C-H Groups as Donors in Hydrogen Bonds: A Historical Overview and Occurrence in Proteins and Nucleic Acids
Abstract
1. Introduction
2. History
2.1. Early Period: Emergence of Concepts
2.2. Spectroscopic Evidence
2.3. The Turning Point: Structural Evidence from Crystallography
2.4. Recent Advances
3. Significance of C-H…O H-Bonds in Biological Macromolecules
3.1. Earliest Hypotheses and Observations
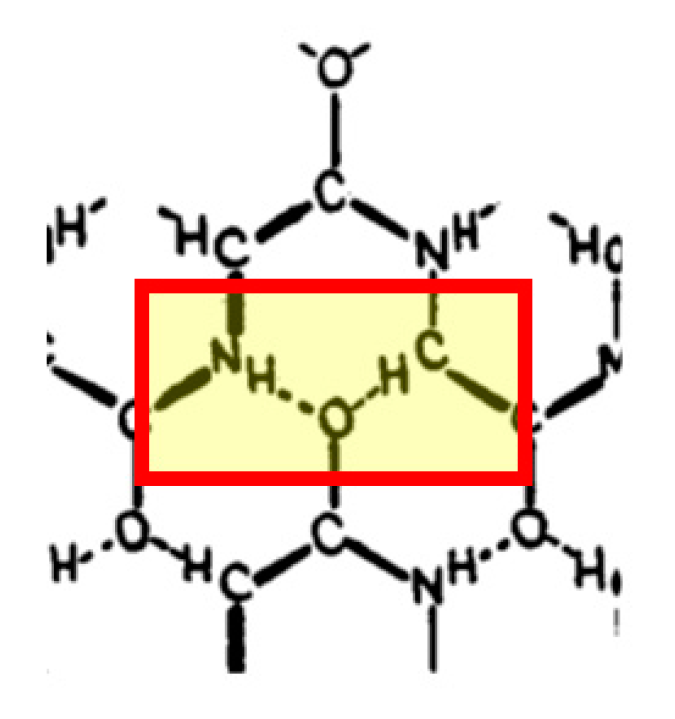
‘The hydrogen atoms of the CHR (i.e., CαHR—ZSD) groups may also form bridges to carbonyl oxygen atoms, since there is good evidence for C-H…O bridges in comparable structures (multiple references to the Copley/Zellhoefer series: ZSD). The possibility will be discussed in connection with the structure of collagen (Huggins later in the paper proposes such a structure—ZSD).’(Figure 1)
‘The distance between the atoms C4 (NB: the convention was later modified so that this is C6 according to accepted numbering—ZSD) in the pyrimidine and O5 ‘in the D-ribose is only 3.24 Å which is considered by the author to be significantly less than the normal van der Waals approach of 3.4–3.5 Å. This would seem to indicate some kind of attraction, possibly of the hydrogen bond type.’ (…) The reason for the formation of the bond may be sought in a possible polarization of the group (CH)4 by the electronegative substituents in the pyrimidine ring.’
3.2. C-H…O Bonds in Proteins
3.2.1. Cα-H Groups as Ubiquitous Donors in H-Bonds in Proteins
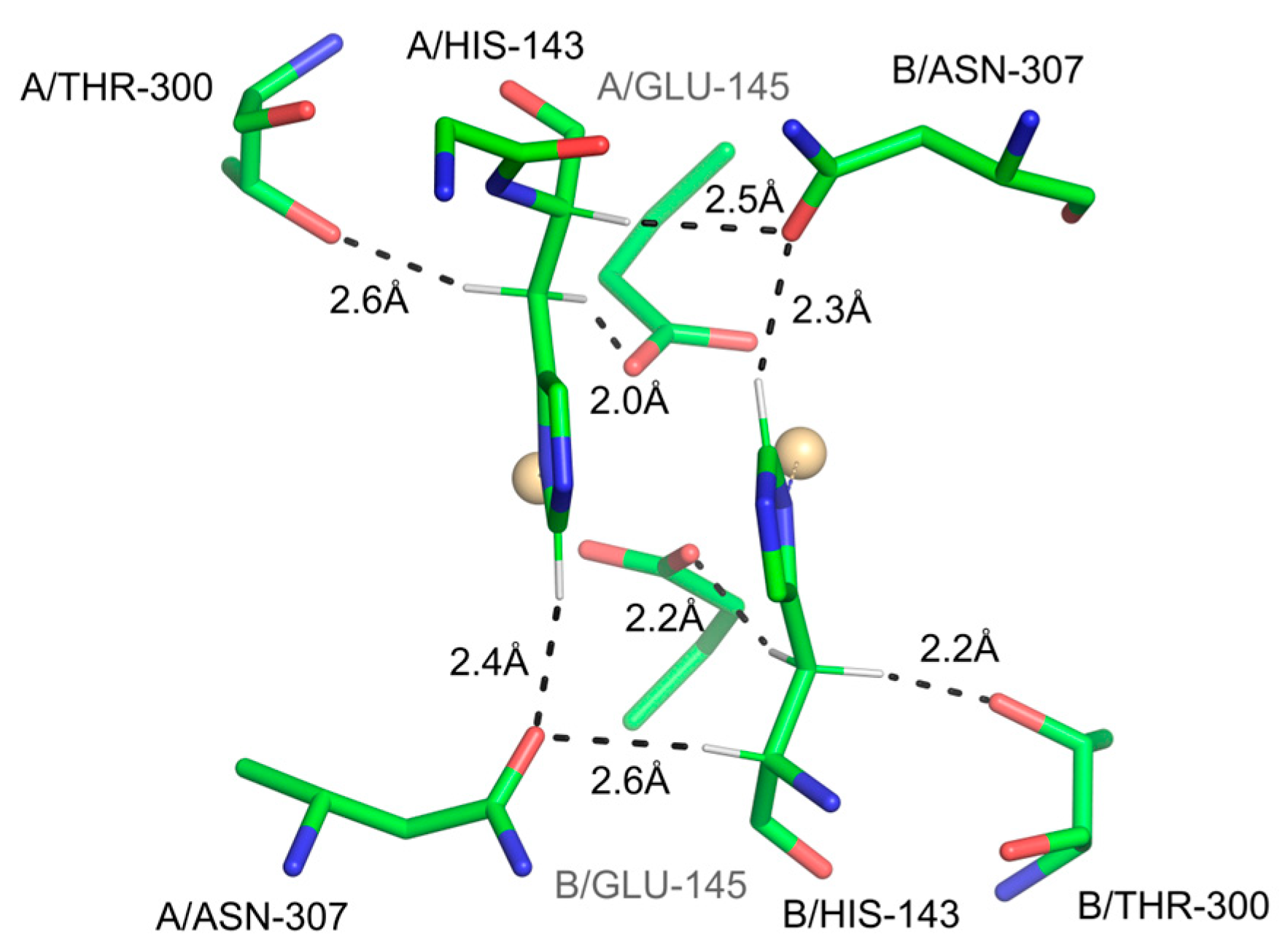
3.2.2. Bonds Involving C-H Methine Groups in His
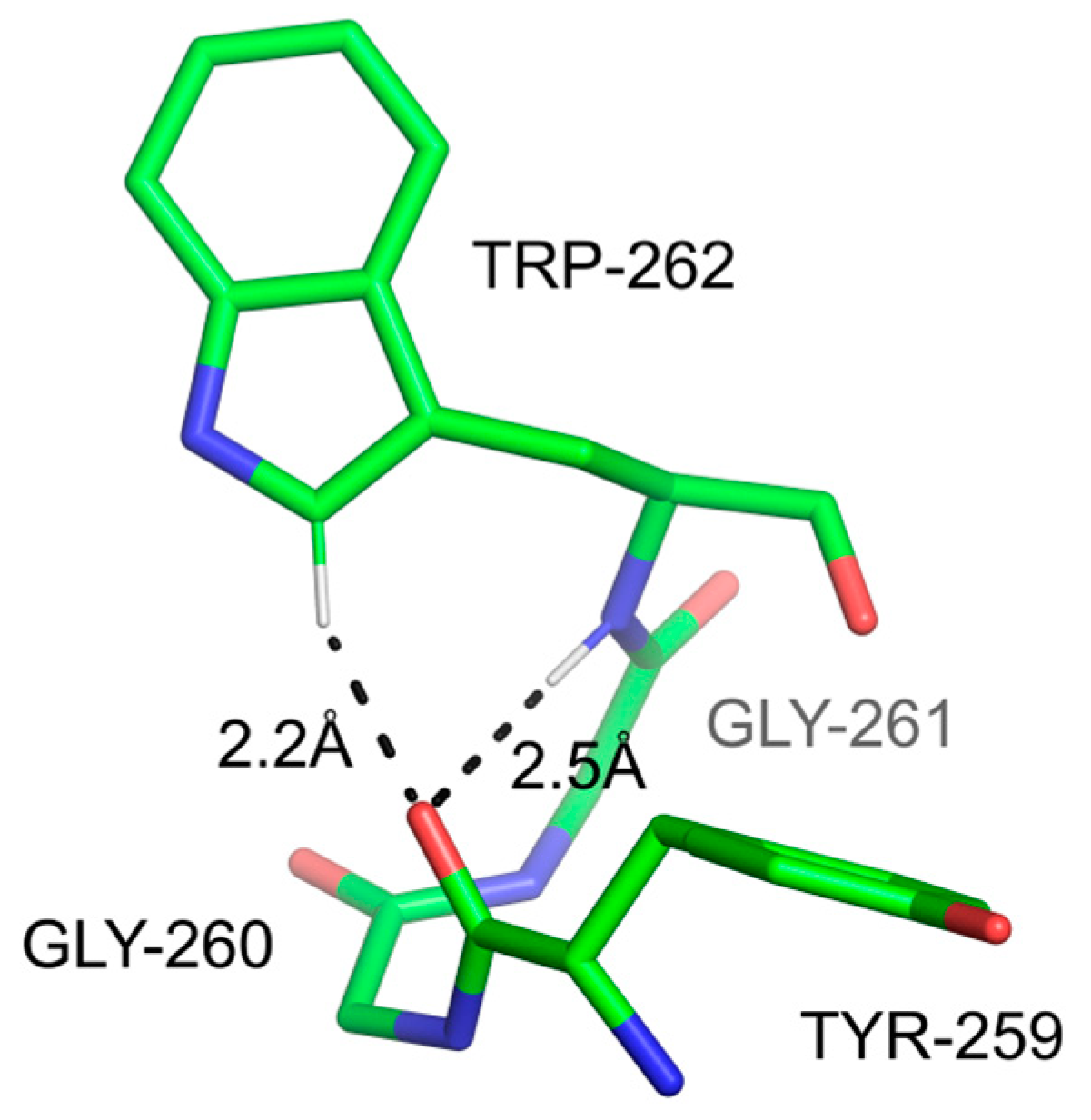
3.2.3. Bonds Involving Cδ1-H Group from Trp
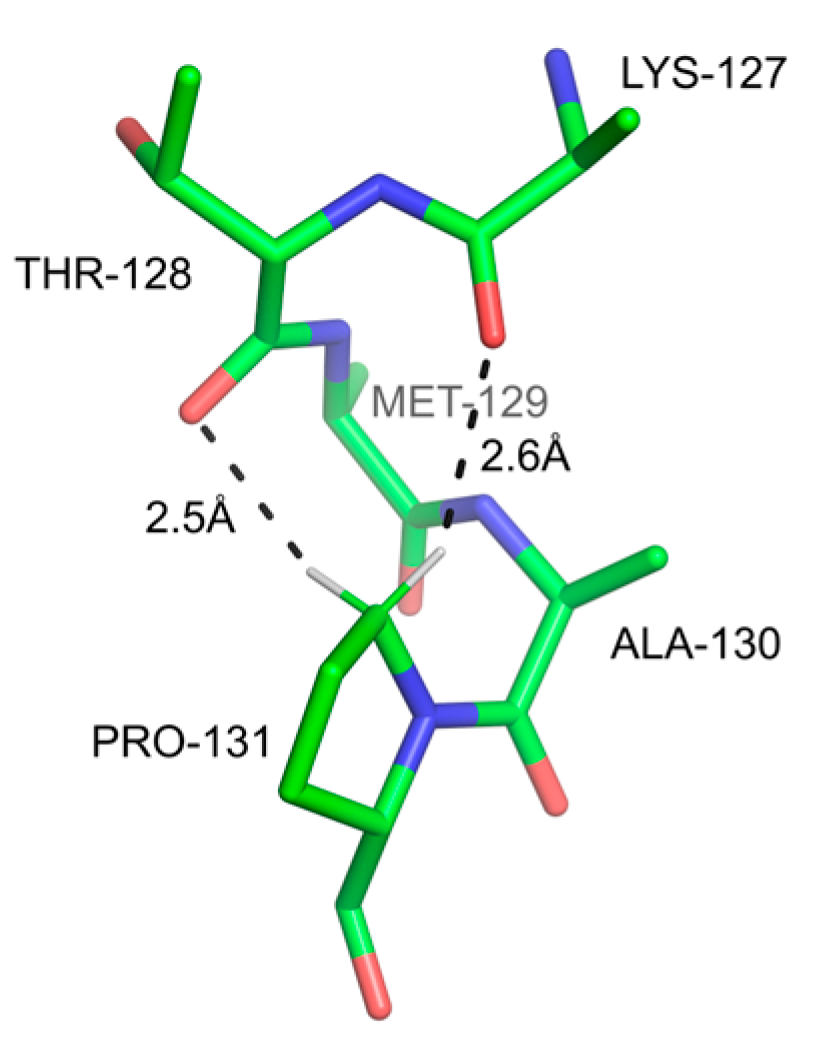
3.2.4. The sp3 C-H Groups
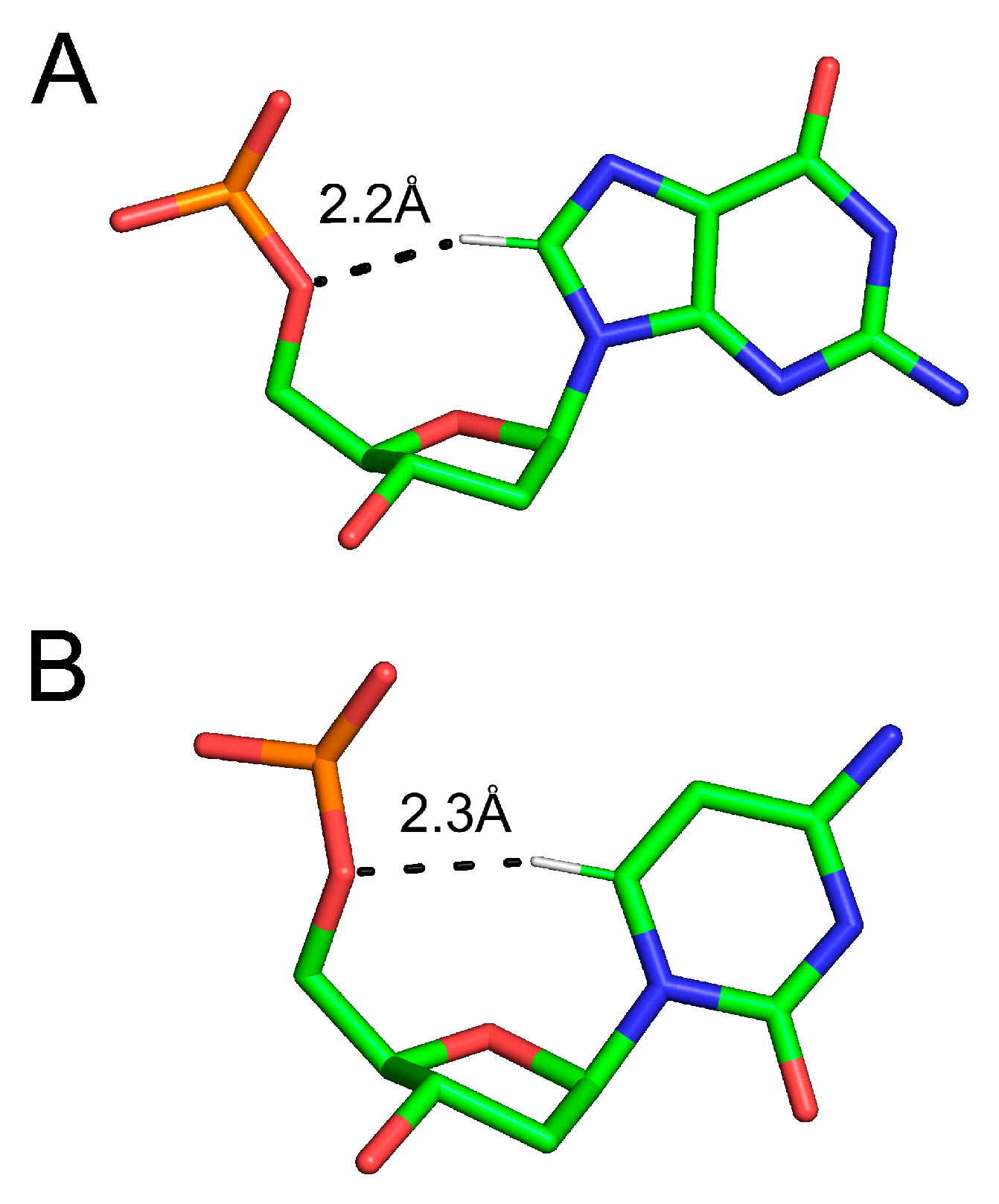
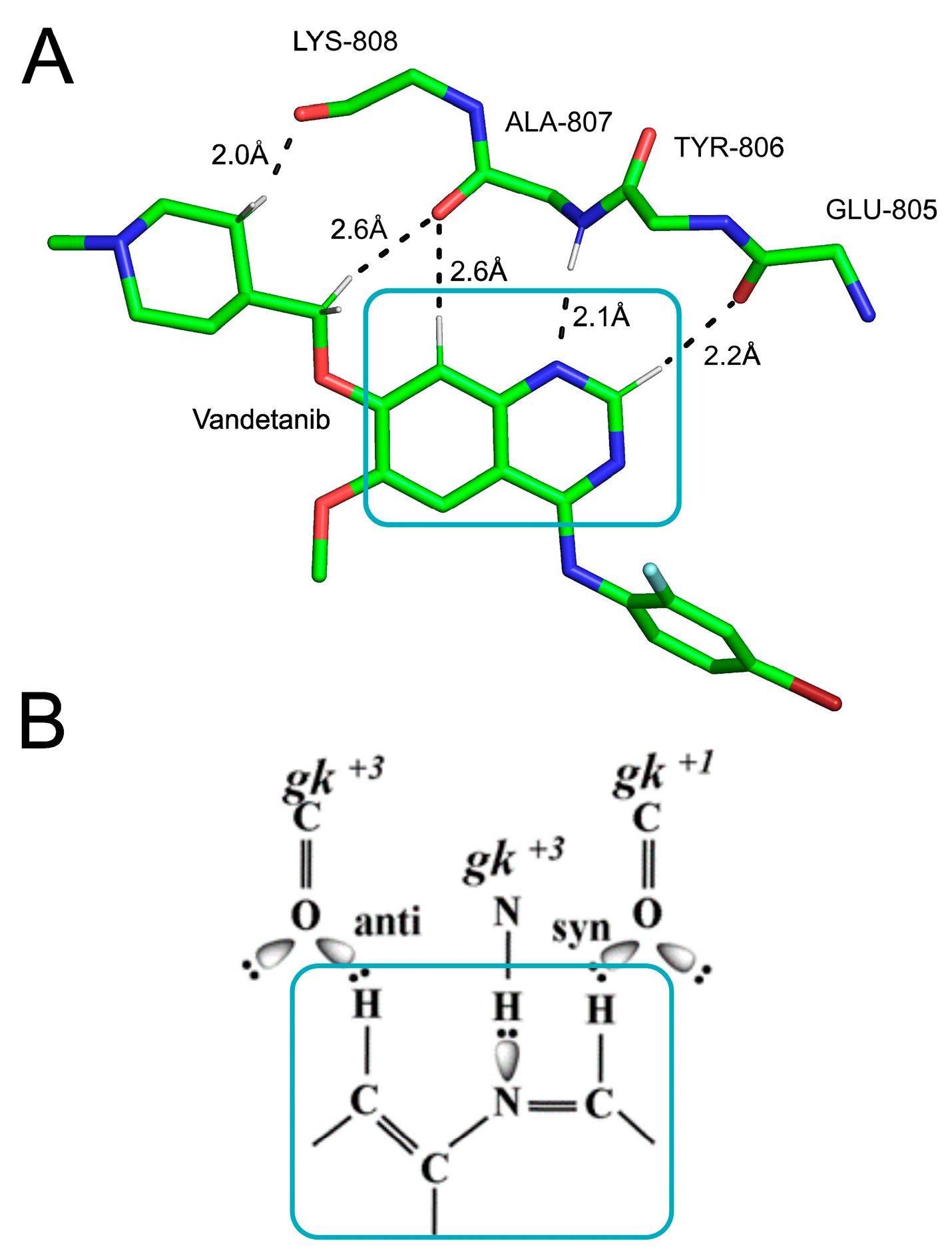
3.2.5. Protein–Ligand Interactions
3.3. Nucleic Acids
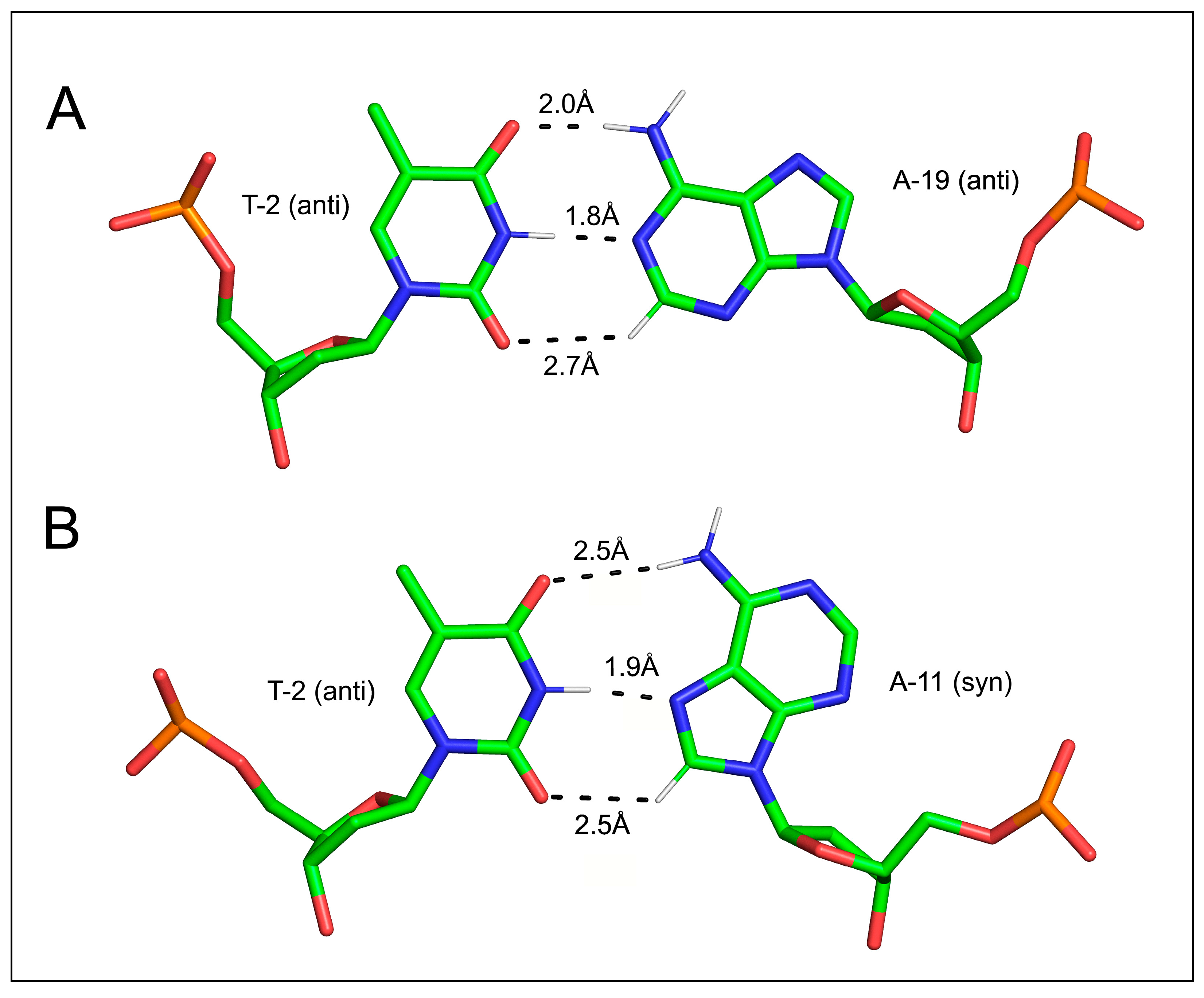
3.3.1. C-H…O Bonds in Base Pairing
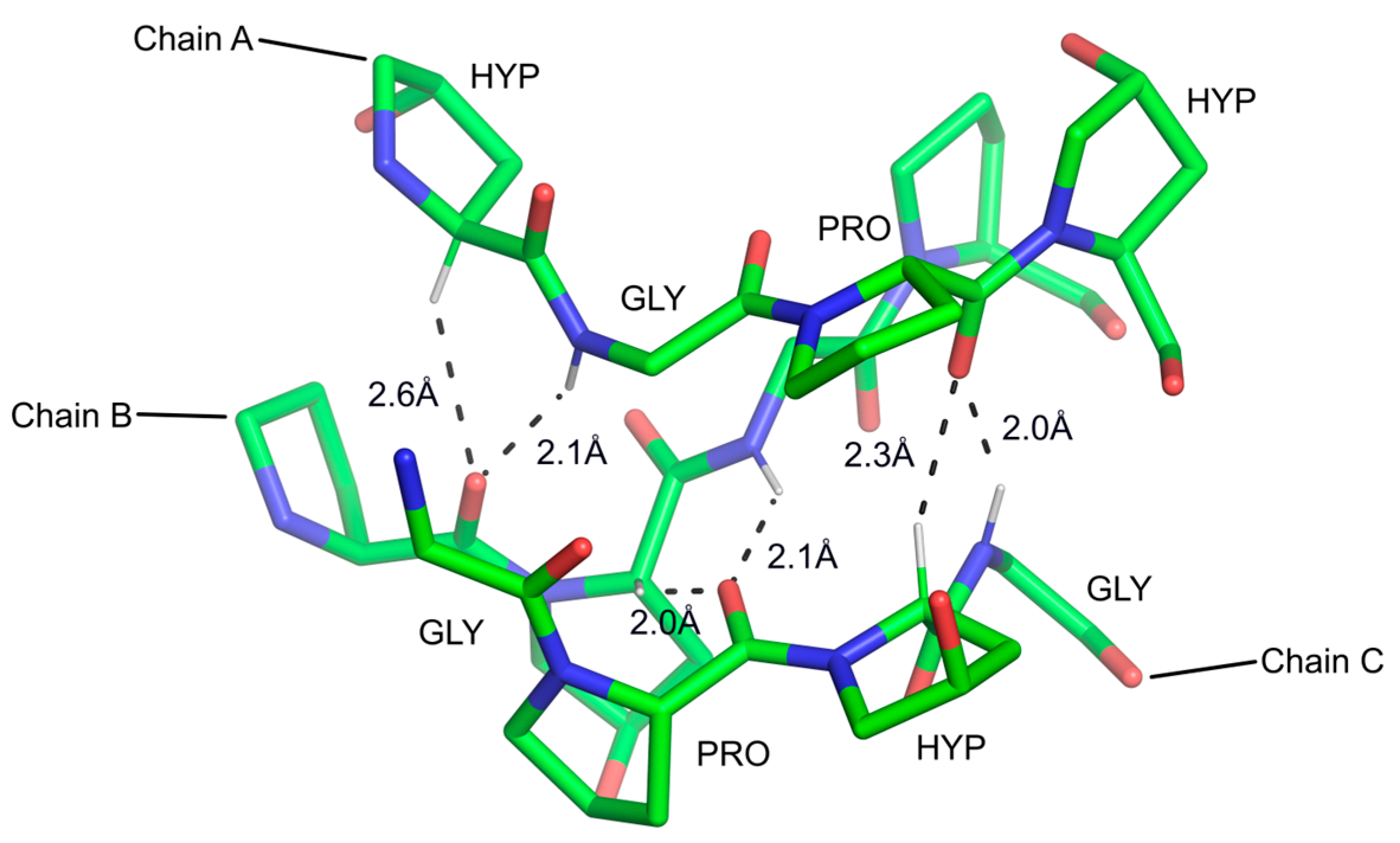
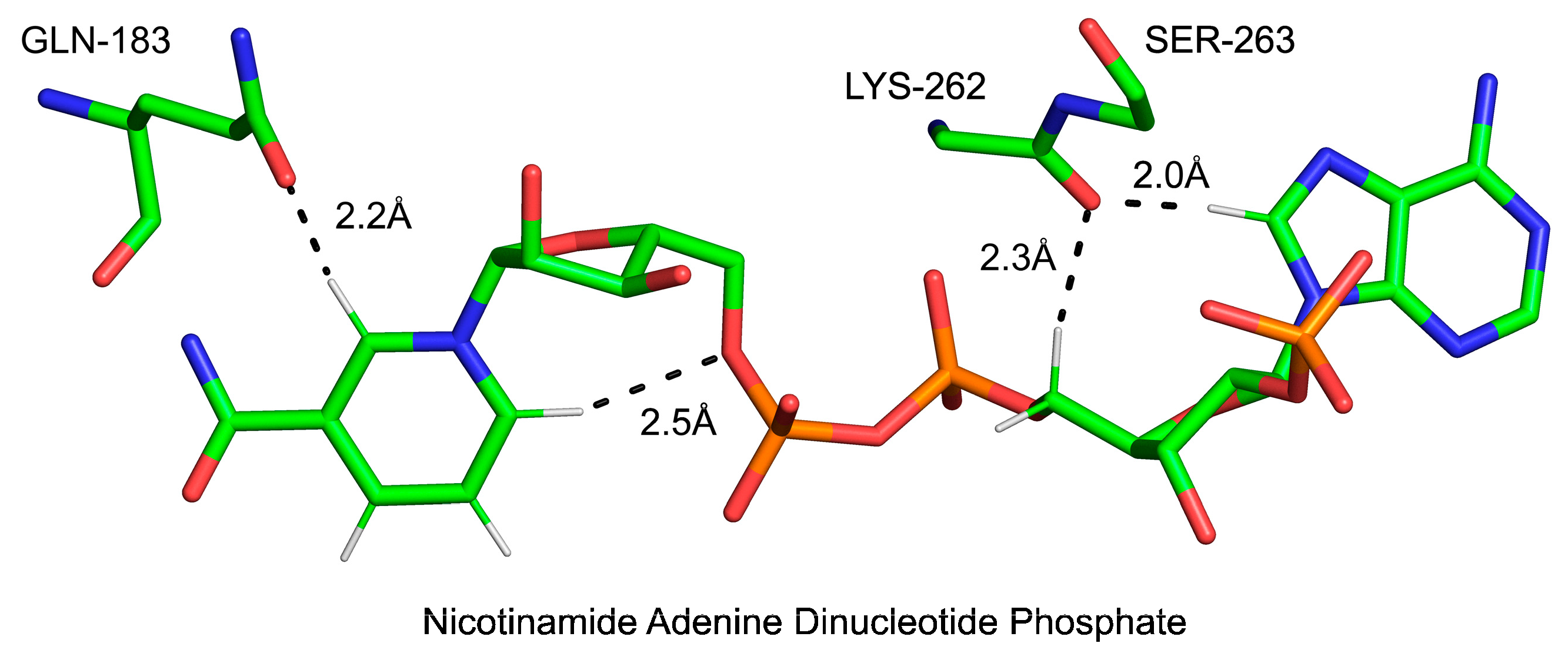
3.3.2. C-H…O Base–Backbone Bonds in NA
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Latimer, W.M.; Rodebush, W.H. Polarity and ionization from the standpoint of the Lewis theory of valence. J. Am. Chem. Soc. 1920, 42, 1419–1433. [Google Scholar]
- Derewenda, Z.S. On the centennials of the discoveries of the hydrogen bond and the structure of the water molecule: The short life and work of Eustace Jean Cuy (1897–1925). Acta Crystallogr. Sect. A Found. Adv. 2021, 77 Pt 5, 362–378. [Google Scholar]
- Weinhold, F.; Klein, R.A. What is a hydrogen bond? Mutually consistent theoretical and experimental criteria for characterizing H-bonding interactions. Mol. Phys. 2012, 110, 565–579. [Google Scholar]
- Quane, D.J. The Reception of the Concept of Hydrogen-Bonding by the Chemical Community 1920–1937. Bull. Hist. Chem. 1990, 7, 3–13. [Google Scholar]
- Huggins, M.L. 50 Years of Hydrogen Bond Theory. Angew. Chem. Int. Ed. 1971, 10, 147–152. [Google Scholar] [CrossRef]
- Huggins, M.L. The Hydrogen-Bond and Other Reminiscences. ChemTech 1980, 10, 422–429. [Google Scholar]
- Pauling, L. The nature of the chemical bond IV The energy of single bonds and the relative electronegativity of atoms. J. Am. Chem. Soc. 1932, 54, 3570–3582. [Google Scholar] [CrossRef]
- Hildebrand, J.H. A Quantitative Treatment of Deviations from Raoult’s Law. Proc. Natl. Acad. Sci. USA 1927, 13, 267–272. [Google Scholar]
- Bowler, M.G.; Bowler, D.R.; Bowler, M.W. Raoult’s law revisited: Accurately predicting equilibrium relative humidity points for humidity control experiments. J. Appl. Crystallogr. 2017, 50 Pt 2, 631–638. [Google Scholar] [PubMed]
- Dolezalek, F. On the theory of binary mixture and concentrated solutions. Z. Phys. Chem. 1908, 64, 727–747. [Google Scholar] [CrossRef]
- Dolezalek, F.; Schulze, A. The theory of binary mixtures and concentrated solutions. IV. The mixture: Ethyl ether chloroform. Z. Phys. Chem. 1913, 83, 45–78. [Google Scholar] [CrossRef]
- Dolezalek, F. The theory of binary mixtures and concentrated solutions. III. Responses to the gentelmen T.S. Patterson and J.J. van Laar. Z. Phys. Chem. 1913, 83, 40–44. [Google Scholar] [CrossRef]
- Lewis, G.N. The atom and the molecule. J. Am. Chem. Soc. 1916, 38, 762–785. [Google Scholar] [CrossRef]
- Wyatt, W.F. Solutions Part III. The transition point of carbon tetrachloride, and compounds of carbon tetrachloride or chloroform with acetone, ether, and benzene. Trans. Faraday Soc. 1929, 25, 48–53. [Google Scholar] [CrossRef]
- Moelwyn-Hughes, E.A.; Sherman, A. Sow kinetic consequences of complex formation in solution. J. Chem. Soc. 1936, 101–110. [Google Scholar] [CrossRef]
- Bernal, J.D.; Megaw, H.D. The function of hydrogen in intermolecular forces. Proc. R. Soc. Lond. Ser. A 1935, 151, 384–420. [Google Scholar] [CrossRef]
- Glasstone, S. The structure of some molecular complexes in the liquid phase. Trans. Faraday Soc. 1937, 33, 200–206. [Google Scholar] [CrossRef]
- Huggins, M.L. Hydrogen bridges in organic compounds. J. Org. Chem. 1936, 1, 407–456. [Google Scholar]
- Zellhoefer, G.F. Solubility of halogenated hydrocarbon refrigerants in organic solvents. Ind. Eng. Chem. 1937, 29, 548–551. [Google Scholar] [CrossRef]
- Leonard, N.J. Marvel, Carl, Shipp—September 11, 1894 January 4, 1988. Org. Synth. 1989, 67, R13–R15. [Google Scholar]
- Zellhoefer, G.F.; Copley, M.J.; Marvel, C.S. Hydrogen bonds involving the C-H link—The solubility of haloforms in donor solvents. J. Am. Chem. Soc. 1938, 60, 1337–1343. [Google Scholar] [CrossRef]
- Zellhoefer, G.F.; Copley, M.J. The heats of mixing of haloforms and polyethylene glycol ethers. J. Am. Chem. Soc. 1938, 60, 1343–1345. [Google Scholar] [CrossRef]
- Copley, M.J.; Zellhoefer, G.F.; Marvel, C.S. Hydrogen bonds involving the C-H link IV The effect of solvent association on solubility. J. Am. Chem. Soc. 1938, 60, 2666–2673. [Google Scholar] [CrossRef]
- Copley, M.J.; Zellhoefer, G.F.; Marvel, C.S. Hydrogen bonds involving the C-H link V The solubility of methylene chloride in donor solvents. J. Am. Chem. Soc. 1938, 60, 2714–2716. [Google Scholar]
- Copley, M.J.; Holley, C.E. Hydrogen bonding by negatively substituted CH groups. VI. Acetylenic compounds. J. Am. Chem. Soc. 1939, 61, 1599–1600. [Google Scholar] [CrossRef]
- Copley, M.J.; Marvel, C.S.; Ginsberg, E. Hydrogen Bonding by S-H. VII. Aryl Mercaptans. J. Am. Chem. Soc. 1939, 61, 3161–3162. [Google Scholar] [CrossRef]
- Copley, M.J.; Zellhoefer, G.F.; Marvel, C.S. Hydrogen Bonds Involving the C-H Link. VIII. The Solubilities of Completely Halogenated Methanes in Organic Solvents. J. Am. Chem. Soc. 1939, 61, 3550–3552. [Google Scholar] [CrossRef]
- Copley, M.J.; Zellhoefer, G.F.; Marvel, C.S. Hydrogen bonds involving the C-H link IX Nitriles and dinitriles as solvents for hydrogen containing halogenated methanes. J. Am. Chem. Soc. 1940, 62, 227–228. [Google Scholar] [CrossRef]
- Marvel, C.S.; Dietz, F.C.; Copley, M.J. Hydrogen bonds involving the C-H link. X. The solubility of donor solutes in halogenated hydrocarbons. J. Am. Chem. Soc. 1940, 62, 2273–2275. [Google Scholar] [CrossRef]
- Marvel, C.S.; Copley, M.J.; Ginsberg, E. Hydrogen bonds involving the C-H link. XI. Effect of structure on bonding of donor and acceptor molecules. J. Am. Chem. Soc. 1940, 62, 3109–3112. [Google Scholar] [CrossRef]
- Marvel, C.S.; Copley, M.J.; Ginsberg, E. Hydrogen bonds involving the C-H <- F link. XII. J. Am. Chem. Soc. 1940, 62, 3263–3264. [Google Scholar]
- Copley, M.J.; Ginsberg, E.; Zellhoefer, G.F.; Marvel, C.S. Hydrogen Bonding and the Solubility of Alcohols and Amines in Organic Solvents. XIII. J. Am. Chem. Soc. 1941, 63, 254–256. [Google Scholar]
- Marvel, S.C.; Harkema, J.; Copley, M.J. Hydrogen Bonds Involving the C-H Link. XIV. Solubility of Donor Solutes in Hydrogen Bonding Solvents. J. Am. Chem. Soc. 1941, 63, 1609. [Google Scholar] [CrossRef]
- Marvel, C.S.; Harkema, J. Hydrogen bonds involving the C-H link. XV. Non-bonding of triphenylmethane hydrogen atoms. J. Am. Chem. Soc. 1941, 63, 2221–2222. [Google Scholar] [CrossRef]
- Hunter, L. The hydrogen bond. Ann. Rep. Progr. Chem. 1946, 43, 141–155. [Google Scholar]
- Gordy, W. Association of unlike molecules through hydrogen bonds. Nature 1938, 142, 831. [Google Scholar] [CrossRef]
- Gordy, W. Infrared absorption studies of hydrogen bonds between unlike molecules. J. Am. Chem. Soc. 1938, 60, 605–612. [Google Scholar] [CrossRef]
- Gordy, W. Spectroscopic evidence of hydrogen bonds: Chloroform and bromoform in donor solvents. J. Chem. Phys. 1939, 7, 163–166. [Google Scholar] [CrossRef]
- Klemperer, W.; Cronyn, M.W.; Maki, A.H.; Pimentel, G.C. Infrared Studies of the Association of Secondary Amides in Various Solvents. J. Am. Chem. Soc. 1954, 76, 5846–5848. [Google Scholar] [CrossRef]
- Huggins, C.M.; Pimentel, G.C.; Shoolery, J.N. Proton Magnetic Resonance Studies of Chloroform in Solution—Evidence for Hydrogen Bonding. J. Chem. Phys. 1955, 23, 1244–1247. [Google Scholar]
- Pimentel, G.C.; McClellan, A.L. The Hydrogen Bond; W.H. Freeman: San Francisco, CA, USA, 1960; 475p. [Google Scholar]
- Campbell, A.N.; Kartzmark, E.M. The Energy of Hydrogen Bonding in the System—Acetone-Chloroform. Can. J. Chem. 1960, 38, 652–655. [Google Scholar]
- Allerhand, A.; Schleyer, P.V. A Survey of C-H Groups as Proton Donors in Hydrogen Bonding. J. Am. Chem. Soc. 1963, 85, 1715–1723. [Google Scholar] [CrossRef]
- Dulmage, W.J.; Lipscomb, W.N. The Crystal Structures of Hydrogen Cyanide, Hcn. Acta Crystallogr. 1951, 4, 330–334. [Google Scholar] [CrossRef]
- Sutor, D.J. Crystal and Molecular Structure of 1,3,7,9-Tetramethyluric Acid. Acta Crystallogr. 1963, 16, 97–104. [Google Scholar]
- Sutor, D.J. C-H⋯O Hydrogen Bond in Crystals. Nature 1962, 195, 68–69. [Google Scholar] [CrossRef]
- Sutor, D.J. Evidence for Existence of C-H⋯O Hydrogen Bonds in Crystals. J. Chem. Soc. 1963, 1105–1110. [Google Scholar] [CrossRef]
- Furberg, S. The Crystal Structure of Cytidine. Acta Crystallogr. 1950, 3, 325–333. [Google Scholar] [CrossRef]
- Shefter, E.; Trueblood, K.N. Crystal and Molecular Structure of D(Plus)-Barium Uridine-5′-Phosphate. Acta Crystallogr. 1965, 18, 1067–1077. [Google Scholar] [CrossRef]
- Sharma, B.D.; Mcconnell, J.F. Crystal and Molecular Structure of Isocytosine. Acta Crystallogr. 1965, 19, 797–806. [Google Scholar] [CrossRef]
- Sundaralingam, M. Stereochemistry of Nucleic Acid Constituents. III. Crystal and Molecular Structure of Adenosine 3′-Phosphate Dihydrate (Adenylic Acid B). Acta Crystallogr. 1966, 21, 495–506. [Google Scholar] [CrossRef]
- Haschemeyer, A.E.; Rich, A. Nucleoside Confromations—An Analysis of Steric Barriers to Rotation About Gylcosidic Bond. J. Mol. Biol. 1967, 27, 369–384. [Google Scholar] [PubMed]
- Donohue, J. The Hydrogen Bond in Organic Crystals. J. Phys. Chem. 1952, 56, 502–510. [Google Scholar]
- Ramachandran, G.N.; Ramakrishnan, C.; Sasisekharan, V. Stereochemistry of polypeptide chain configurations. J. Mol. Biol. 1963, 7, 95–99. [Google Scholar]
- Schwalbe, C.H. June Sutor and the C-H⋯O hydrogen bonding controversy. Crystallogr. Rev. 2012, 18, 189–204. [Google Scholar]
- Desiraju, G.R. The C-H⋯O Hydrogen-Bond in Crystals—What Is It. Acc. Chem. Res. 1991, 24, 290–296. [Google Scholar] [CrossRef]
- Olympia, P.L. Some theoretical aspects of C-H⋯O bonding. Chem. Phys. Lett. 1970, 5, 593–596. [Google Scholar]
- Green, R.D. Hydrogen Bonding by C-H Groups; Macmillan: London, UK, 1974; 207p. [Google Scholar]
- Kollman, P.; Mckelvey, J.; Johansson, A.; Rothenberg, S. Theoretical Studies of Hydrogen-Bonded Dimers—Complexes Involving Hf, H2o, Nh3, Hcl, H2s, Ph3, Hcn, Hnc, Hcp, Ch2nh, H2cs, H2co, Ch4, Cf3h, C2h2, C2h4, C6h6, F-, and H3o+. J. Am. Chem. Soc. 1975, 97, 955–965. [Google Scholar] [CrossRef]
- Gay, R.; Vanderkooi, G. Hydrogen-Bonding of Phosphodiesters to Water, Methanol, and Methylamine as Studied by the Cndo-2 Method. J. Chem. Phys. 1981, 75, 2281–2289. [Google Scholar]
- Umeyama, H.; Morokuma, K. Origin of Hydrogen-Bonding—Energy Decomposition Study. J. Am. Chem. Soc. 1977, 99, 1316–1332. [Google Scholar] [CrossRef]
- Taylor, R.; Kennard, O. Crystallographic Evidence for the Existence of C-H⋯O, C-H⋯N, and C-H⋯C1 Hydrogen-Bonds. J. Am. Chem. Soc. 1982, 104, 5063–5070. [Google Scholar] [CrossRef]
- Sarma, J.A.R.P.; Desiraju, G.R. The Role of Cl=Cl and C-H=O Interactions in the Crystal Engineering of 4-a Short-Axis Structures. Acc. Chem. Res. 1986, 19, 222–228. [Google Scholar]
- Sarma, J.A.R.P.; Desiraju, G.R. C-H⋯O Interactions and the Adoption of 4-a Short-Axis Crystal-Structures by Oxygenated Aromatic-Compounds. J. Chem. Soc. Perkin Trans. 1987, 2, 1195–1202. [Google Scholar]
- Desiraju, G.R. Crystal Engineering: From Molecule to Crystal. J. Am. Chem. Soc. 2013, 135, 9952–9967. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal engineering. From molecules to crystals. Acta Crystallogr. Sect. A Found. Adv. 2011, 67, C18–C19. [Google Scholar] [CrossRef]
- Budesinsky, M.; Fiedler, P.; Arnold, Z. Triformylmethane—An Efficient Preparation, Some Derivatives, and Spectra. Synthesis 1989, 1989, 858–860. [Google Scholar]
- Boldeskul, I.E.; Tsymbal, I.F.; Ryltsev, E.V.; Latajka, Z.; Barnes, A.J. Reversal of the usual nu(C-H/D) spectral shift of haloforms in some hydrogen-bonded complexes. J. Mol. Struct. 1997, 437, 167–171. [Google Scholar]
- Hobza, P.; Havlas, Z. Blue-shifting hydrogen bonds. Chem. Rev. 2000, 100, 4253–4264. [Google Scholar]
- Alabugin, I.V.; Manoharan, M.; Peabody, S.; Weinhold, F. Electronic basis of improper hydrogen bonding: A subtle balance of hyperconjugation and rehybridization. J. Am. Chem. Soc. 2003, 125, 5973–5987. [Google Scholar]
- Joseph, J.; Jemmis, E.D. Red-, blue-, or no-shift in hydrogen bonds: A unified explanation. J. Am. Chem. Soc. 2007, 129, 4620–4632. [Google Scholar] [PubMed]
- Scheiner, S.; Kar, T. Spectroscopic and structural signature of the CH-O hydrogen bond. J. Phys. Chem. A 2008, 112, 11854–11860. [Google Scholar] [PubMed]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Definition of the hydrogen bond (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 1637–1641. [Google Scholar]
- Vaz, P.D.; Nolasco, M.M.; Gil, F.P.S.C.; Ribeiro-Claro, P.J.A.; Tomikinson, J. Hydrogen-Bond Dynamics of C-H⋯O Interactions: The Chloroform⋯Acetone Case. Chem.-Eur. J. 2010, 16, 9010–9017. [Google Scholar] [PubMed]
- Kankanamge, S.R.G.; Ma, J.B.; Mackin, R.T.; Leonik, F.M.; Taylor, C.M.; Rubtsov, I.V.; Kuroda, D.G. Proving and Probing the Presence of the Elusive C-H⋯O Hydrogen Bond in Liquid Solutions at Room Temperature. Angew. Chem. Int. Ed. 2020, 59, 17012–17017. [Google Scholar]
- Pullanchery, S.; Kulik, S.; Rehl, B.; Hassanali, A.; Roke, S. Charge transfer across C-H⋯O hydrogen bonds stabilizes oil droplets in water. Science 2021, 374, 1366–1370. [Google Scholar]
- Yu, Y.Q.; Fan, W.; Wang, Y.X.; Zhou, X.G.; Sun, J.; Liu, S.L. C-H⋯O Interaction in Methanol-Water Solution Revealed from Raman Spectroscopy and Theoretical Calculations. J. Phys. Chem. B 2017, 121, 8179–8187. [Google Scholar]
- Shi, L.X.; Min, W. Vibrational Solvatochromism Study of the C-H···O Improper Hydrogen Bond. J. Phys. Chem. B 2023, 127, 3798–3805. [Google Scholar]
- Adhav, V.A.; Saikrishnan, K. The Realm of Unconventional Noncovalent Interactions in Proteins: Their Significance in Structure and Function. Acs Omega 2023, 8, 22268–22284. [Google Scholar]
- Danovich, D.; Shaik, S.; Neese, F.; Echeverria, J.; Aullon, G.; Alvarez, S. Understanding the Nature of the CH⋯HC Interactions in Alkanes. J. Chem. Theory Comput. 2013, 9, 1977–1991. [Google Scholar]
- Watson, J.D.; Crick, F.H. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature 1953, 171, 737–738. [Google Scholar]
- Franklin, R.E.; Gosling, R.G. Molecular Configuration in Sodium Thymonucleate. Nature 1953, 171, 740–741. [Google Scholar]
- Wilkins, M.H.F.; Stokes, A.R.; Wilson, H.R. Molecular Structure of Deoxypentose Nucleic Acids. Nature 1953, 171, 738–740. [Google Scholar] [CrossRef]
- Wing, R.; Drew, H.; Takano, T.; Broka, C.; Tanaka, S.; Itakura, K.; Dickerson, R.E. Crystal structure analysis of a complete turn of B-DNA. Nature 1980, 287, 755–758. [Google Scholar] [CrossRef]
- Kypr, J.; Vorlickova, M. Experiment acknowledged the Watson-Crick hypothesis: A review of B-DNA double helix structural features at atomic resolution. Gen. Physiol. Biophys. 1985, 4, 471–482. [Google Scholar]
- Bernal, J.D.; Crowfoot, D. X-ray photographs of crystalline pepsin. Nature 1934, 133, 794–795. [Google Scholar]
- Astbury, W.T.; Atkin, W.R. X-ray interpretation of the molecular structure of gelatine. Nature 1933, 132, 348. [Google Scholar]
- Kendrew, J.C.; Bodo, G.; Dintzis, H.M.; Parrish, R.G.; Wyckoff, H.; Phillips, D.C. A three-dimensional model of the myoglobin molecule obtained by X-ray analysis. Nature 1958, 181, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L.; Corey, R.B. Compound Helical Configurations of Polypeptide Chains—Structure of Proteins of the Alpha-Keratin Type. Nature 1953, 171, 59–61. [Google Scholar] [CrossRef]
- Pauling, L.; Corey, R.B. The Structure of Hair, Muscle, and Related Proteins. Proc. Natl. Acad. Sci. USA 1951, 37, 261–271. [Google Scholar]
- Pauling, L.; Corey, R.B. The Structure of Fibrous Proteins of the Collagen-Gelatin Group. Proc. Natl. Acad. Sci. USA 1951, 37, 272–281. [Google Scholar] [CrossRef]
- Pauling, L.; Corey, R.B.; Branson, H.R. The Structure of Proteins—2 Hydrogen-Bonded Helical Configurations of the Polypeptide Chain. Proc. Natl. Acad. Sci. USA 1951, 37, 205–211. [Google Scholar]
- Huggins, M.L. The structure of fibrous proteins. Chem. Rev. 1943, 32, 195–218. [Google Scholar] [CrossRef]
- Furberg, S. An X-ray Study of the Stereochemistry of the Nucleosides. Acta Chem. Scand 1950, 4, 751–761. [Google Scholar]
- Wilds, C.J.; Wawrzak, Z.; Krishnamurthy, R.; Eschenmoser, A.; Egli, M. Crystal structure of a B-form DNA duplex containing (L)-alpha-threofuranosyl (3′ -> 2′) nucleosides: A four-carbon sugar is easily accommodated into the backbone of DNA. J. Am. Chem. Soc. 2002, 124, 13716–13721. [Google Scholar] [CrossRef]
- Crick, F.H.C.; Watson, J.D. The Complementary Structure of Deoxyribonucleic Acid. Proc. R. Soc. Lond. Ser.-A 1954, 223, 80–96. [Google Scholar]
- Shefter, E.; Barlow, M.; Sparks, R.A.; Trueblood, K.N. Crystal and Molecular Structure of a Dinucleoside Phosphate—Beta-Adenosine-2′-Beta-Uridine-5′-Phosphoric Acid. Acta Crystallogr. Sect. B Struct. Cryst. Crystal Chem. 1969, 25, 895–909. [Google Scholar] [CrossRef]
- Seeman, N.C.; Sussman, J.L.; Berman, H.N.; Kim, S.H. Nucleic acid conformation: Crystal structure of a naturally occurring dinucleoside phosphate (UpA). Nat. New Biol. 1971, 233, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Saenger, W. Structure and function of nucleosides and nucleotides. Angew. Chem. Int. Ed. Engl. 1973, 12, 591–601. [Google Scholar]
- Jack, A.; Ladner, J.E.; Klug, A. Crystallographic refinement of yeast phenylalanine transfer RNA at 2–5A resolution. J. Mol. Biol. 1976, 108, 619–649. [Google Scholar]
- Dragelj, J.L.; Stankovic, I.M.; Bozinovski, D.M.; Meyer, T.; Veljkovic, D.Z.; Medakovic, V.B.; Knapp, E.W.; Zaric, S.D. C-H/O Interactions of Aromatic CH Donors within Proteins: A Crystallographic Study. Cryst. Growth Des. 2016, 16, 1948–1957. [Google Scholar]
- Scheiner, S. Theoretical Analysis of the Contributions Made by CH··OH Bonds to Protein Structure. Curr. Org. Chem. 2010, 14, 106–128. [Google Scholar]
- Steiner, T.; Saenger, W. Role of C-H⋯O Hydrogen-Bonds in the Coordination of Water-Molecules—Analysis of Neutron-Diffraction Data. J. Am. Chem. Soc. 1993, 115, 4540–4547. [Google Scholar] [CrossRef]
- Baker, E.N. Structure of actinidin, after refinement at 1.7 A resolution. J. Mol. Biol. 1980, 141, 441–484. [Google Scholar] [PubMed]
- Walter, R.L.; Thiel, D.J.; Barna, S.L.; Tate, M.W.; Wall, M.E.; Eikenberry, E.F.; Gruner, S.M.; Ealick, S.E. High-resolution macromolecular structure determination using CCD detectors and synchrotron radiation. Structure 1995, 3, 835–844. [Google Scholar] [PubMed]
- Derewenda, Z.S.; Lee, L.; Derewenda, U. The occurrence of C-H⋯O hydrogen bonds in proteins. J. Mol. Biol. 1995, 252, 248–262. [Google Scholar] [CrossRef]
- Fabiola, G.F.; Krishnaswamy, S.; Nagarajan, V.; Pattabhi, V. C-H⋯O hydrogen bonds in beta-sheets. Acta Crystallogr. Sect. D Struct. Biol. 1997, 53 Pt 3, 316–320. [Google Scholar]
- Bella, J.; Brodsky, B.; Berman, H.M. Hydration Structure of a Collagen Peptide. Structure 1995, 3, 893–906. [Google Scholar] [PubMed]
- Bella, J.; Berman, H.M. Crystallographic evidence for C-alpha-H⋯O=C hydrogen bonds in a collagen triple helix. J. Mol. Biol. 1996, 264, 734–742. [Google Scholar] [CrossRef]
- Kang, B.S.; Devedjiev, Y.; Derewenda, U.; Derewenda, Z.S. The PDZ2 domain of syntenin at ultra-high resolution: Bridging the gap between macromolecular and small molecule crystallography. J. Mol. Biol. 2004, 338, 483–493. [Google Scholar]
- Laulumaa, S.; Kursula, P. Sub-Atomic Resolution Crystal Structures Reveal Conserved Geometric Outliers at Functional Sites. Molecules 2019, 24, 3044. [Google Scholar] [CrossRef]
- Addlagatta, A.; Krzywda, S.; Czapinska, H.; Otlewski, J.; Jaskolski, M. Ultrahigh-resolution structure of a BPTI mutant. Acta Crystallogr. Sect. D Struct. Biol. 2001, 57, 649–663. [Google Scholar] [CrossRef]
- Vargas, R.; Garza, J.; Dixon, D.A.; Hay, B.P. How strong is the C-alpha-H⋯O=C hydrogen bond? J. Am. Chem. Soc. 2000, 122, 4750–4755. [Google Scholar]
- Scheiner, S.; Kar, T.; Gu, Y.L. Strength of the CαH··O hydrogen bond of amino acid residues. J. Biol. Chem. 2001, 276, 9832–9837. [Google Scholar]
- Scheiner, S. Contributions of NH···O and CH···O hydrogen bonds to the stability of beta-sheets in proteins. J. Phys. Chem. B 2006, 110, 18670–18679. [Google Scholar] [PubMed]
- Scheiner, S. Relative strengths of NH··O and CH··O hydrogen bonds between polypeptide chain segments. J. Phys. Chem. B 2005, 109, 16132–16141. [Google Scholar] [PubMed]
- Cordier, F.; Barfield, M.; Grzesiek, S. Direct observation of C-alpha-H-alpha⋯O=C hydrogen bonds in proteins by interresidue (h3)J(CalphaC′) scalar couplings. J. Am. Chem. Soc. 2003, 125, 15750–15751. [Google Scholar]
- Manikandan, K.; Ramakumar, S. The occurrence of C-H⋯O hydrogen bonds in alpha-helices and helix termini in globular proteins. Proteins-Struct. Funct. Bioinform. 2004, 56, 768–781. [Google Scholar]
- Mottamal, M.; Lazaridis, T. The contribution of C-alpha-H⋯O hydrogen bonds to membrane protein stability depends on the position of the amide. Biochemistry 2005, 44, 1607–1613. [Google Scholar]
- Senes, A.; Ubarretxena-Belandia, I.; Engelman, D.M. The C alpha-H⋯O hydrogen bond: A determinant of stability and specificity in transmembrane helix interactions. Proc. Natl. Acad. Sci. USA 2001, 98, 9056–9061. [Google Scholar] [CrossRef]
- Senes, A.; Engel, D.E.; DeGrado, W.F. Folding of helical membrane proteins: The role of polar, GxxxG-like and proline motifs. Curr. Opin. Struct. Biol. 2004, 14, 465–479. [Google Scholar]
- Dhar, J.; Chakrabarti, P.; Saini, H.; Raghava, G.P.S.; Kishore, R. omega-Turn: A novel beta-turn mimic in globular proteins stabilized by main-chain to side-chain C-H⋯O interaction. Proteins-Struct. Funct. Bioinform. 2015, 83, 203–214. [Google Scholar]
- Otero, L.H.; Rojas-Altuve, A.; Llarrull, L.I.; Carrasco-Lopez, C.; Kumarasiri, M.; Lastochkin, E.; Fishovitz, J.; Dawley, M.; Hesek, D.; Lee, M.; et al. How allosteric control of Staphylococcus aureus penicillin binding protein 2a enables methicillin resistance and physiological function. Proc. Natl. Acad. Sci. USA 2013, 110, 16808–16813. [Google Scholar]
- Newberry, R.W.; Raines, R.T. Secondary Forces in Protein Folding. ACS Chem. Biol. 2019, 14, 1677–1686. [Google Scholar] [PubMed]
- Buncel, E.; Clement, O.; Onyido, I. Metal ion effects in isotopic hydrogen exchange in biologically important heterocycles. Acc. Chem. Res. 2000, 33, 672–678. [Google Scholar]
- Scheiner, S.; Kar, T.; Pattanayak, J. Comparison of various types of hydrogen bonds involving aromatic amino acids. J. Am. Chem. Soc. 2002, 124, 13257–13264. [Google Scholar]
- Nanda, V.; Schmiedekamp, A. Are aromatic carbon donor hydrogen bonds linear in proteins? Proteins-Struct. Funct. Bioinform. 2008, 70, 489–497. [Google Scholar]
- Steinert, R.M.; Kasireddy, C.; Heikes, M.E.; Mitchell-Koch, K.R. Newly identified C–H⋯O hydrogen bond in histidine. Phys. Chem. Chem. Phys. 2022, 24, 19233–19251. [Google Scholar]
- Derewenda, Z.S.; Derewenda, U.; Kobos, P.M. (His)C epsilon-H⋯O=C < hydrogen bond in the active sites of serine hydrolases. J. Mol. Biol. 1994, 241, 83–93. [Google Scholar]
- Ash, E.L.; Sudmeier, J.L.; Day, R.M.; Vincent, M.; Torchilin, E.V.; Haddad, K.C.; Bradshaw, E.M.; Sanford, D.G.; Bachovchin, W.W. Unusual H-1 NMR chemical shifts support (His) C-epsilon 1-H⋯O=C H-bond: Proposal for reaction-driven ring flip mechanism in serine protease catalysis. Proc. Natl. Acad. Sci. USA 2000, 97, 10371–10376. [Google Scholar]
- Yasutake, Y.; Konishi, K.; Muramatsu, S.; Yoshida, K.; Aburatani, S.; Sakasegawa, S.I.; Tamura, T. Bacterial triacylglycerol lipase is a potential cholesterol esterase: Identification of a key determinant for sterol-binding specificity. Int. J. Biol. Macromol. 2021, 167, 578–586. [Google Scholar]
- Petrella, R.J.; Karplus, M. The role of carbon-donor hydrogen bonds in stabilizing tryptophan conformations. Proteins 2004, 54, 716–726. [Google Scholar] [PubMed]
- Sekula, B.; Ruszkowski, M.; Dauter, Z. S-adenosylmethionine synthases in plants: Structural characterization of type I and II isoenzymes from Arabidopsis thaliana and Medicago truncatula. Int. J. Biol. Macromol. 2020, 151, 554–565. [Google Scholar]
- Chakrabarti, P.; Chakrabarti, S. C-H⋯O hydrogen bond involving proline residues in alpha-helices. J. Mol. Biol. 1998, 284, 867–873. [Google Scholar]
- Wishart, D.S.; Sykes, B.D.; Richards, F.M. Relationship between Nuclear-Magnetic-Resonance Chemical-Shift and Protein Secondary Structure. J. Mol. Biol. 1991, 222, 311–333. [Google Scholar]
- Arnold, M.R.; Kremer, W.; Ludemann, H.D.; Kalbitzer, H.R. H-1-NMR parameters of common amino acid residues measured in aqueous solutions of the linear tetrapeptides Gly-Gly-X-Ala at pressures between 0.1 and 200 MPa. Biophys. Chem. 2002, 96, 129–140. [Google Scholar]
- Bundi, A.; Wuthrich, K. H-1-Nmr Parameters of the Common Amino-Acid Residues Measured in Aqueous-Solutions of the Linear Tetrapeptides H-Gly-Gly-X-L-Ala-Oh. Biopolymers 1979, 18, 285–297. [Google Scholar] [CrossRef]
- Guo, H.B.; Beahm, R.F.; Guo, H. Stabilization and destabilization of the C-delta-h⋯O=C hydrogen bonds involving proline residues in helices. J. Phys. Chem. B 2004, 108, 18065–18072. [Google Scholar]
- Vijay-Kumar, S.; Cook, W.J. Structure of a sarcoplasmic calcium-binding protein from Nereis diversicolor refined at 2.0 A resolution. J. Mol. Biol. 1992, 224, 413–426. [Google Scholar] [CrossRef]
- Daniecki, N.J.; Bhatt, M.R.; Yap, G.P.A.; Zondlo, N.J. Proline C-H Bonds as Loci for Proline Assembly via C-H/O Interactions. ChemBioChem 2022, 23, e202200409. [Google Scholar] [PubMed]
- Jiang, L.; Lai, L. CH⋯O hydrogen bonds at protein-protein interfaces. J. Biol. Chem. 2002, 277, 37732–37740. [Google Scholar]
- Pierce, A.C.; Sandretto, K.L.; Bemis, G.W. Kinase inhibitors and the case for CH⋯O hydrogen bonds in protein-ligand binding. Proteins 2002, 49, 567–576. [Google Scholar]
- Panigrahi, S.K. Strong and weak hydrogen bonds in protein-ligand complexes of kinases: A comparative study. Amino Acids 2008, 34, 617–633. [Google Scholar]
- Ferguson, F.M.; Gray, N.S. Kinase inhibitors: The road ahead. Nat. Rev. Drug Discov. 2018, 17, 353–377. [Google Scholar]
- Attwood, M.M.; Fabbro, D.; Sokolov, A.V.; Knapp, S.; Schioth, H.B. Trends in kinase drug discovery: Targets, indications and inhibitor design. Nat. Rev. Drug Discov. 2021, 20, 839–861. [Google Scholar]
- Derewenda, Z.S.; Hawro, I.; Derewenda, U. C–H⋯O hydrogen bonds in kinase-inhibitor interfaces. IUBMB Life 2020, 72, 1233–1242. [Google Scholar]
- Xing, L.; Klug-Mcleod, J.; Rai, B.; Lunney, E.A. Kinase hinge binding scaffolds and their hydrogen bond patterns. Bioorg. Med. Chem. 2015, 23, 6520–6527. [Google Scholar]
- Knowles, P.P.; Murray-Rust, J.; Kjaer, S.; Scott, R.P.; Hanrahan, S.; Santoro, M.; Ibanez, C.F.; McDonald, N.Q. Structure and chemical inhibition of the RET tyrosine kinase domain. J. Biol. Chem. 2006, 281, 33577–33587. [Google Scholar] [PubMed]
- Bukrejewska, M.; Derewenda, U.; Radwanska, M.; Engel, D.A.; Derewenda, Z.S. Crystal structures of the methyltransferase and helicase from the ZIKA 1947 MR766 Uganda strain. Acta Crystallogr. Sect. D Struct. Biol. 2017, 73 Pt 9, 767–774. [Google Scholar]
- Fanfrlik, J.; Kolar, M.; Kamlar, M.; Hurny, D.; Ruiz, F.X.; Cousido-Siah, A.; Mitschler, A.; Rezac, J.; Munusamy, E.; Lepsik, M.; et al. Modulation of aldose reductase inhibition by halogen bond tuning. ACS Chem. Biol. 2013, 8, 2484–2492. [Google Scholar] [PubMed]
- Creeth, J.M.; Gulland, J.M.; Jordan, D.O. Deoxypentose nucleic acids; viscosity and streaming birefringence of solutions of the sodium salt of the deoxypentose nucleic acid of calf thymus. J. Chem. Soc. 1947, 25, 1141–1145. [Google Scholar]
- Harding, S.E.; Channell, G.; Phillips-Jones, M.K. The discovery of hydrogen bonds in DNA and a re-evaluation of the 1948 Creeth two-chain model for its structure. Biochem. Soc. Trans. 2018, 46, 1171–1182. [Google Scholar]
- Watson, J.D.; Crick, F.H. Genetical implications of the structure of deoxyribonucleic acid. Nature 1953, 171, 964–967. [Google Scholar] [CrossRef]
- Wain-Hobson, S. The third Bond. Nature 2006, 439, 539. [Google Scholar] [CrossRef]
- Pauling, L.; Corey, R.B. Specific Hydrogen-Bond Formation between Pyrimidines and Purines in Deoxyribonucleic Acids. Arch. Biochem. Biophys. 1956, 65, 164–181. [Google Scholar] [CrossRef]
- O’Brien, E.J. The crystal structure of a complex of 9-Ethyl guanine with 1-Methyl cytosine. J. Mol. Biol. 1963, 7, 107–110. [Google Scholar]
- Hoogsteen, K. The Structure of Crystals Containing a Hydrogen-Bonded Complex of 1-Methylthymine and 9-Methyladenine. Acta Crystallogr. 1959, 12, 822–823. [Google Scholar] [CrossRef]
- Felsenfeld, G.; Rich, A. Studies on the formation of two- and three-stranded polyribonucleotides. Biochim. Biophys. Acta 1957, 26, 457–468. [Google Scholar]
- Courtois, Y.; Fromageot, P.; Guschlbauer, W. Protonated polynucleotide structures. 3. An optical rotatory dispersion study of the protonation of DNA. Eur. J. Biochem. 1968, 6, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Quigley, G.J.; Ughetto, G.; van der Marel, G.A.; van Boom, J.H.; Wang, A.H.; Rich, A. Non-Watson-Crick G.C and A.T base pairs in a DNA-antibiotic complex. Science 1986, 232, 1255–1258. [Google Scholar] [CrossRef] [PubMed]
- Leonard, G.A.; Mcauleyhecht, K.; Brown, T.; Hunter, W.N. Do C-H⋯O Hydrogen-Bonds Contribute to the Stability of Nucleic-Acid Base-Pairs. Acta Crystallogr. Sect. D Struct. Biol. 1995, 51, 136–139. [Google Scholar]
- Starikov, E.B.; Steiner, T. Computational support for the suggested contribution of C-H⋯O=C interactions to the stability of nucleic acid base pairs. Acta Crystallogr. Sect. D Struct. Biol. 1997, 53 Pt 3, 345–347. [Google Scholar]
- Asensio, A.; Kobko, N.; Dannenberg, J.J. Cooperative hydrogen-bonding in adenine-thymine and guanine-cytosine base pairs. Density functional theory and Moller-Plesset molecular orbital study. J. Phys. Chem. A 2003, 107, 6441–6443. [Google Scholar] [CrossRef]
- Zhou, P.P.; Qiu, W.Y. Red-shifted hydrogen bonds and blue-shifted van der Waals contact in the standard Watson-Crick adenine-thymine base pair. J. Phys. Chem. A 2009, 113, 10306–10320. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathi, R.; Amutha, R.; Subramanian, V.; Nair, B.U.; Ramasami, T. Bader’s and reactivity descriptors’ analysis of DNA base pairs. J. Phys. Chem. A 2004, 108, 3817–3828. [Google Scholar] [CrossRef]
- Yurenko, Y.P.; Zhurakivsky, R.O.; Samijlenko, S.P.; Hovorun, D.M. Intramolecular CH⋯O hydrogen bonds in the AI and BI DNA-like conformers of canonical nucleosides and their Watson-Crick pairs. Quantum chemical and AIM analysis. J. Biomol. Struct. Dyn. 2011, 29, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Brovarets, O.O.; Yurenko, Y.P.; Hovorun, D.M. Intermolecular CH⋯O/N H-bonds in the biologically important pairs of natural nucleobases: A thorough quantum-chemical study. J. Biomol. Struct. Dyn. 2014, 32, 993–1022. [Google Scholar]
- Fick, R.J.; Liu, A.Y.; Nussbaumer, F.; Kreutz, C.; Rangadurai, A.; Xu, Y.; Sommer, R.D.; Shi, H.; Scheiner, S.; Stelling, A.L. Probing the Hydrogen-Bonding Environment of Individual Bases in DNA Duplexes with Isotope-Edited Infrared Spectroscopy. J. Phys. Chem. B 2021, 125, 7613–7627. [Google Scholar] [CrossRef]
- Quintano, M.; Delgado, A.A.A.; Moura, R.T.; Freindorf, M.; Kraka, E. Local mode analysis of characteristic vibrational coupling in nucleobases and Watson-Crick base pairs of DNA. Electron. Struct. 2022, 4, 044005. [Google Scholar] [CrossRef]
- Beiranvand, N.; Freindorf, M.; Kraka, E. Hydrogen Bonding in Natural and Unnatural Base Pairs-A Local Vibrational Mode Study. Molecules 2021, 26, 2268. [Google Scholar] [CrossRef]
- Hoshika, S.; Leal, N.A.; Kim, M.J.; Kim, M.S.; Karalkar, N.B.; Kim, H.J.; Bates, A.M.; Watkins, N.E., Jr.; SantaLucia, H.A.; Meyer, A.J.; et al. Hachimoji DNA and RNA: A genetic system with eight building blocks. Science 2019, 363, 884–887. [Google Scholar] [CrossRef]
- Das, S.; Roy, S.; Bhattacharyya, D. Understanding the role of non-Watson-Crick base pairs in DNA-protein recognition: Structural and energetic aspects using crystallographic database analysis and quantum chemical calculation. Biopolymers 2022, 113, e23492. [Google Scholar] [CrossRef]
- Halder, S.; Bhattacharyya, D. RNA structure and dynamics: A base pairing perspective. Prog. Biophys. Mol. Biol. 2013, 113, 264–283. [Google Scholar] [CrossRef] [PubMed]
- Poltev, V.; Anisimov, V.M.; Dominguez, V.; Ruiz, A.; Deriabina, A.; Gonzalez, E.; Garcia, D.; Rivas, F. Understanding the Origin of Structural Diversity of DNA Double Helix. Computation 2021, 9, 98. [Google Scholar] [CrossRef]
- Neidle, S. Beyond the double helix: DNA structural diversity and the PDB. J. Biol. Chem. 2021, 296, 100553. [Google Scholar] [CrossRef]
- Bansal, A.; Kaushik, S.; Kukreti, S. Non-canonical DNA structures: Diversity and disease association. Front. Genet. 2022, 13, 959258. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.; Neidle, S.; Berman, H.M. Conformations of the sugar-phosphate backbone in helical DNA crystal structures. Biopolymers 1997, 42, 113–124. [Google Scholar] [CrossRef]
- Berman, H.M. Crystal studies of B-DNA: The answers and the questions. Biopolymers 1997, 44, 23–44. [Google Scholar] [CrossRef]
- Yurenko, Y.P.; Zhurakivsky, R.O.; Samijlenko, S.P.; Ghomi, M.; Hovorun, D.M. The whole of intramolecular H-bonding in the isolated DNA nucleoside thymidine. AIM electron density topological study. Chem. Phys. Lett. 2007, 447, 140–146. [Google Scholar] [CrossRef]
- Berger, I.; Egli, M.; Rich, A. Inter-strand C-H⋯O hydrogen bonds stabilizing four-stranded intercalated molecules: Stereoelectronic effects of 04′ in cytosine-rich DNA. Proc. Natl. Acad. Sci. USA 1996, 93, 12116–12121. [Google Scholar] [CrossRef]
- Auffinger, P.; LouiseMay, S.; Westhof, E. Molecular dynamics simulations of the anticodon hairpin of tRNA(Asp): Structuring effects of C-H⋯O hydrogen bonds and of long-range hydration forces. J. Am. Chem. Soc. 1996, 118, 1181–1189. [Google Scholar] [CrossRef]
- Brandl, M.; Lindauer, K.; Meyer, M.; Suhnel, J. C-H⋯O and C-H⋯N interactions in RNA structures. Theor. Chem. Acc. 1999, 101, 103–113. [Google Scholar] [CrossRef]
- Witts, R.N.; Hopson, E.C.; Koballa, D.E.; Van Boening, T.A.; Hopkins, N.H.; Patterson, E.V.; Nagan, M.C. Backbone-Base Interactions Critical to Quantum Stabilization of Transfer RNA Anticodon Structure. J. Phys. Chem. B 2013, 117, 7489–7497. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derewenda, Z.S. C-H Groups as Donors in Hydrogen Bonds: A Historical Overview and Occurrence in Proteins and Nucleic Acids. Int. J. Mol. Sci. 2023, 24, 13165. https://doi.org/10.3390/ijms241713165
Derewenda ZS. C-H Groups as Donors in Hydrogen Bonds: A Historical Overview and Occurrence in Proteins and Nucleic Acids. International Journal of Molecular Sciences. 2023; 24(17):13165. https://doi.org/10.3390/ijms241713165
Chicago/Turabian StyleDerewenda, Zygmunt Stanislaw. 2023. "C-H Groups as Donors in Hydrogen Bonds: A Historical Overview and Occurrence in Proteins and Nucleic Acids" International Journal of Molecular Sciences 24, no. 17: 13165. https://doi.org/10.3390/ijms241713165
APA StyleDerewenda, Z. S. (2023). C-H Groups as Donors in Hydrogen Bonds: A Historical Overview and Occurrence in Proteins and Nucleic Acids. International Journal of Molecular Sciences, 24(17), 13165. https://doi.org/10.3390/ijms241713165



