A.; Ramakrishnan, S.; Thinon, E.; Yuan, X.; Yount, J.S.; Hang, H.C. Mass-tag labeling reveals site-specific and endogenous levels of protein S-fatty acylation. Proc. Natl. Acad. Sci. USA 2016, 113, 4302–4307. [Google Scholar] [CrossRef]





| Gene | Forward 5′→3′ | Reverse 5′→3′ |
|---|---|---|
| GAPDH | GGAGCGAGATCCCTCCAAAAT | GGCTGTTGTCATACTTCTCATGG |
| RAB27A | GCTTTGGGAGACTCTGGTGTA | TCAATGCCCACTGTTGTGATAAA |
| EGFR | AGGCACGAGTAACAAGCTCAC | ATGAGGACATAACCAGCCACC |
| ZDHHC13 | ACCCCACTCTTATTGATGGAGA | TGTCTGCCCATTTACATCTGTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Yang, J.-G.; Ren, J.-G.; Xia, H.-F.; Chen, G.-H.; Fu, Q.-Y.; Zhang, L.-Z.; Liu, H.-M.; Wang, K.-M.; Xie, Q.-H.; et al. Overexpression of RAB27A in Oral Squamous Cell Carcinoma Promotes Tumor Migration and Invasion via Modulation of EGFR Membrane Stability. Int. J. Mol. Sci. 2023, 24, 13103. https://doi.org/10.3390/ijms241713103
Huang J, Yang J-G, Ren J-G, Xia H-F, Chen G-H, Fu Q-Y, Zhang L-Z, Liu H-M, Wang K-M, Xie Q-H, et al. Overexpression of RAB27A in Oral Squamous Cell Carcinoma Promotes Tumor Migration and Invasion via Modulation of EGFR Membrane Stability. International Journal of Molecular Sciences. 2023; 24(17):13103. https://doi.org/10.3390/ijms241713103
Chicago/Turabian StyleHuang, Jue, Jie-Gang Yang, Jian-Gang Ren, Hou-Fu Xia, Gao-Hong Chen, Qiu-Yun Fu, Lin-Zhou Zhang, Hai-Ming Liu, Kui-Ming Wang, Qi-Hui Xie, and et al. 2023. "Overexpression of RAB27A in Oral Squamous Cell Carcinoma Promotes Tumor Migration and Invasion via Modulation of EGFR Membrane Stability" International Journal of Molecular Sciences 24, no. 17: 13103. https://doi.org/10.3390/ijms241713103






