Bipolar Membrane Electrodialysis for Direct Conversion of L-Ornithine Monohydrochloride to L-Ornithine
Abstract
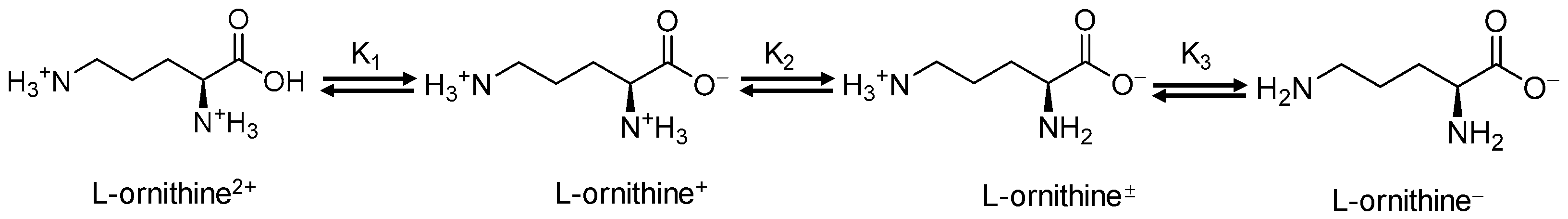
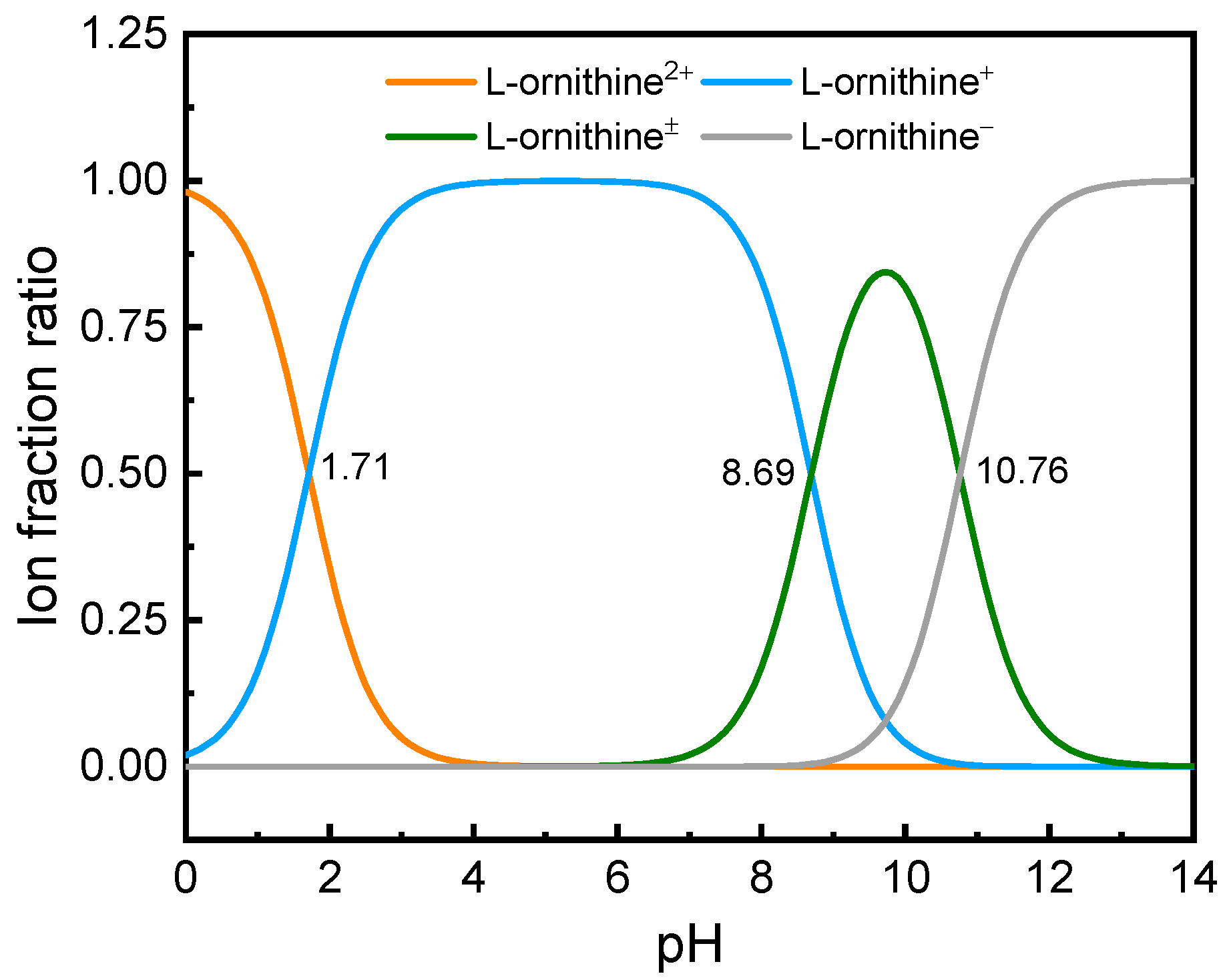
:1. Introduction
2. Results and Discussion
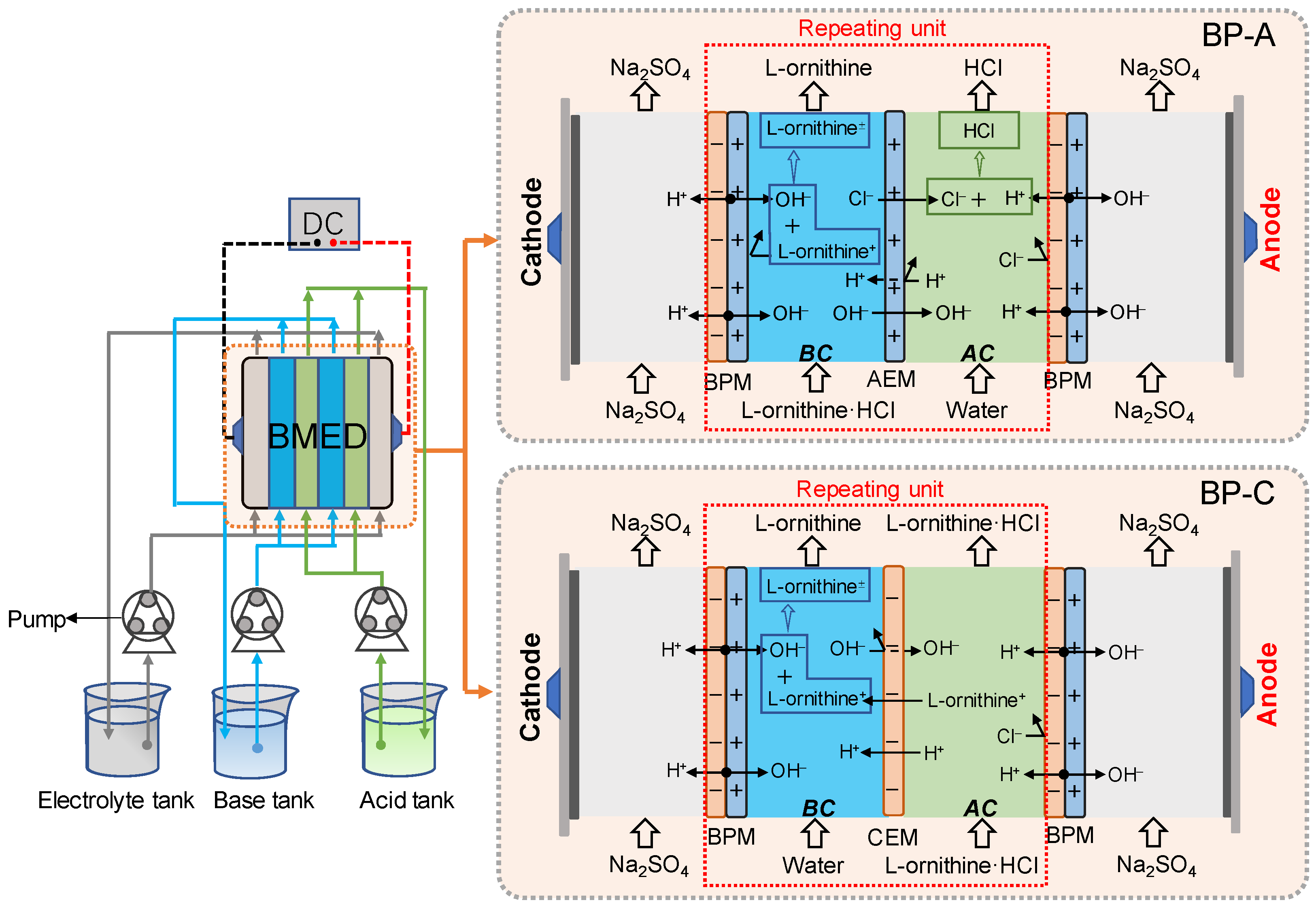
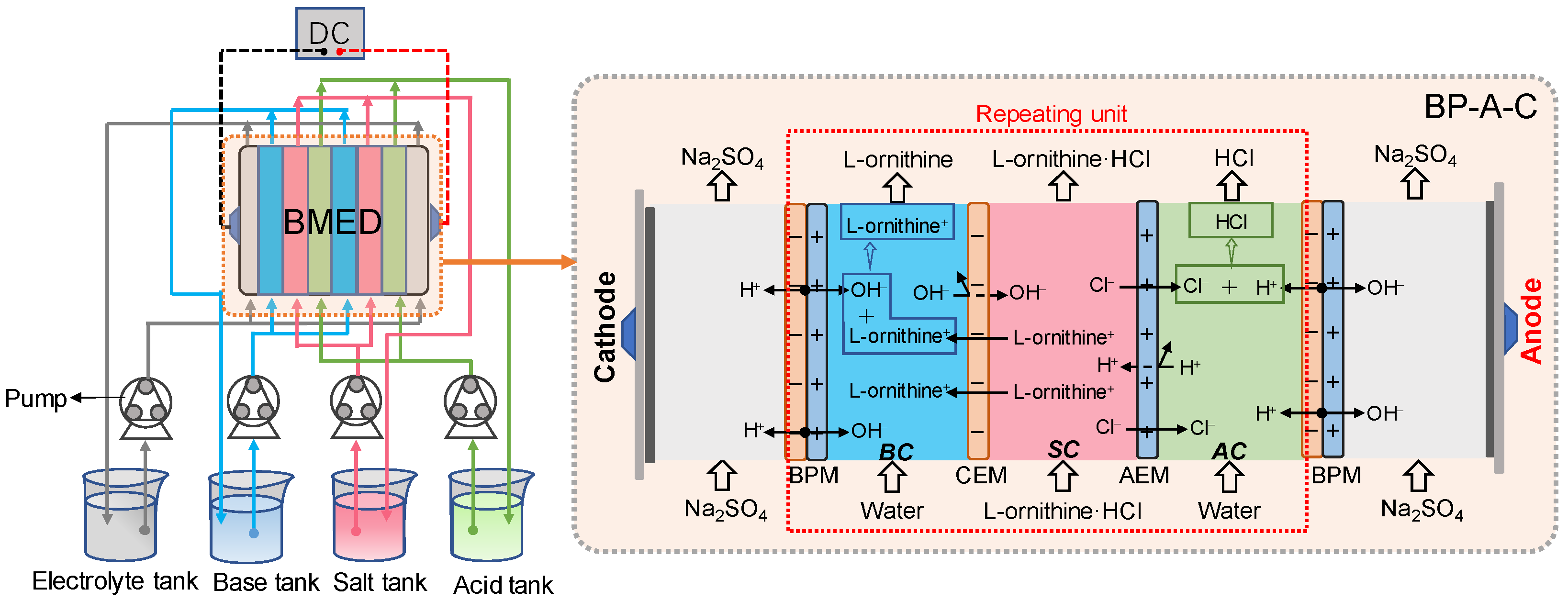
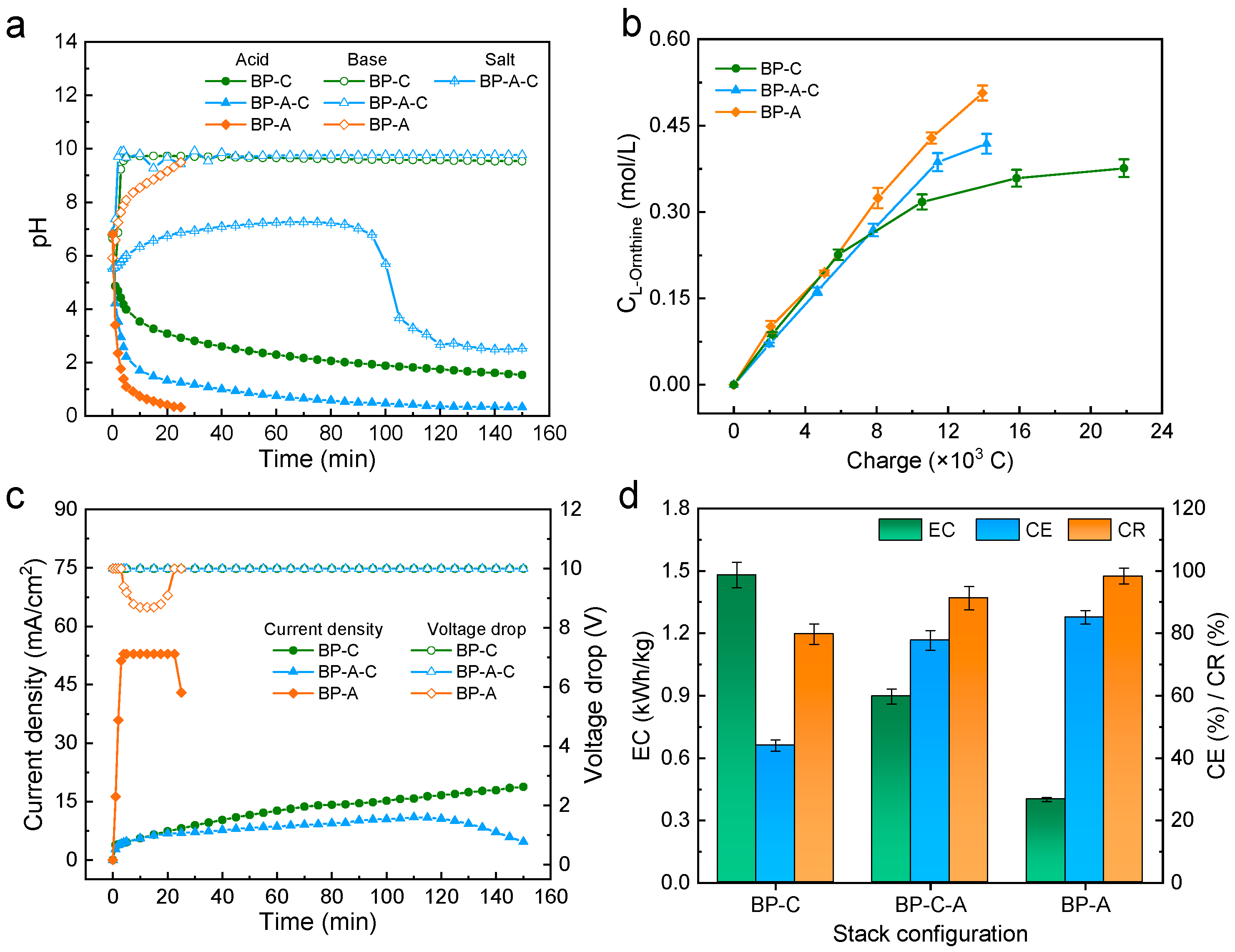
2.1. Effect of the Stack Configuration
2.2. Effect of the Voltage Drop across the Membrane Stack
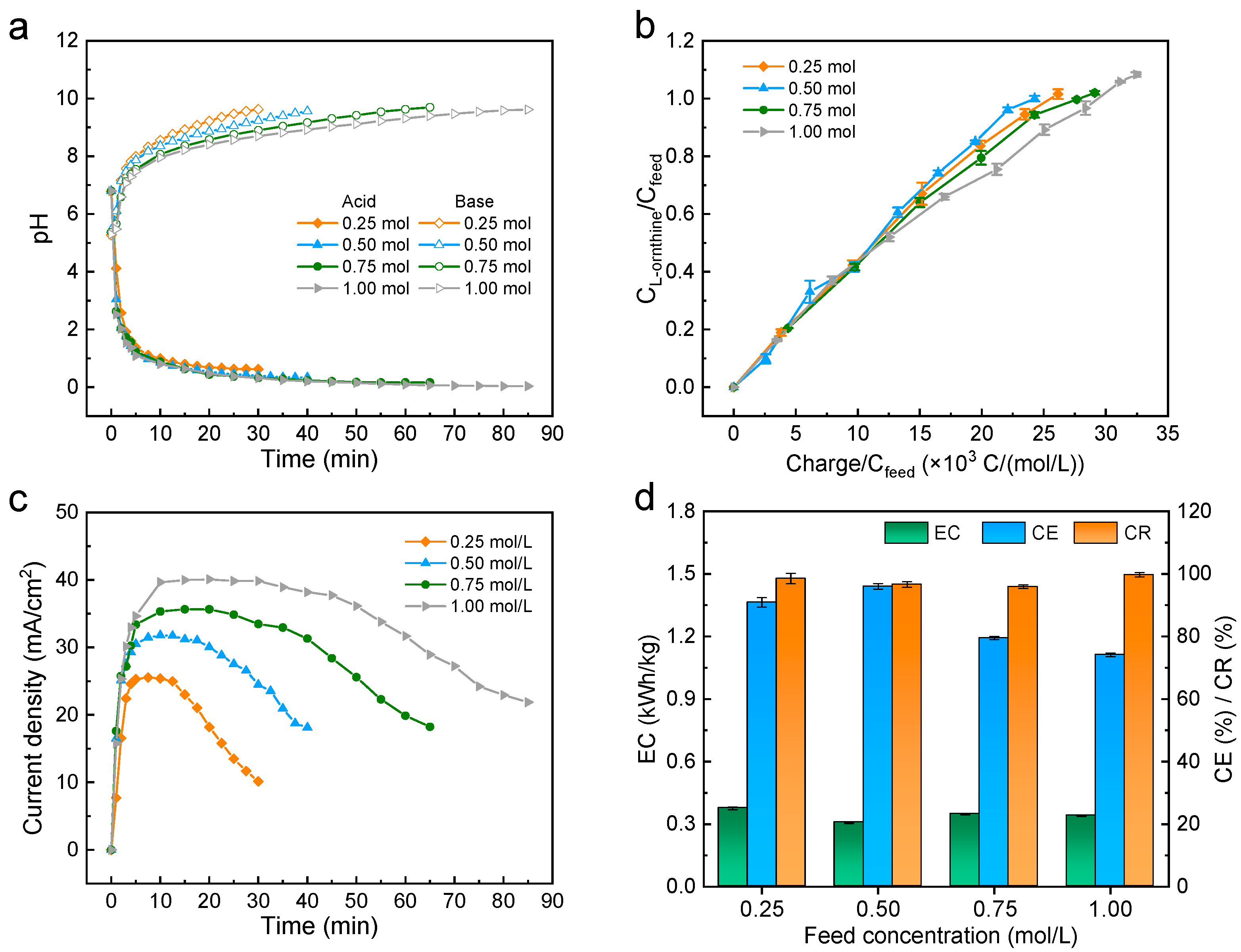
2.3. Effect of the Feed Concentration
3. Materials and Methods
3.1. Materials
3.2. Experimental Setup
3.3. Experimental Procedure
3.4. Sample Analysis and Data Calculation
3.4.1. Sample Analysis
3.4.2. Conversion Ratio, Current Efficiency and Energy Consumption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nie, L.; He, Y.; Hu, L.; Zhu, X.; Wu, X.; Zhang, B. Improvement in l-ornithine production from mannitol via transcriptome-guided genetic engineering in Corynebacterium glutamicum. Biotechnol. Biofuels Bioprod. 2022, 15, 97. [Google Scholar] [CrossRef] [PubMed]
- Hirofuto, M.; Kunio Saito, S. Ornithine Aspartate and a Process for Its Preparation. U.S. Patent 3,360,549, 26 December 1967. [Google Scholar]
- Canbay, A.; Sowa, J.-P. l-Ornithine l-Aspartate (LOLA) as a Novel Approach for Therapy of Non-alcoholic Fatty Liver Disease. Drugs 2019, 79, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, R.F. l-Ornithine l-Aspartate (LOLA) for the Treatment of Hepatic Encephalopathy in Cirrhosis: Novel Insights and Translation to the Clinic. Drugs 2019, 79, 1–3. [Google Scholar] [CrossRef]
- Jain, A.; Sharma, B.C.; Mahajan, B.; Srivastava, S.; Kumar, A.; Sachdeva, S.; Sonika, U.; Dalal, A. L-ornithine L-aspartate in acute treatment of severe hepatic encephalopathy: A double-blind randomized controlled trial. Hepatology 2022, 75, 1194–1203. [Google Scholar] [CrossRef]
- Shioya, S. Optimization and control in fed-batch bioreactors. In Modern Biochemical Engineering; Springer: Berlin/Heidelberg, Germany, 1992; pp. 111–142. [Google Scholar]
- Makryaleas, K.; Drauz, K. Method for the Preparation of Salts of L-Ornithine. U.S. Patent 5,405,761, 11 April 1995. [Google Scholar]
- Makryaleas, K.; Drauz, K.; Bommarius, A. Method for the Preparation of D-Arginine and L-Ornithine. U.S. Patent 5,591,613, 7 January 1997. [Google Scholar]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics, Internet Version 2005; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Xu, T.; Huang, C. Electrodialysis-based separation technologies: A critical review. AIChE J. 2008, 54, 3147–3159. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, M.; Huang, S.; Wang, J.; Zhang, X. Limiting concentration during batch electrodialysis process for concentrating high salinity solutions: A theoretical and experimental study. Desalination 2021, 498, 114793. [Google Scholar] [CrossRef]
- Liu, W.; He, J.; Yan, J.; Tian, Z.; Li, Q.; Wang, H.; Li, C.; Wang, Y.; Yan, H. Simultaneous salt recovery and zwitterionic stachydrine purification from saline eluent via two-stage electrodialysis system. Sep. Purif. Technol. 2023, 310, 123142. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Jiang, C.; Xu, T. Recovery of gamma-aminobutyric acid (GABA) from reaction mixtures containing salt by electrodialysis. Sep. Purif. Technol. 2016, 170, 353–359. [Google Scholar] [CrossRef]
- Jaroszek, H.; Dydo, P. Potassium nitrate synthesis by electrodialysis-metathesis: The effect of membrane type. J. Membr. Sci. 2018, 549, 28–37. [Google Scholar] [CrossRef]
- Sharma, P.P.; Gahlot, S.; Rajput, A.; Patidar, R.; Kulshrestha, V. Efficient and Cost Effective Way for the Conversion of Potassium Nitrate from Potassium Chloride Using Electrodialysis. ACS Sustain. Chem. Eng. 2016, 4, 3220–3227. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Yue, M.; Ji, W. Recovery of L-lysine from L-lysine monohydrochloride by ion substitution using ion-exchange membrane. Desalination 2011, 271, 163–168. [Google Scholar] [CrossRef]
- Kumar, M.; Ali Khan, M.; Al Othman, Z. Electro-membrane reactor for the conversion of lysine monohydrochloride to lysine by in situ ion substitution and separation. J. Chem. Technol. Biotechnol. 2013, 88, 910–918. [Google Scholar] [CrossRef]
- Fu, R.; Wang, H.; Yan, J.; Li, R.; Wang, B.; Jiang, C.; Wang, Y.; Xu, T. A cost-effective and high-efficiency online ED-BMED integrated system enables the conversion of 3.5 wt% NaCl aqueous solution into 6.20 mol/L NaOH. Chem. Eng. Sci. 2023, 270, 118523. [Google Scholar] [CrossRef]
- Cassaro, C.; Virruso, G.; Culcasi, A.; Cipollina, A.; Tamburini, A.; Micale, G. Electrodialysis with Bipolar Membranes for the Sustainable Production of Chemicals from Seawater Brines at Pilot Plant Scale. ACS Sustain. Chem. Eng. 2023, 11, 2989–3000. [Google Scholar] [CrossRef]
- Aghajanyan, A.E.; Tsaturyan, A.O.; Hambardzumyan, A.A.; Saghyan, A.S. Obtaining the zwitterionic form of L-lysine from L-lysine monohydrochloride by electrodialysis. Membr. Water Treat. 2013, 4, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Yue, M.; Wang, L. Production of l-lysine from l-lysine monohydrochloride by bipolar membrane electrodialysis. Desalination Water Treat. 2012, 41, 105–113. [Google Scholar] [CrossRef]
- Eliseev, T.V.; Krisilova, E.V.; Shaposhnik, V.A.; Bukhovets, A.E. Recovery and concentration of basic amino acids by electrodialysis with bipolar membranes. Desalination Water Treat. 2010, 14, 196–200. [Google Scholar] [CrossRef]
- Readi, O.M.K.; Gironès, M.; Wiratha, W.; Nijmeijer, K. On the Isolation of Single Basic Amino Acids with Electrodialysis for the Production of Biobased Chemicals. Ind. Eng. Chem. Res. 2013, 52, 1069–1078. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, C.; Sun, M.; Zhang, X.; Wu, C.; Wu, Y. Separation of mixed amino acids by BMED process using porous SPES and SPSf cation exchange membranes. Sep. Purif. Technol. 2017, 188, 539–547. [Google Scholar] [CrossRef]
- Pärnamäe, R.; Mareev, S.; Nikonenko, V.; Melnikov, S.; Sheldeshov, N.; Zabolotskii, V.; Hamelers, H.V.M.; Tedesco, M. Bipolar membranes: A review on principles, latest developments, and applications. J. Membr. Sci. 2021, 617, 118538. [Google Scholar] [CrossRef]
- Tedesco, M.; Hamelers, H.V.M.; Biesheuvel, P.M. Nernst-Planck transport theory for (reverse) electrodialysis: I. Effect of co-ion transport through the membranes. J. Membr. Sci. 2016, 510, 370–381. [Google Scholar] [CrossRef]
- Nightingale, E.R. Phenomenological Theory of Ion Solvation. Effective Radii of Hydrated Ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Agmon, N. The Grotthuss mechanism. Chem. Phys. Lett. 1995, 244, 456–462. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Abraham, M.H.; Zissimos, A.M. Fast Calculation of van der Waals Volume as a Sum of Atomic and Bond Contributions and Its Application to Drug Compounds. J. Org. Chem. 2003, 68, 7368–7373. [Google Scholar] [CrossRef] [PubMed]
- Sheng, F.; Wu, B.; Li, X.; Xu, T.; Shehzad, M.A.; Wang, X.; Ge, L.; Wang, H.; Xu, T. Efficient Ion Sieving in Covalent Organic Framework Membranes with Sub-2-Nanometer Channels. Adv. Mater. 2021, 33, 2104404. [Google Scholar] [CrossRef]
- Huang, C.; Xu, T.; Yang, X. Regenerating Fuel-Gas Desulfurizing Agents by Using Bipolar Membrane Electrodialysis (BMED): Effect of Molecular Structure of Alkanolamines on the Regeneration Performance. Environ. Sci. Technol. 2007, 41, 984–989. [Google Scholar] [CrossRef]
- Yan, H.; Wu, L.; Wang, Y.; Irfan, M.; Jiang, C.; Xu, T. Ammonia capture from wastewater with a high ammonia nitrogen concentration by water splitting and hollow fiber extraction. Chem. Eng. Sci. 2020, 227, 115934. [Google Scholar] [CrossRef]
- Chen, W.; Shen, H.; Gong, Y.; Li, P.; Cheng, C. Anion exchange membranes with efficient acid recovery obtained by quaternized poly epichlorohydrin and polyvinyl alcohol during diffusion dialysis. J. Membr. Sci. 2023, 674, 121514. [Google Scholar] [CrossRef]
- McGovern, R.K.; Weiner, A.M.; Sun, L.; Chambers, C.G.; Zubair, S.M.; Lienhard, V.J.H. On the cost of electrodialysis for the desalination of high salinity feeds. Appl. Energy 2014, 136, 649–661. [Google Scholar] [CrossRef]
- Wang, W.; Fu, R.; Liu, Z.; Wang, H. Low-resistance anti-fouling ion exchange membranes fouled by organic foulants in electrodialysis. Desalination 2017, 417, 1–8. [Google Scholar] [CrossRef]
- Yan, J.; Yan, H.; Wang, H.; Li, Q.; Zhang, H.; Jiang, C.; Ye, B.; Wang, Y.; Xu, T. Bipolar membrane electrodialysis for clean production of L-10-camphorsulfonic acid: From laboratory to industrialization. AIChE J. 2022, 68, e17490. [Google Scholar] [CrossRef]
- Nichka, V.; Mareev, S.; Pismenskaya, N.; Nikonenko, V.; Bazinet, L. Mathematical Modeling of the Effect of Pulsed Electric Field Mode and Solution Flow Rate on Protein Fouling during Bipolar Membrane Electroacidificaiton of Caseinate Solution. Membranes 2022, 12, 193. [Google Scholar] [CrossRef] [PubMed]

| Properties of the Considered Ions | L-Ornithine+ (C5H13N2O2+) | Cl− | H+ | OH− |
|---|---|---|---|---|
| Molar weight (g/mol) | 133.2 | 35.5 | 1.00 | 17.00 |
| Van der Waals Volume (Å3) | a 133.32 | - | - | - |
| Bare ion radius (Å) | b 3.17 | c 1.81 | c 0.28 | c 1.76 |
| Hydrated ion radius (Å) | - | c 3.58 | c 2.82 | c 3.00 |
| Diffusion coefficient (m2/s) | d 8.71 × 10−10 | e 1.33 × 10−9 | f 9.31 × 10−9 | f 5.27 × 10−9 |
| Membrane Name | Thickness (μm) | IEC (meq/g) | Water Uptake (%) | Burst Strength (MPa) | Area Resistance (Ω·cm2) | Transport Number (%) |
| CIS [12] | 70 | 0.90–1.10 | 20–30 | ≥0.22 | ≤4.0 | ≥95 |
| AIS [12] | 70 | 0.90–1.10 | 20–30 | ≥0.17 | ≤4.0 | ≥98 |
| Membrane Name | Thickness (μm) | Water Splitting Voltage Drop (V) | Water Splitting Efficiency | Burst Strength (MPa) | ||
| a BP-1E | 280 | 1.0 | ≥0.98 | ≥0.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Liu, W.; Hao, J.; Ma, X.; Zheng, Z.; Fang, Y.; Liang, Y.; Tian, Z.; Sun, L.; Li, C.; et al. Bipolar Membrane Electrodialysis for Direct Conversion of L-Ornithine Monohydrochloride to L-Ornithine. Int. J. Mol. Sci. 2023, 24, 13174. https://doi.org/10.3390/ijms241713174
He J, Liu W, Hao J, Ma X, Zheng Z, Fang Y, Liang Y, Tian Z, Sun L, Li C, et al. Bipolar Membrane Electrodialysis for Direct Conversion of L-Ornithine Monohydrochloride to L-Ornithine. International Journal of Molecular Sciences. 2023; 24(17):13174. https://doi.org/10.3390/ijms241713174
Chicago/Turabian StyleHe, Jinfeng, Wenlong Liu, Jianrong Hao, Xixi Ma, Zhiyi Zheng, Yinghan Fang, Yuxin Liang, Zhihao Tian, Li Sun, Chuanrun Li, and et al. 2023. "Bipolar Membrane Electrodialysis for Direct Conversion of L-Ornithine Monohydrochloride to L-Ornithine" International Journal of Molecular Sciences 24, no. 17: 13174. https://doi.org/10.3390/ijms241713174
APA StyleHe, J., Liu, W., Hao, J., Ma, X., Zheng, Z., Fang, Y., Liang, Y., Tian, Z., Sun, L., Li, C., & Yan, H. (2023). Bipolar Membrane Electrodialysis for Direct Conversion of L-Ornithine Monohydrochloride to L-Ornithine. International Journal of Molecular Sciences, 24(17), 13174. https://doi.org/10.3390/ijms241713174







