From Biomarkers to the Molecular Mechanism of Preeclampsia—A Comprehensive Literature Review
Abstract
:1. Introduction
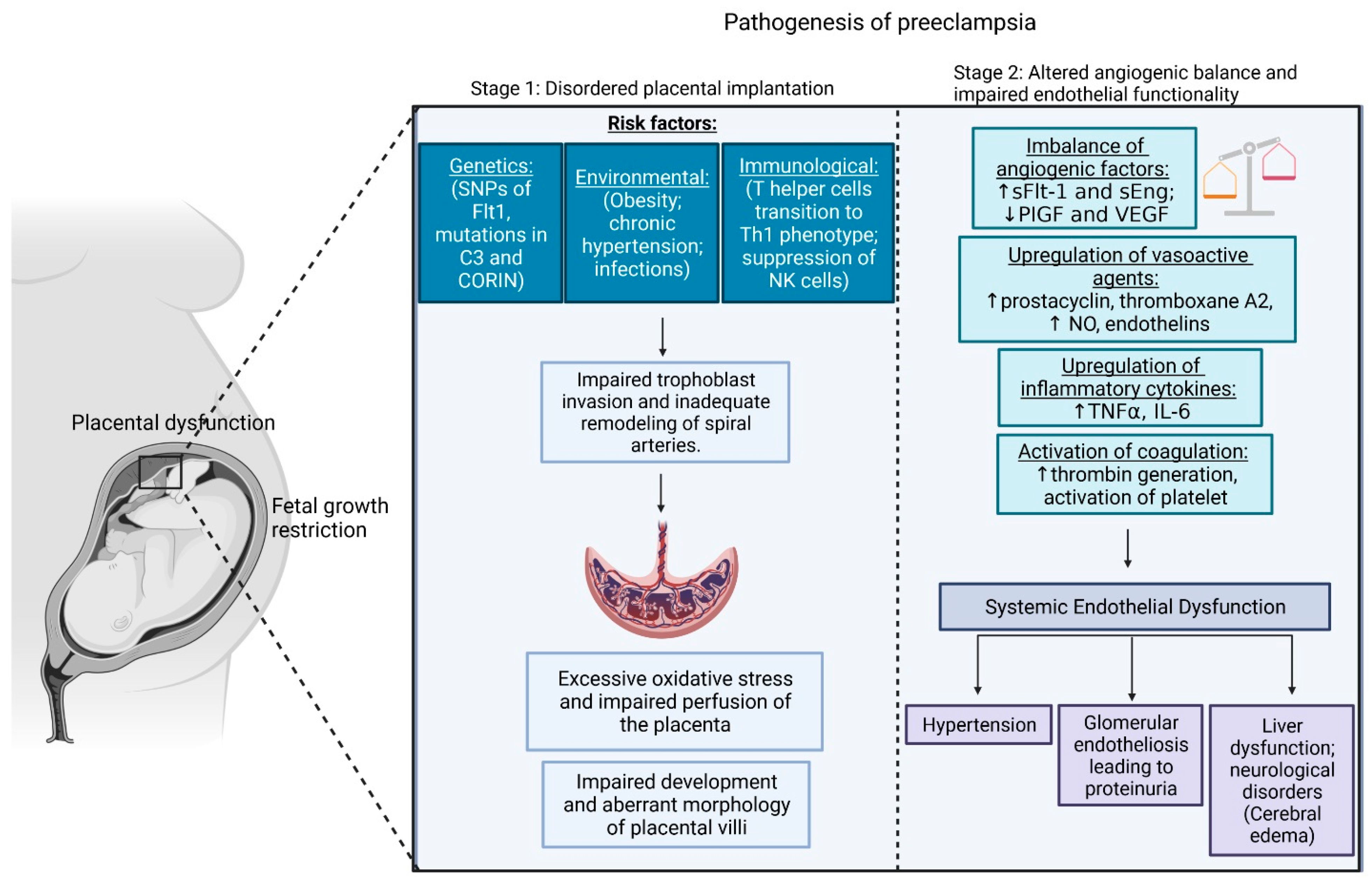
2. Pathogenesis of PE
2.1. Early-Onset PE
2.2. Late-Onset PE
3. Biomarkers and Their Molecular Pathway Development and Prediction of PE
3.1. Pregnancy-Associated Plasma Protein-A (PAPP-A)
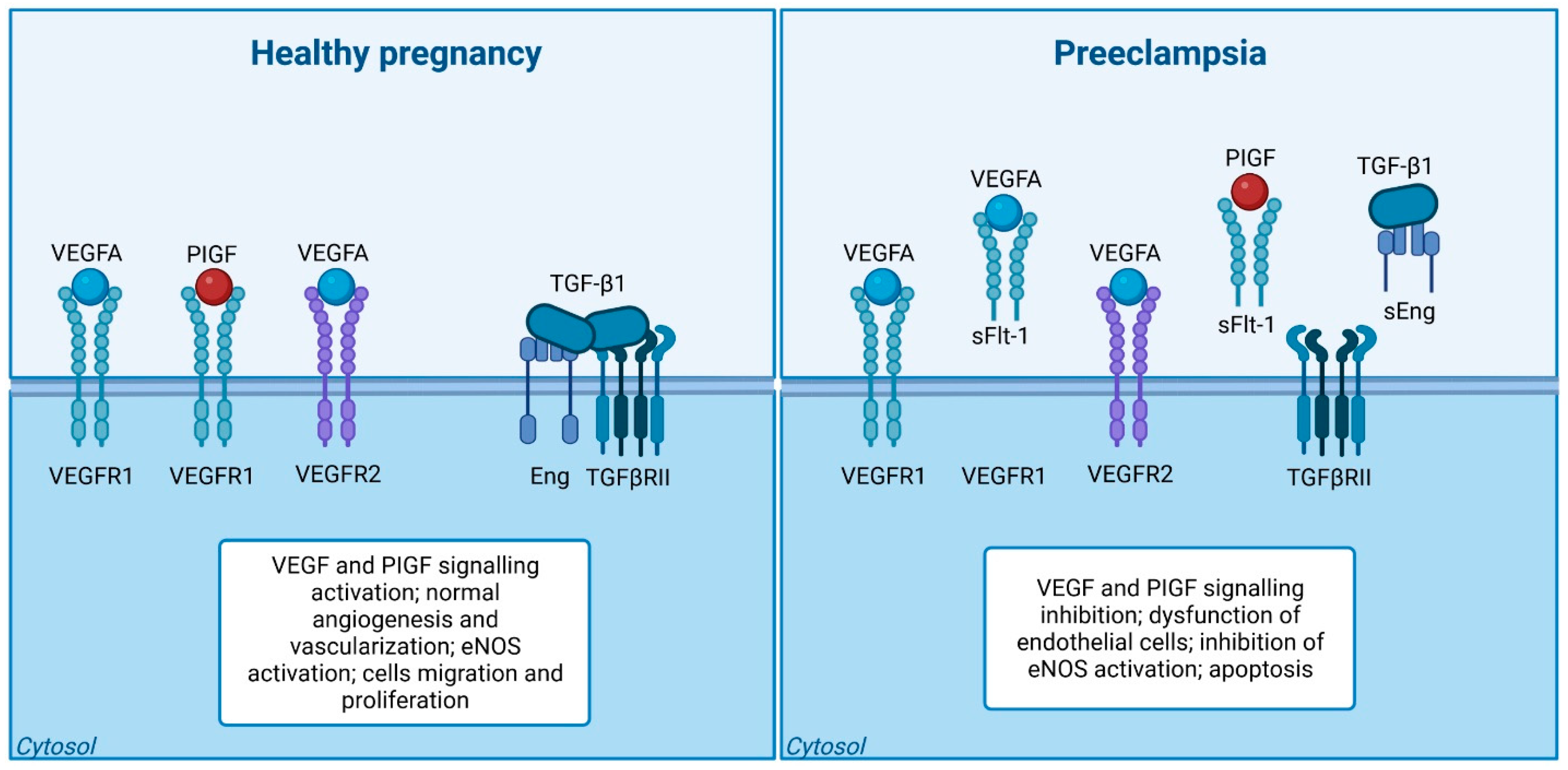
3.2. Placental Growth Factor (PlGF) and Vascular Endothelial Growth Factor (VEGF)
3.3. Soluble FMS-Like Tyrosine Kinase-1 (sFlt-1)
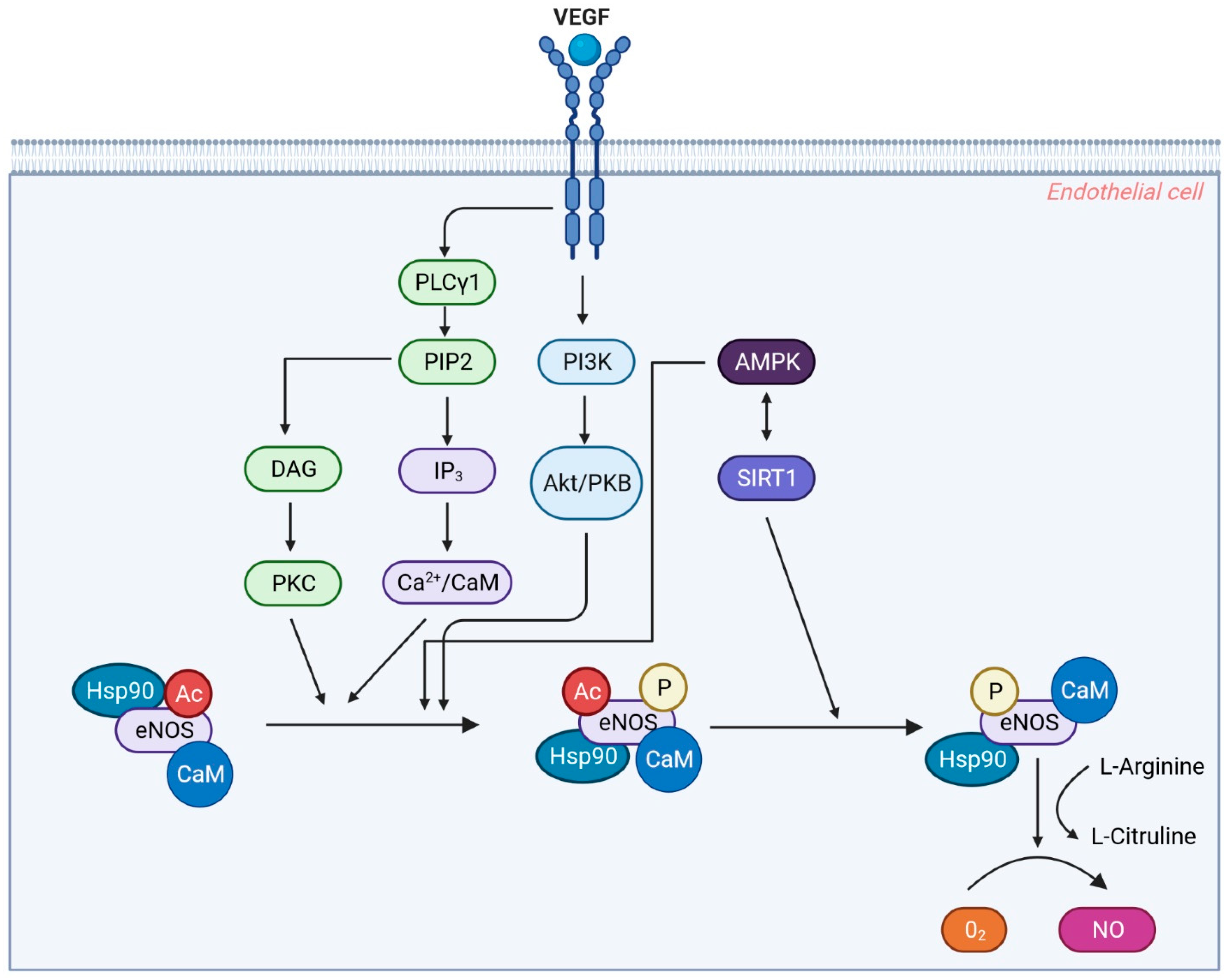
3.4. Endothelium-Derived Nitric Oxide (NO)
3.5. Placental Protein 13 (PP-13)
3.6. Growth Differentiation Factor 15 (GDF15)
3.7. A disintegrin and Metalloprotease 12 (ADAM-12)
3.8. β-Human Chorionic Gonadotropin (β-hCG)
3.9. Inhibin Alpha (Inhibin-A)
3.10. Soluble Endoglin (sEng)
| Biomarker Name (Units) | Authors (Year)/ Country | Control (Healthy Population) Mean (Range) | EOP Mean (Range) | LOP Mean (Range) | Any Stage of PE | Trimester (GW) |
|---|---|---|---|---|---|---|
| PAPP-A (MoM) | Sonek et al. (2018) [71]/USA | 1.00 (0.69–1.50) | 0.62 (0.50–0.86) | 0.97 (0.57–1.47) | NA | 1st (11–13 GW) |
| Hu et al. (2021) [72]/Chinese | 1.01 (0.69–1.44) | 0.70 (0.45–1.09) | 0.86 (0.60–1.26) at term | NA | 1st (11–13 GW) | |
| PIGF (MoM) | Sonek et al. (2018) [71]/USA | 1.01 (0.81–1.27) | 0.68 (0.38–1.17) | 1.07 (0.84–1.28) | NA | 1st (11–13 GW) |
| Hu et al. (2021) [72]/Chinese | 0.99 (0.73–1.32) | 0.91 (0.63–1.18) | 0.83 (0.53–1.09) | NA | 1st (11–13 GW) | |
| sVEGFR-1 (pg/mL) | Kusanovic et al. (2009)/USA | 1612 (245–10595.5) | NA | NA | 1637.4 (325.1–17768.9) | NA |
| sFlt-1 (pg/mL) | Widmer et al. (2015) [73]/Multicentric (Argentina, Colombia, Peru, India, Italy, Kenya, Switzerland and Thailand) | 2230 (1490–3340) | 2030 (1300–2930) | NA | 1890 (1210–2840) | 1st–2nd (<20 GW) |
| 2280 (1480–3580) | 2510 (1460–4310) | NA | 2260 (1400–3650) | 2nd (23–27 GW) | ||
| 3760 (2520–5800) | NA | NA | 7905 (4750–13,620) | 3rd (32–35 GW) | ||
| PP-13 (MoM) | Romera et al. ((2008) [74]/USA | 1.00 (0.83–1.10) | 0.26 (0.10–0.40) | 0.24 (0.11–0.40) | 0.59 (0.41–0.83) | NA |
| β-hCG (MoM) | Hanchard et al. (2020) [75]/Australia | 2.27 ± 0.90 | NA | NA | 2.68 ± 1.10 | NA |
| Inhibin-A (MoM) | Farzaneh et al. ((2019) [60]/NA | 1.67 ± 0.59 | NA | NA | 1.98 ± 0.81 | NA |
| sEng (pg/mL) | Widmer et al. (2015) [73]/Multicentric (Argentina, Colombia, Peru, India, Italy, Kenya, Switzerland and Thailand) | 5.0 (3.9–6.4) | 5.8 (4.3–8.4) | NA | 5.5 (4.3–7.6) | 1st–2nd (<20 GW) |
| 4.5 (3.5–5.6) | 5.4 (4.2–7.1) | NA | 5.5 (4.2–7.2) | 2nd (23–27 GW) | ||
| 7.7 (5.6–11.0) | 5.8 (4.3–8.4) | NA | 17.4 (9.8–35.5) | 3rd (32–35 GW) |
| Biomarker in Combinations (Units) | Authors (Year)/ Country | Control (Healthy Population) Mean (Range) | EOP Mean (Range) | LOP Mean (Range) | Any Stage of PE | Trimester (GW) | Reliability Parameter |
|---|---|---|---|---|---|---|---|
| sFlt-1/PlGF | Widmer et al. (2015) [73] /Multicentric (Argentina, Colombia, Peru, India, Italy, Kenya, Switzerland and Thailand) | 27.9 (12.7–62.2) | 30.7 (14.7–81.7) | NA | 32.8 (16.1–80.9) | 1st–2nd (<20 GW) | Cut-off <117 Specificity: 90% Sensitivity: 10% |
| Biomarker Name | Country/no of Literature Assessed | Authors (Year) | Control Size (Mean ± SD) | PE sample Size (Mean ± SD) | Control Value (Mean ± SD) | PE Sample Value (Mean ± SD) | Ratio of Control to the PE |
|---|---|---|---|---|---|---|---|
| PIGF (pg/mL) | USA/n = 3 | Levine et al. (2004); Holston. (2009); Young et al. (2009) [76,77,78] | 629.3 ± 814.7 | 21.7 ± 15.9 | 561.3 ± 155.2 | 180.8 ± 83.2 | 3.1 |
| Germany/n = 2 | Schmidt et al. (2009); Engels et al. (2013) [79] | 114 ± 99.0 | 35.5 ± 40.3 | 288.4 ± 293.9 | 55.2 ± 17.7 | 5.2 | |
| China/n = 3 | Ouyang et al. (2009); Ding et al. (2018); Wang et al. (2021) [80,81,82] | 182 ± 153.1 | 89.3 ± 43.8 | 263 ± 33.3 | 132.4 ± 103.8 | 1.2 | |
| India/n = 2 | Aggarwal et al. (2012); Kumar et al. (2023) [83,84] | 77.5 ± 43.1 | 75.5 ± 48.8 | 368.8 ± 182.2 | 86.2 ± 14.3 | 4.3 | |
| sFlt1 (pg/mL) | USA/n = 4 | [76,77,78,85] | 482.5 ± 727.1 | 26.8 ± 16.5 | 4978.5 ± 3831.3 | 12460 ± 8764.3 | - |
| China/n = 3 | Ouyang et al. (2009); Ding et al. (2018); Wang et al. (2021) [80,81,82] | 182.3 ± 153.1 | 89.3 ± 43.8 | 2041.2 ± 464.9 | 4524.4 ± 2428.3 | - | |
| India/n = 2 | Aggarwal et al. (2012); Kumar et al. (2023) [83,84] | 77.5 ± 43.1 | 75.5 ± 48.8 | 5704.5 ± 573.5 | 27650 ± 19586.7 | - | |
| sFlt1/PIGF | USA /n = 2 | Levine et al. (2004); Young et al. (2009) [76,78] | 802 ± 1077.6 | 25.5 ± 20.5 | 16.5 ± 16.3 | 183 ± 145.7 | - |
| China/n = 2 | Ouyang et al. (2009); Wang et al. (2021) [80,82] | 98.5 ± 68.6 | 66.0 ± 24.0 | 6.7 ± 0.14 | 16.7 ± 4.8 | - | |
| India/n = 2 | Aggarwal et al. (2012); Kumar et al. (2023) [83,84] | 77.5 ± 43.1 | 75.5 ± 48.8 | 147.9 ± 181.7 | 670.8 ± 112.7 | - | |
| sEng | USA/n = 3 | Levine et al. (2004); Holston., (2009); Young et al. (2009) [76,77,78] [76,77,78] | 619.3 ± 825.1 | 30.3 ± 16.7 | 9.9 ± 1.3 | 36.1 ± 15.7 | - |
| Chinese/n = 3 | Wang et al. (2021); Fang et al. (2010); Zhang et al. (2020) [82,86,87] | 27.7 ± 12.5 | 46.3 ± 7.1 | 10.2 ± 1.1 | 25.5 ± 11.9 | - | |
| India/n = 2 | Sachan et al. (2016); Archana et al. (2018) [77] | 35.0 ± 7.1 | 32.5 ± 3.5 | 4.0 ± 2.7 | 11.9 ± 4.5 | - |
4. Conclusions
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poon, L.C.; Shennan, A.; Hyett, J.A.; Kapur, A.; Hadar, E.; Divakar, H.; McAuliffe, F.; da Silva Costa, F.; von Dadelszen, P.; McIntyre, H.D.; et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on Pre-Eclampsia: A Pragmatic Guide for First-Trimester Screening and Prevention. Int. J. Gynecol. Obstet. 2019, 145, S173–S221. [Google Scholar] [CrossRef] [PubMed]
- Tessema, K.F.; Gebremeskel, F.; Getahun, F.; Chufamo, N.; Misker, D. Individual and Obstetric Risk Factors of Preeclampsia among Singleton Pregnancy in Hospitals of Southern Ethiopia. Int. J. Hypertens. 2021, 2021, 7430827. [Google Scholar] [CrossRef]
- Rana, S.; Lemoine, E.; Granger, J.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- Gyselaers, W. Preeclampsia Is a Syndrome with a Cascade of Pathophysiologic Events. J. Clin. Med. 2020, 9, 2245. [Google Scholar] [CrossRef]
- Tanner, M.S.; Davey, M.-A.; Mol, B.W.; Rolnik, D.L. The Evolution of the Diagnostic Criteria of Preeclampsia-Eclampsia. Am. J. Obs. Gynecol. 2022, 226, S835–S843. [Google Scholar] [CrossRef]
- Turbeville, H.R.; Sasser, J.M. Preeclampsia beyond pregnancy: Long-term consequences for mother and child. Am. J. Physiol.-Ren. Physiol. 2020, 318, F1315–F1326. [Google Scholar] [CrossRef]
- Burton, G.J.; Redman, C.W.; Roberts, J.M.; Moffett, A. Pre-Eclampsia: Pathophysiology and Clinical Implications. BMJ 2019, 366, l2381. [Google Scholar] [CrossRef]
- Ullah, Z.; Bashir, F.; Fatima, K.; Ahmed, S.; Tahir, B.H.; Irshad, F. Changes in Serum Adiponectin and Serum Leptin Levels Can Predict Pre-Eclampsia in Pregnant Women: A Prospective Study. Pak. J. Med. Health Sci. 2022, 16, 610–613. [Google Scholar] [CrossRef]
- Tan, M.Y.; Syngelaki, A.; Poon, L.C.; Rolnik, D.L.; O’Gorman, N.; Delgado, J.L.; Akolekar, R.; Konstantinidou, L.; Tsavdaridou, M.; Galeva, S.; et al. Screening for Pre-Eclampsia by Maternal Factors and Biomarkers at 11–13 Weeks’ Gestation. Ultrasound Obstet. Gynecol. 2018, 52, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Monte, S. Biochemical Markers for Prediction of Preclampsia: Review of the Literature. J. Prenat Med. 2011, 5, 69–77. [Google Scholar] [PubMed]
- Zhou, S.; Li, J.; Yang, W.; Xue, P.; Yin, Y.; Wang, Y.; Tian, P.; Peng, H.; Jiang, H.; Xu, W.; et al. Noninvasive Preeclampsia Prediction Using Plasma Cell-Free RNA Signatures. Am. J. Obs. Gynecol. 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Ding, Y. Predictive Performance of Placental Protein 13 for Screening Preeclampsia in the First Trimester: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 370. [Google Scholar] [CrossRef] [PubMed]
- Young, B.C.; Levine, R.J.; Karumanchi, S.A. Pathogenesis of Preeclampsia. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 173–192. [Google Scholar] [CrossRef]
- Perry, T.E.; George, S.A.; Lee, B.; Wahr, J.; Randle, D.; Sigurðsson, G. A guide for pre-procedural imaging for transcatheter aortic valve replacement patients. Perioper. Med. 2020, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Phipps, E.; Prasanna, D.; Brima, W.; Jim, B. Preeclampsia: Updates in Pathogenesis, Definitions, and Guidelines. Clin. J. Am. Soc. Nephrol. 2016, 11, 1102–1113. [Google Scholar] [CrossRef] [PubMed]
- Konstantopoulos, A.; Sfakianoudis, K.; Simopoulou, M.; Kontogeorgi, A.; Rapani, A.; Grigoriadis, S.; Pantou, A.; Bathrellos, N.; Grammatis, A.; Pantos, K. Early Onset Preeclampsia Diagnosis Prior to the 20th Week of Gestation in a Twin Pregnancy Managed via Selective Reduction of an Intrauterine Growth Restriction Fetus: A Case Report and Literature Review. Diagnostics 2020, 10, 531. [Google Scholar] [CrossRef]
- Jensen, F.; Wallukat, G.; Herse, F.; Budner, O.; El-Mousleh, T.; Costa, S.-D.; Dechend, R.; Zenclussen, A.C. CD19+CD5+ Cells as Indicators of Preeclampsia. Hypertension 2012, 59, 861–868. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, S.; Zhao, Y.; Wang, H.; Pan, Q.; Shao, Q. Decidual Natural Killer Cells: A Good Nanny at the Maternal-Fetal Interface During Early Pregnancy. Front. Immunol. 2021, 12, 663660. [Google Scholar] [CrossRef]
- Wei, X.; Yang, X. The Central Role of Natural Killer Cells in Preeclampsia. Front. Immunol. 2023, 14, 1009867. [Google Scholar] [CrossRef]
- Sojka, D.K.; Yang, L.; Yokoyama, W.M. Uterine Natural Killer Cells. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Fukui, A.; Funamizu, A.; Yokota, M.; Yamada, K.; Nakamua, R.; Fukuhara, R.; Kimura, H.; Mizunuma, H. Uterine and Circulating Natural Killer Cells and Their Roles in Women with Recurrent Pregnancy Loss, Implantation Failure and Preeclampsia. J. Reprod. Immunol. 2011, 90, 105–110. [Google Scholar] [CrossRef]
- Fukui, A.; Yokota, M.; Funamizu, A.; Nakamua, R.; Fukuhara, R.; Yamada, K.; Kimura, H.; Fukuyama, A.; Kamoi, M.; Tanaka, K.; et al. Changes of NK Cells in Preeclampsia. Am. J. Reprod. Immunol. 2012, 67, 278–286. [Google Scholar] [CrossRef]
- Venkatesha, S.; Toporsian, M.; Lam, C.; Hanai, J.; Mammoto, T.; Kim, Y.M.; Bdolah, Y.; Lim, K.-H.; Yuan, H.-T.; Libermann, T.A.; et al. Soluble Endoglin Contributes to the Pathogenesis of Preeclampsia. Nat. Med. 2006, 12, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Gathiram, P.; Moodley, J. Pre-Eclampsia: Its Pathogenesis and Pathophysiolgy. Cardiovasc. J. Afr. 2016, 27, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Kalousová, M.; Muravská, A.; Zima, T. Pregnancy-Associated Plasma Protein A (PAPP-A) and Preeclampsia. Adv. Clin. Chem. 2014, 63, 169–209. [Google Scholar] [CrossRef]
- Fruscalzo, A.; Cividino, A.; Rossetti, E.; Maurigh, A.; Londero, A.P.; Driul, L. First Trimester PAPP-A Serum Levels and Long-Term Metabolic Outcome of Mothers and Their Offspring. Sci. Rep. 2020, 10, 5131. [Google Scholar] [CrossRef] [PubMed]
- Luewan, S.; Teja-intr, M.; Sirichotiyakul, S.; Tongsong, T. Low Maternal Serum Pregnancy-Associated Plasma Protein-A as a Risk Factor of Preeclampsia. Singap. Med. J. 2018, 59, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Creswell, L.; O’Gorman, N.; Palmer, K.R.; da Silva Costa, F.; Rolnik, D.L. Perspectives on the Use of Placental Growth Factor (PlGF) in the Prediction and Diagnosis of Pre-Eclampsia: Recent Insights and Future Steps. Int. J. Women’s Health 2023, 15, 255–271. [Google Scholar] [CrossRef]
- Chau, K.; Hennessy, A.; Makris, A. Placental Growth Factor and Pre-Eclampsia. J. Hum. Hypertens. 2017, 31, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Malamitsi-Puchner, A.; Boutsikou, T.; Economou, E.; Sarandakou, A.; Makrakis, E.; Hassiakos, D.; Creatsas, G. Vascular Endothelial Growth Factor and Placenta Growth Factor in Intrauterine Growth-Restricted Fetuses and Neonates. Mediat. Inflamm 2005, 2005, 293–297. [Google Scholar] [CrossRef]
- Rath, G.; Aggarwal, R.; Jawanjal, P.; Tripathi, R.; Batra, A. HIF-1 Alpha and Placental Growth Factor in Pregnancies Complicated With Preeclampsia: A Qualitative and Quantitative Analysis. J. Clin. Lab. Anal. 2016, 30, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Mutter, W.P.; Karumanchi, S.A. Molecular Mechanisms of Preeclampsia. Microvasc. Res. 2008, 75, 1–8. [Google Scholar] [CrossRef]
- Armaly, Z.; Jadaon, J.E.; Jabbour, A.; Abassi, Z.A. Preeclampsia: Novel Mechanisms and Potential Therapeutic Approaches. Front. Physiol. 2018, 9, 973. [Google Scholar] [CrossRef] [PubMed]
- Zimna, A.; Kurpisz, M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. BioMed Res. Int. 2015, 2015, e549412. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, A.; Dunk, C.; Jiang, J.; Shams, M.; Li, X.F.; Acevedo, C.; Weich, H.; Whittle, M.; Ahmed, A. Hypoxia Down-Regulates Placenta Growth Factor, Whereas Fetal Growth Restriction up-Regulates Placenta Growth Factor Expression: Molecular Evidence for “Placental Hyperoxia” in Intrauterine Growth Restriction. Lab. Invest. 1999, 79, 151–170. [Google Scholar]
- Ahmed, A.; Dunk, C.; Ahmad, S.; Khaliq, A. Regulation of Placental Vascular Endothelial Growth Factor (VEGF) and Placenta Growth Factor (PIGF) and Soluble Flt-1 by Oxygen—A Review. Placenta 2000, 21 (Suppl. A), S16–S24. [Google Scholar] [CrossRef]
- Lee, E.S.; Oh, M.-J.; Jung, J.W.; Lim, J.-E.; Seol, H.-J.; Lee, K.-J.; Kim, H.-J. The Levels of Circulating Vascular Endothelial Growth Factor and Soluble Flt-1 in Pregnancies Complicated by Preeclampsia. J. Korean Med. Sci. 2007, 22, 94–98. [Google Scholar] [CrossRef]
- Duhig, K.E.; Myers, J.; Seed, P.T.; Sparkes, J.; Lowe, J.; Hunter, R.M.; Shennan, A.H.; Chappell, L.C.; Bahl, R.; Bambridge, G.; et al. Placental Growth Factor Testing to Assess Women with Suspected Pre-Eclampsia: A Multicentre, Pragmatic, Stepped-Wedge Cluster-Randomised Controlled Trial. Lancet 2019, 393, 1807–1818. [Google Scholar] [CrossRef]
- Nikuei, P.; Rajaei, M.; Roozbeh, N.; Mohseni, F.; Poordarvishi, F.; Azad, M.; Haidari, S. Diagnostic Accuracy of SFlt1/PlGF Ratio as a Marker for Preeclampsia. BMC Pregnancy Childbirth 2020, 20, 80. [Google Scholar] [CrossRef]
- Roberts, J.M.; Rajakumar, A. Preeclampsia and Soluble Fms-Like Tyrosine Kinase 1. J. Clin. Endocrinol. Metab. 2009, 94, 2252–2254. [Google Scholar] [CrossRef]
- Maynard, S.E.; Min, J.-Y.; Merchan, J.; Lim, K.-H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess Placental Soluble Fms-like Tyrosine Kinase 1 (SFlt1) May Contribute to Endothelial Dysfunction, Hypertension, and Proteinuria in Preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef]
- Ahmed, A. New Insights into the Etiology of Preeclampsia: Identification of Key Elusive Factors for the Vascular Complications. Thromb. Res. 2011, 127 (Suppl. 3), S72–S75. [Google Scholar] [CrossRef]
- Duda, D.G.; Fukumura, D.; Jain, R.K. Role of ENOS in Neovascularization: NO for Endothelial Progenitor Cells. Trends Mol. Med. 2004, 10, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Ives, C.W.; Sinkey, R.; Rajapreyar, I.; Tita, A.T.N.; Oparil, S. Preeclampsia—Pathophysiology and Clinical Presentations. J. Am. Coll. Cardiol. 2020, 76, 1690–1702. [Google Scholar] [CrossRef]
- Huppertz, B.; Meiri, H.; Gizurarson, S.; Osol, G.; Sammar, M. Placental Protein 13 (PP13): A New Biological Target Shifting Individualized Risk Assessment to Personalized Drug Design Combating Pre-Eclampsia. Hum. Reprod. Update 2013, 19, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Gadde, R.; CD, D.; Sheela, S. Placental Protein 13. J. Circ. Biomark. 2018, 7, 184945441878615. [Google Scholar] [CrossRef] [PubMed]
- Vasilache, I.-A.; Carauleanu, A.; Socolov, D.; Matasariu, R.; Pavaleanu, I.; Nemescu, D. Predictive Performance of First Trimester Serum Galectin-13/PP-13 in Preeclampsia Screening: A Systematic Review and Meta-analysis. Exp. Ther. Med. 2022, 23, 370. [Google Scholar] [CrossRef] [PubMed]
- El Sherbiny, W.S.; Soliman, A.; Nasr, A.S. Placental Protein 13 as an Early Predictor in Egyptian Patients with Preeclampsia, Correlation to Risk, and Association with Outcome. J. Investig. Med. 2012, 60, 818–822. [Google Scholar] [CrossRef]
- Sugulle, M.; Dechend, R.; Herse, F.; Weedon-Fekjaer, M.S.; Johnsen, G.M.; Brosnihan, K.B.; Anton, L.; Luft, F.C.; Wollert, K.C.; Kempf, T.; et al. Circulating and Placental Growth-Differentiation Factor 15 in Preeclampsia and in Pregnancy Complicated by Diabetes Mellitus. Hypertension 2009, 54, 106–112. [Google Scholar] [CrossRef]
- Cruickshank, T.; MacDonald, T.M.; Walker, S.P.; Keenan, E.; Dane, K.; Middleton, A.; Kyritsis, V.; Myers, J.; Cluver, C.; Hastie, R.; et al. Circulating Growth Differentiation Factor 15 Is Increased Preceding Preeclampsia Diagnosis: Implications as a Disease Biomarker. J. Am. Heart Assoc. 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Wertaschnigg, D.; Rolnik, D.L.; Nie, G.; Teoh, S.S.Y.; Syngelaki, A.; da Silva Costa, F.; Nicolaides, K.H. Second- and Third-trimester Serum Levels of Growth-differentiation Factor-15 in Prediction of Pre-eclampsia. Ultrasound Obstet. Gynecol. 2020, 56, 879–884. [Google Scholar] [CrossRef]
- Laigaard, J.; Sørensen, T.; Placing, S.; Holck, P.; Fröhlich, C.; Wøjdemann, K.R.; Sundberg, K.; Shalmi, A.-C.; Tabor, A.; Nørgaard-Pedersen, B.; et al. Reduction of the Disintegrin and Metalloprotease ADAM12 in Preeclampsia. Obstet. Gynecol. 2005, 106, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.C.Y.; Chelemen, T.; Granvillano, O.; Pandeva, I.; Nicolaides, K.H. First-Trimester Maternal Serum a Disintegrin and Metalloprotease 12 (ADAM12) and Adverse Pregnancy Outcome. Obstet. Gynecol. 2008, 112, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Andres, F.; Wong, G.P.; Walker, S.P.; MacDonald, T.M.; Keenan, E.; Cannon, P.; Nguyen, T.-V.; Hannan, N.J.; Tong, S.; Kaitu’u-Lino, T.J. A Disintegrin and Metalloproteinase 12 (ADAM12) Is Reduced at 36 Weeks’ Gestation in Pregnancies Destined to Deliver Small for Gestational Age Infants. Placenta 2022, 117, 1–4. [Google Scholar] [CrossRef]
- Goetzinger, K.R.; Zhong, Y.; Cahill, A.G.; Odibo, L.; Macones, G.A.; Odibo, A.O. Efficiency of First-Trimester Uterine Artery Doppler, A-Disintegrin and Metalloprotease 12, Pregnancy-Associated Plasma Protein A, and Maternal Characteristics in the Prediction of Preeclampsia. J. Ultrasound Med. 2013, 32, 1593–1600. [Google Scholar] [CrossRef]
- Choudhury, K.M.; Das, M.; Ghosh, S.; Bhattacharya, D.; Ghosh, T.K. Value of Serum β-hCG in Pathogenesis of Pre-Eclampsia. J. Clin. Gynecol. Obstet. 2012, 1, 71–75. [Google Scholar] [CrossRef]
- Åsvold, B.O.; Vatten, L.J.; Tanbo, T.G.; Eskild, A. Concentrations of Human Chorionic Gonadotrophin in Very Early Pregnancy and Subsequent Pre-Eclampsia: A Cohort Study. Hum. Reprod. 2014, 29, 1153–1160. [Google Scholar] [CrossRef]
- Sirikunalai, P.; Wanapirak, C.; Sirichotiyakul, S.; Tongprasert, F.; Srisupundit, K.; Luewan, S.; Traisrisilp, K.; Tongsong, T. Associations between Maternal Serum Free Beta Human Chorionic Gonadotropin (β-HCG) Levels and Adverse Pregnancy Outcomes. J. Obstet. Gynaecol. 2016, 36, 178–182. [Google Scholar] [CrossRef]
- Goetzinger, K.R.; Singla, A.; Gerkowicz, S.; Dicke, J.M.; Gray, D.L.; Odibo, A.O. Predicting the Risk of Pre-Eclampsia between 11 and 13 Weeks’ Gestation by Combining Maternal Characteristics and Serum Analytes, PAPP-A and Free β-HCG. Prenat. Diagn. 2010, 30, 1138–1142. [Google Scholar] [CrossRef]
- Farzaneh, F.; Sharifi, M.; Nourinasab, N.; Younesi, S. Value of α-Fetoprotein, β-HCG, Inhibin A, and UE3 at Second Trimester for Early Screening of Preeclampsia. Asian Pac. J. Reprod. 2019, 8, 30. [Google Scholar] [CrossRef]
- Yue, C.-Y.; Zhang, C.-Y.; Ni, Y.-H.; Ying, C.-M. Are Serum Levels of Inhibin A in Second Trimester Predictors of Adverse Pregnancy Outcome? PLoS ONE 2020, 15, e0232634. [Google Scholar] [CrossRef] [PubMed]
- Muttukrishna, S.; North, R.A.; Morris, J.; Schellenberg, J.-C.; Taylor, R.S.; Asselin, J.; Ledger, W.; Groome, N.; Redman, C.W.G. Serum Inhibin A and Activin A Are Elevated Prior to the Onset of Pre-Eclampsia. Hum. Reprod. 2000, 15, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Neuman, R.I.; Alblas van der Meer, M.M.; Nieboer, D.; Saleh, L.; Verdonk, K.; Kalra, B.; Kumar, A.; Alpadi, K.; van den Meiracker, A.H.; Visser, W.; et al. PAPP-A2 and Inhibin A as Novel Predictors for Pregnancy Complications in Women With Suspected or Confirmed Preeclampsia. J. Am. Heart Assoc. 2020, 9, e018219. [Google Scholar] [CrossRef]
- Ree, P.H.; Hahn, W.B.; Chang, S.W.; Jung, S.H.; Kang, J.H.; Cha, D.H.; Kang, M.S.; Huh, J.Y. Early Detection of Preeclampsia Using Inhibin A and Other Second-Trimester Serum Markers. Fetal Diagn. Ther. 2011, 29, 280–286. [Google Scholar] [CrossRef]
- Paiwattananupant, K.; Phupong, V. Serum Inhibin A Level in Preeclampsia and Normotensive Pregnancy. Hypertens. Pregnancy 2008, 27, 337–343. [Google Scholar] [CrossRef]
- Margioula-Siarkou, G.; Margioula-Siarkou, C.; Petousis, S.; Margaritis, K.; Alexandratou, M.; Dinas, K.; Sotiriadis, A.; Mavromatidis, G. Soluble Endoglin Concentration in Maternal Blood as a Diagnostic Biomarker of Preeclampsia: A Systematic Review and Meta-Analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 258, 366–381. [Google Scholar] [CrossRef]
- Perucci, L.O.; Gomes, K.B.; Freitas, L.G.; Godoi, L.C.; Alpoim, P.N.; Pinheiro, M.B.; Miranda, A.S.; Teixeira, A.L.; Dusse, L.M.; Sousa, L.P. Soluble Endoglin, Transforming Growth Factor-Beta 1 and Soluble Tumor Necrosis Factor Alpha Receptors in Different Clinical Manifestations of Preeclampsia. PLoS ONE 2014, 9, e97632. [Google Scholar] [CrossRef]
- Muhammed, L.T.; Ali, E.A.; Hameed, H. Role Of Soluble Endoglin In The Diagnosis Of Preeclampsia Severity In Iraqi Women. Syst. Rev. Pharm. 2021, 12, 301–305. [Google Scholar]
- MacDonald, T.M.; Walker, S.P.; Hannan, N.J.; Tong, S.; Kaitu’u-Lino, T.J. Clinical Tools and Biomarkers to Predict Preeclampsia. eBioMedicine 2022, 75, 103780. [Google Scholar] [CrossRef]
- Huang, T.; Rashid, S.; Priston, M.; Rasasakaram, E.; Mak-Tam, E.; Gibbons, C.; Mei-Dan, E.; Bedford, H.M. Prenatal Screening for Preeclampsia: The Roles of Placental Growth Factor and Pregnancy–Associated Plasma Protein A in the First Trimester and Placental Growth Factor and Soluble Fms-like Tyrosine Kinase 1–Placental Growth Factor Ratio in the Early Second. AJOG Glob. Rep. 2023, 3, 100193. [Google Scholar] [CrossRef] [PubMed]
- Sonek, J.; Krantz, D.; Carmichael, J.; Downing, C.; Jessup, K.; Haidar, Z.; Ho, S.; Hallahan, T.; Kliman, H.J.; McKenna, D. First-Trimester Screening for Early and Late Preeclampsia Using Maternal Characteristics, Biomarkers, and Estimated Placental Volume. Am. J. Obstet. Gynecol. 2018, 218, 126.e1–126.e13. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Gao, J.; Liu, J.; Meng, H.; Hao, N.; Song, Y.; Ma, L.; Luo, W.; Sun, J.; Gao, W.; et al. Prospective Evaluation of First-trimester Screening Strategy for Preterm Pre-eclampsia and Its Clinical Applicability in China. Ultrasound Obstet. Gynecol. 2021, 58, 529–539. [Google Scholar] [CrossRef]
- Widmer, M.; Cuesta, C.; Khan, K.S.; Conde-Agudelo, A.; Carroli, G.; Fusey, S.; Karumanchi, S.A.; Lapaire, O.; Lumbiganon, P.; Sequeira, E.; et al. Accuracy of Angiogenic Biomarkers at ≤20weeks’ Gestation in Predicting the Risk of Pre-Eclampsia: A WHO Multicentre Study. Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2015, 5, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Kusanovic, J.P.; Than, N.G.; Erez, O.; Gotsch, F.; Espinoza, J.; Edwin, S.; Chefetz, I.; Gomez, R.; Nien, J.K.; et al. First-Trimester Maternal Serum PP13 in the Risk Assessment for Preeclampsia. Am. J. Obstet. Gynecol. 2008, 199, 122.e1–122.e11. [Google Scholar] [CrossRef]
- Hanchard, T.J.; Vries, B.S.; Quinton, A.E.; Sinosich, M.; Hyett, J.A. Ultrasound Features Prior to 11 Weeks’ Gestation and First-trimester Maternal Factors in Prediction of Hypertensive Disorders of Pregnancy. Ultrasound Obstet. Gynecol. 2020, 55, 629–636. [Google Scholar] [CrossRef]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.-H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating Angiogenic Factors and the Risk of Preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef]
- Holston, A.M.; Qian, C.; Yu, K.F.; Epstein, F.H.; Karumanchi, S.A.; Levine, R.J. Circulating Angiogenic Factors in Gestational Proteinuria without Hypertension. Am. J. Obstet. Gynecol. 2009, 200, 392.e1–392.e10. [Google Scholar] [CrossRef] [PubMed]
- Young, B.; Levine, R.; Salahuddin, S.; Qian, C.; Lim, K.-H.; Karumanchi, S.A.; Rana, S. The use of angiogenic biomarkers to differentiate non-HELLP related thrombocytopenia from HELLP syndrome. J. Matern. Neonatal Med. 2009, 23, 366–370. [Google Scholar] [CrossRef]
- Schmidt, M.; Dogan, C.; Birdir, C.; Kuhn, U.; Gellhaus, A.; Kimmig, R.; Kasimir-Bauer, S. Placental Growth Factor: A Predictive Marker for Preeclampsia? Gynakol. Geburtshilfliche Rundsch. 2009, 49, 94–99. [Google Scholar] [CrossRef]
- Ouyang, Y.-Q.; Li, S.-J.; Zhang, Q.; Xiang, W.-P.; Shen, H.-L.; Chen, H.-P.; Chen, H.; Chen, H.-Z. Plasma SFlt-1-to-PlGF Ratio Is Correlated with Inflammatory but Not with Oxidative Stress in Chinese Preeclamptic Women. Arch. Gynecol. Obstet. 2009, 280, 91–97. [Google Scholar] [CrossRef]
- Ding, G.; Liping, L.; Moli, D.; Wuliyeti, A.; Shaohe, Z.; Huijuan, W.; Chen, P.; Chen, C.; Guiqin, B. A Study of the Association between the SFlt-1/PIGF Ratio and Preeclampsia in Xinjiang Uygur Autonomous Region of China. Artif. Cells Nanomed. Biotechnol. 2018, 46, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, H.; Liu, X.; Zhao, S.; Zheng, Y.; Jia, Z.; Chen, L.; Zhang, C.; Xie, X.; Zhong, J.; et al. Predictive Values of Various Serum Biomarkers in Women with Suspected Preeclampsia: A Prospective Study. J. Clin. Lab. Anal. 2021, 35. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.K.; Chandel, N.; Jain, V.; Jha, V. The Relationship between Circulating Endothelin-1, Soluble Fms-like Tyrosine Kinase-1 and Soluble Endoglin in Preeclampsia. J. Hum. Hypertens. 2012, 26, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Das, V.; Agarwal, A.; Agrawal, S. Correlation of SFlt/PlGF Ratio with Severity of Preeclampsia in an Indian Population. AJOG Glob. Rep. 2023, 3, 100177. [Google Scholar] [CrossRef] [PubMed]
- Chaiworapongsa, T.; Romero, R.; Kim, Y.M.; Kim, G.J.; Kim, M.R.; Espinoza, J.; Bujold, E.; Gonçalves, L.; Gomez, R.; Edwin, S.; et al. Plasma Soluble Vascular Endothelial Growth Factor Receptor-1 Concentration Is Elevated Prior to the Clinical Diagnosis of Pre-Eclampsia. J. Matern.-Fetal Neonatal Med. 2005, 17, 3–18. [Google Scholar] [CrossRef]
- Fang, M.; He, Y.; Li, H.; Wu, M.; Shi, X.; Du, H. Alterations of Serum and Placental Endoglin in Pre-Eclampsia. J. Int. Med. Res. 2010, 38, 43–51. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Zhou, C.; You, Z.; Zhang, J.; Cao, G. The Diagnosis Values of Serum STAT4 and SEng in Preeclampsia. J. Clin. Lab. Anal. 2020, 34, e23073. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rybak-Krzyszkowska, M.; Staniczek, J.; Kondracka, A.; Bogusławska, J.; Kwiatkowski, S.; Góra, T.; Strus, M.; Górczewski, W. From Biomarkers to the Molecular Mechanism of Preeclampsia—A Comprehensive Literature Review. Int. J. Mol. Sci. 2023, 24, 13252. https://doi.org/10.3390/ijms241713252
Rybak-Krzyszkowska M, Staniczek J, Kondracka A, Bogusławska J, Kwiatkowski S, Góra T, Strus M, Górczewski W. From Biomarkers to the Molecular Mechanism of Preeclampsia—A Comprehensive Literature Review. International Journal of Molecular Sciences. 2023; 24(17):13252. https://doi.org/10.3390/ijms241713252
Chicago/Turabian StyleRybak-Krzyszkowska, Magda, Jakub Staniczek, Adrianna Kondracka, Joanna Bogusławska, Sebastian Kwiatkowski, Tomasz Góra, Michał Strus, and Wojciech Górczewski. 2023. "From Biomarkers to the Molecular Mechanism of Preeclampsia—A Comprehensive Literature Review" International Journal of Molecular Sciences 24, no. 17: 13252. https://doi.org/10.3390/ijms241713252






