Sodium Nitrite Attenuates Reduced Activity of Vascular Matrix Metalloproteinase-2 and Vascular Hyper-Reactivity and Increased Systolic Blood Pressure Induced by the Placental Ischemia Model of Preeclampsia in Anesthetized Rats
Abstract
:1. Introduction
2. Results
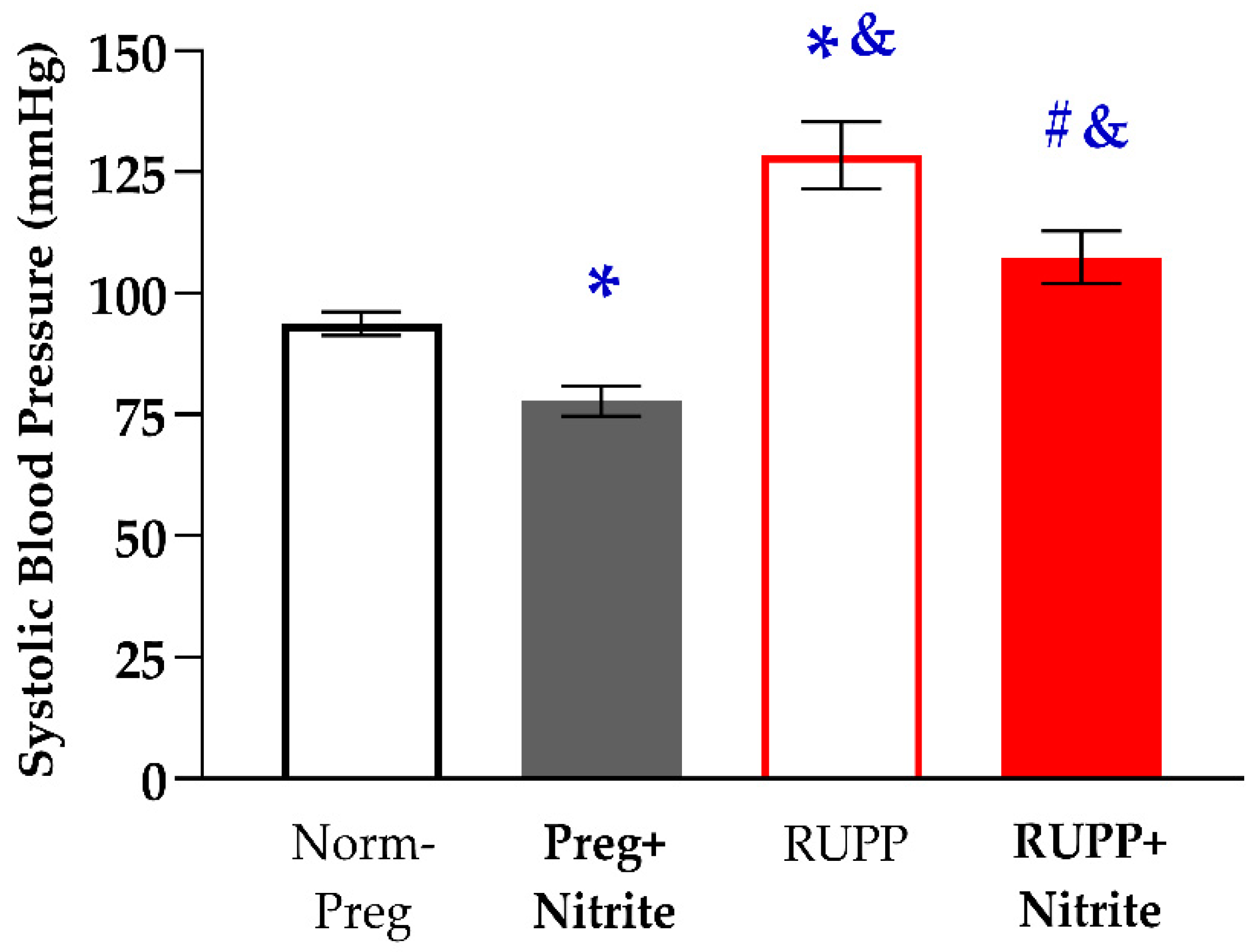
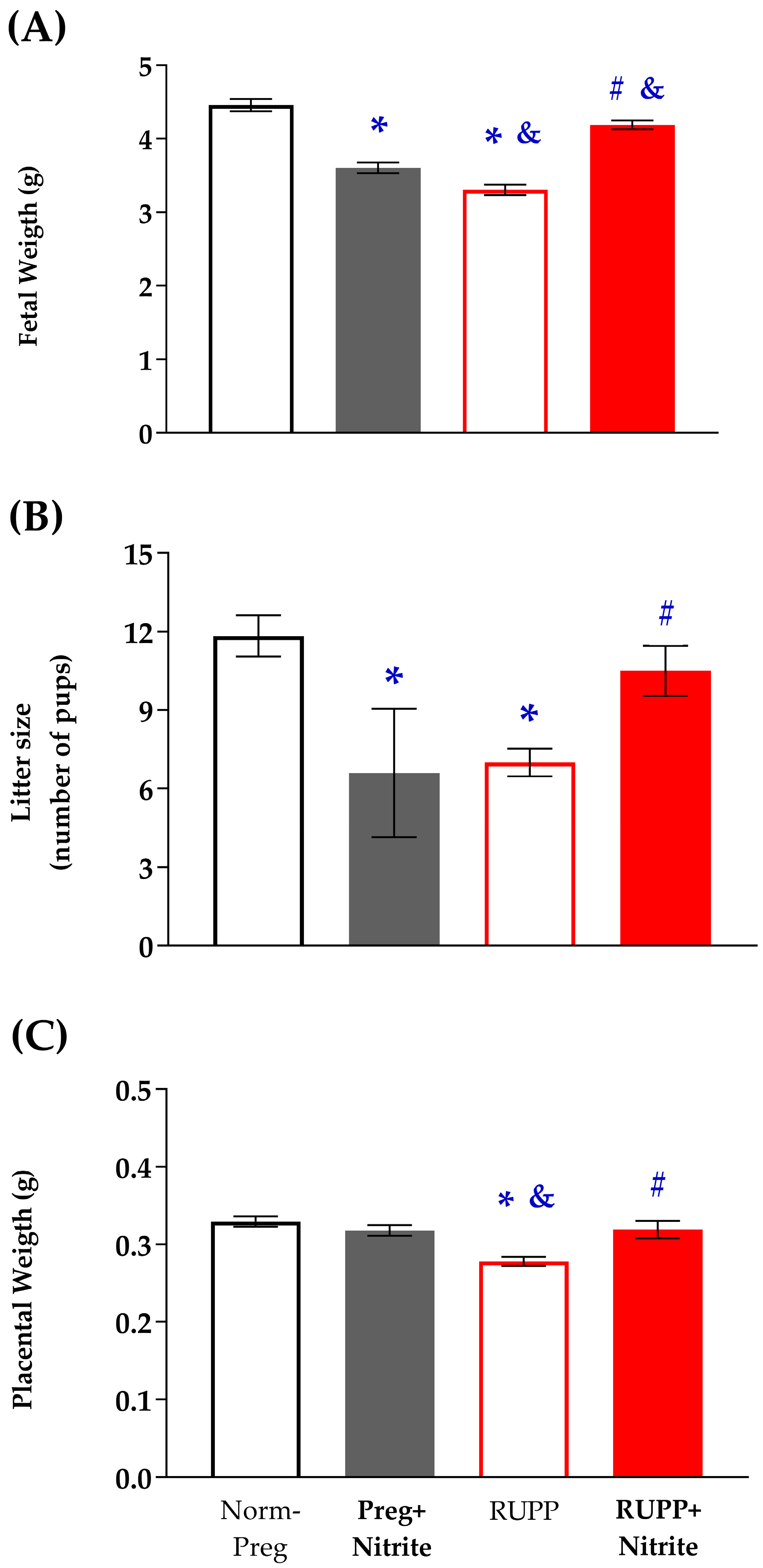
2.1. Sodium Nitrite Effects on Systolic Blood Pressure and Fetal and Placental Parameters
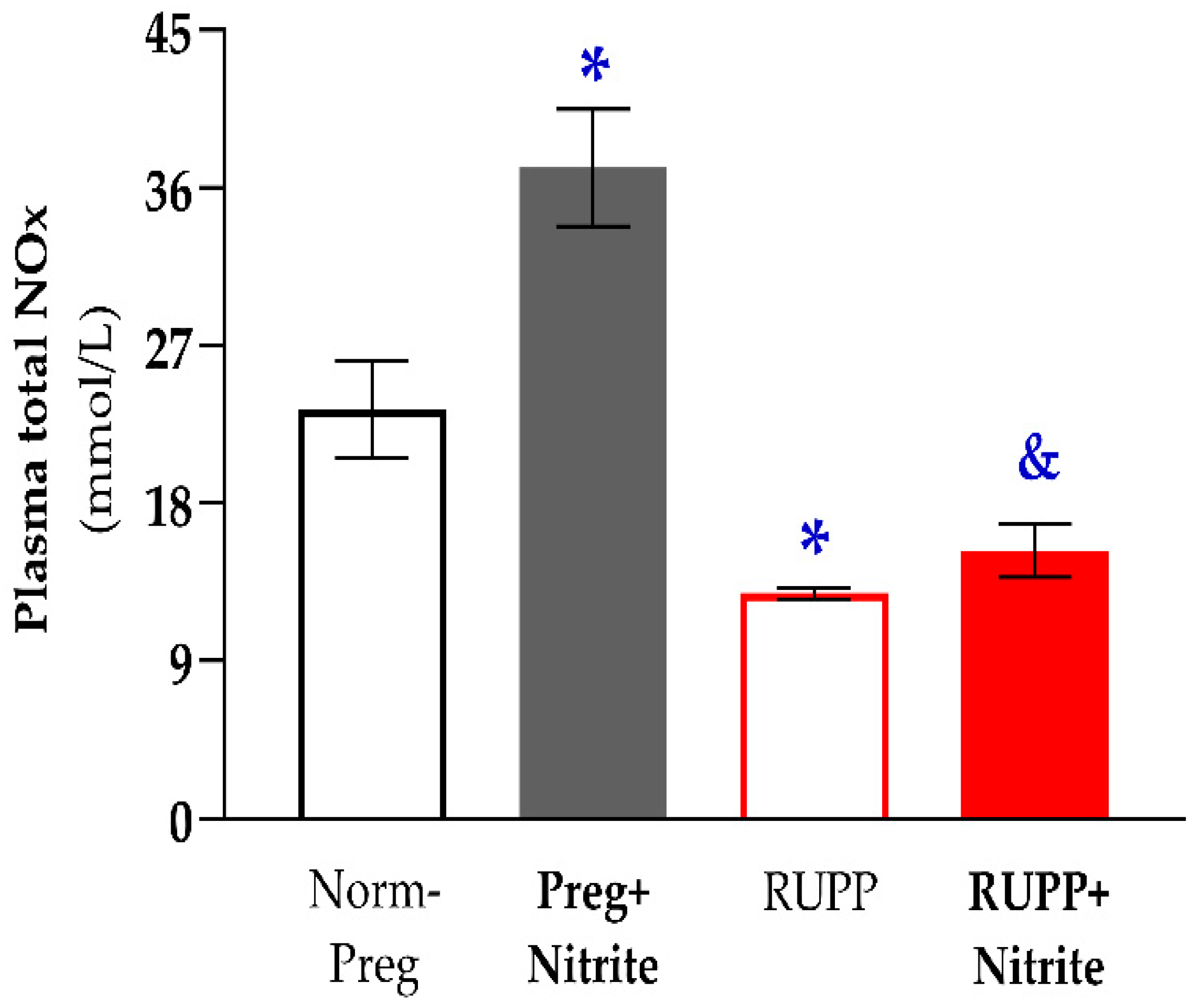
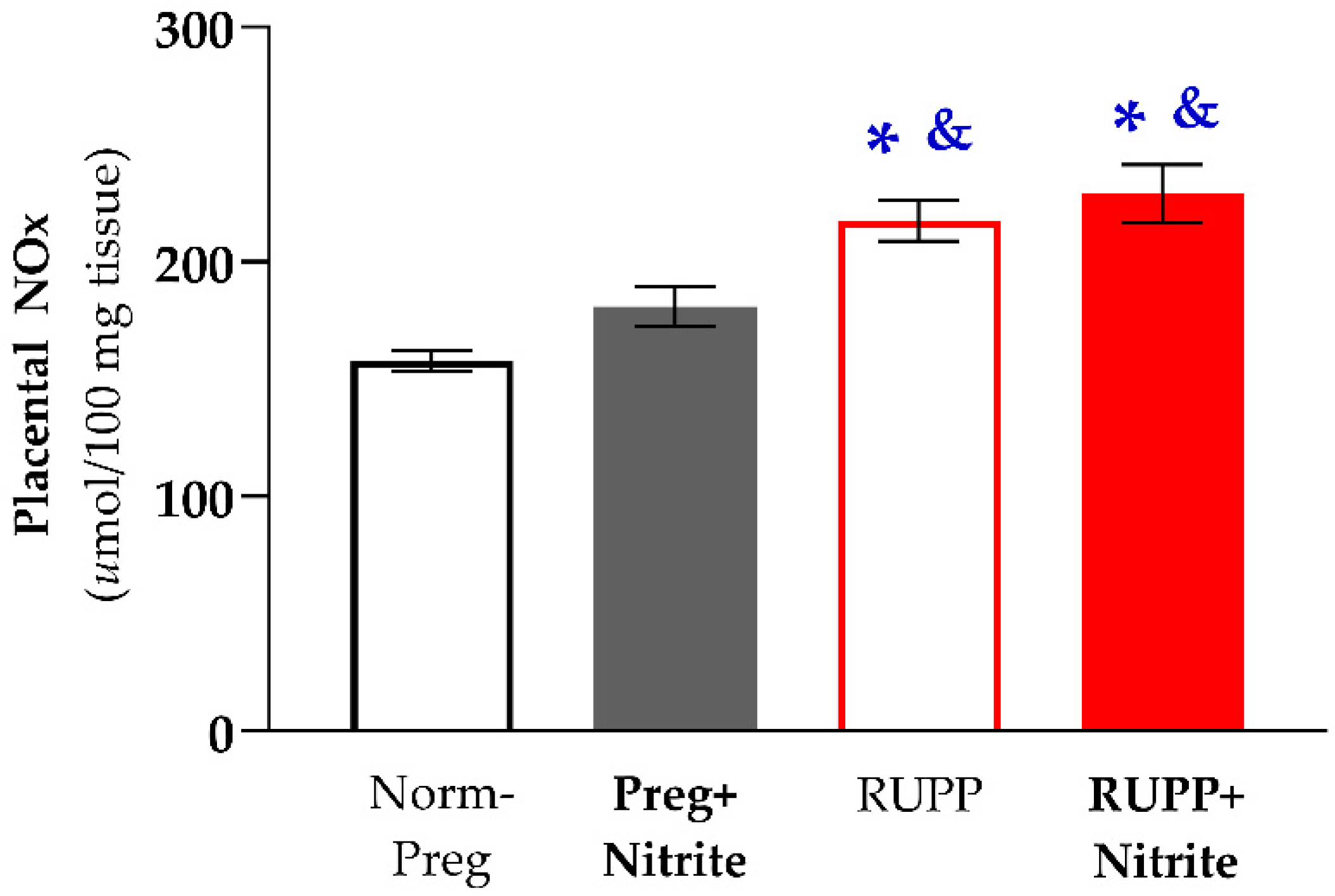
2.2. Sodium Nitrite Treatment and Circulating and Placental Levels of NO Metabolites
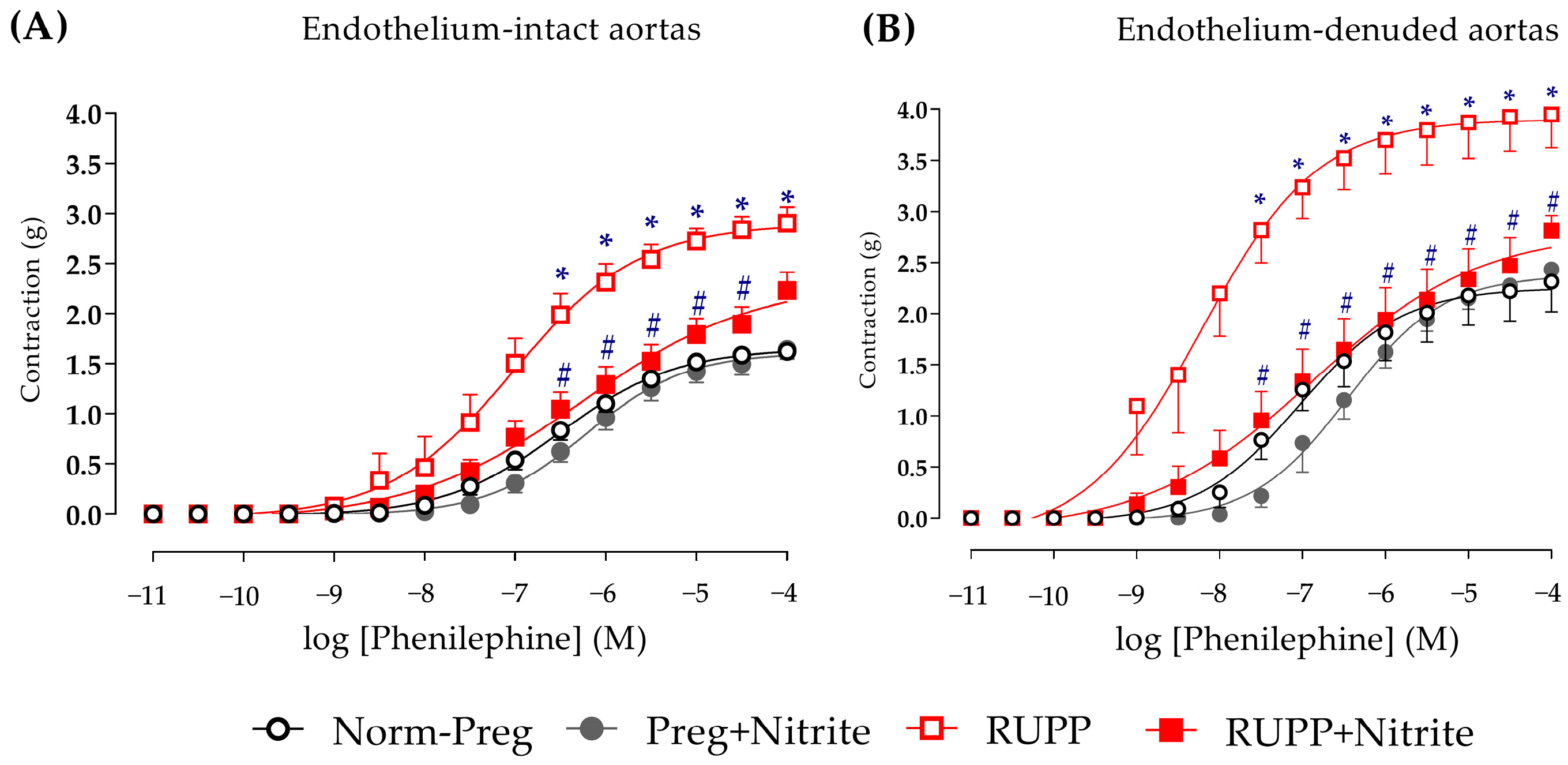
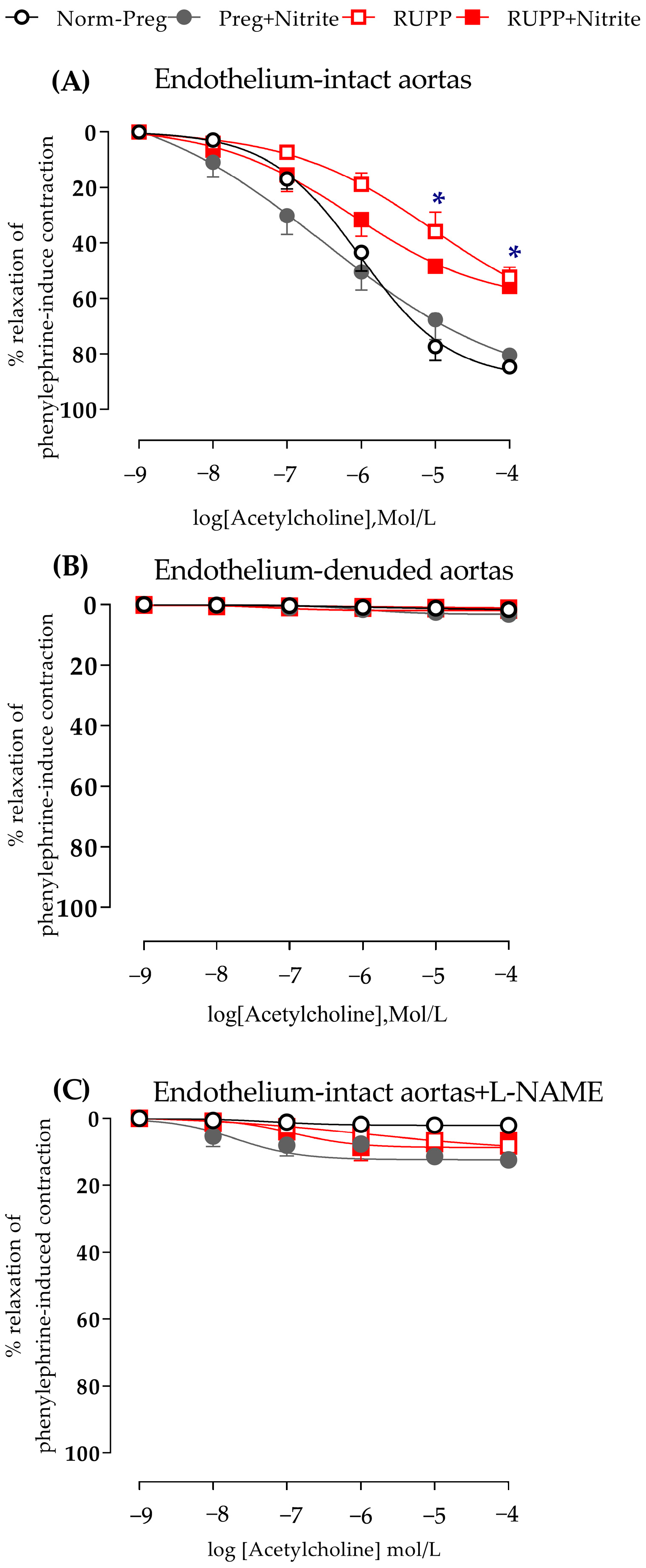
2.3. Sodium Nitrite Effects on Vascular Hyperreactivity and Endothelial Dysfunction
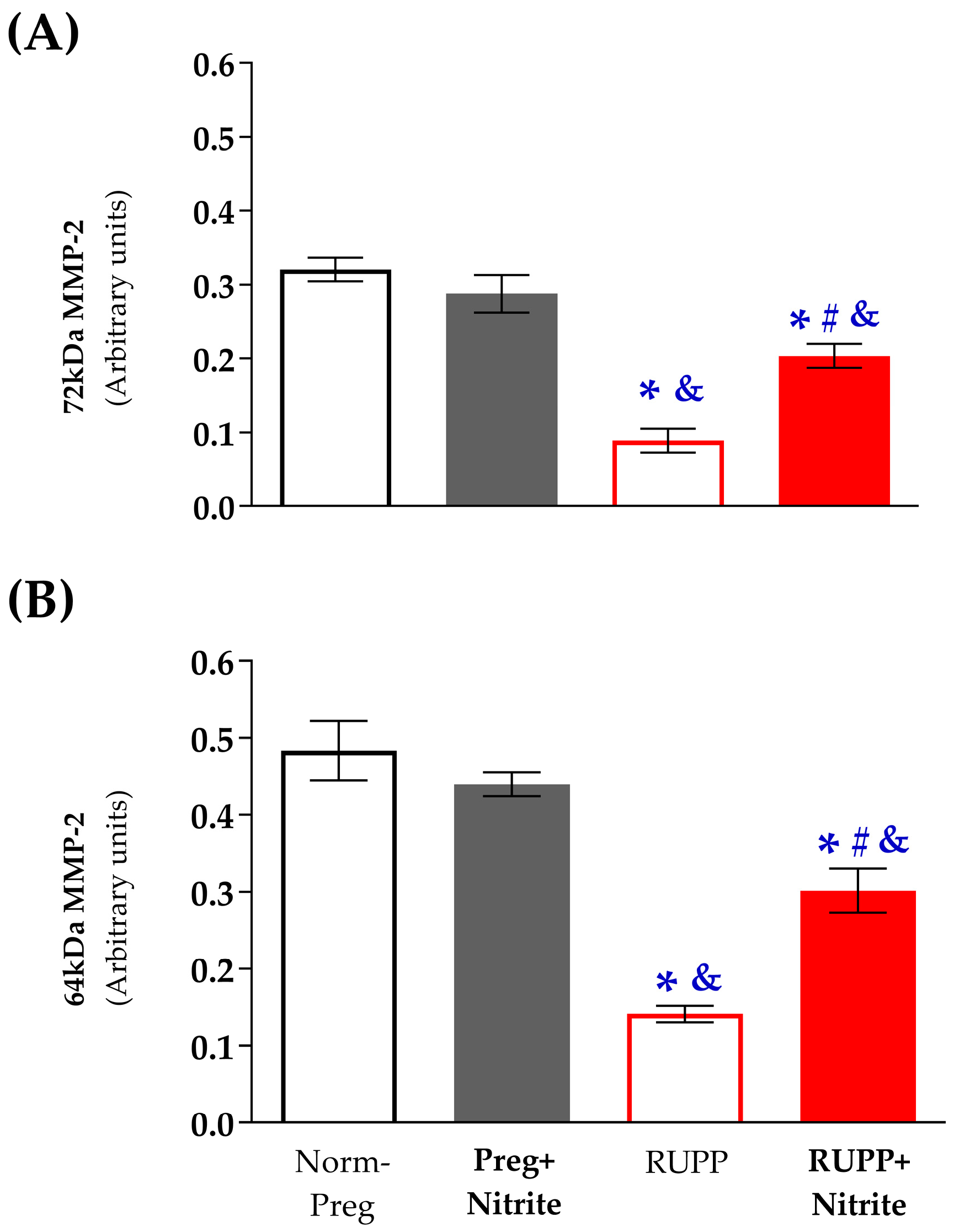
2.4. Sodium Nitrite Effects on the Gelatinolytic Activity of Vascular MMP-2
3. Discussion
4. Materials and Methods
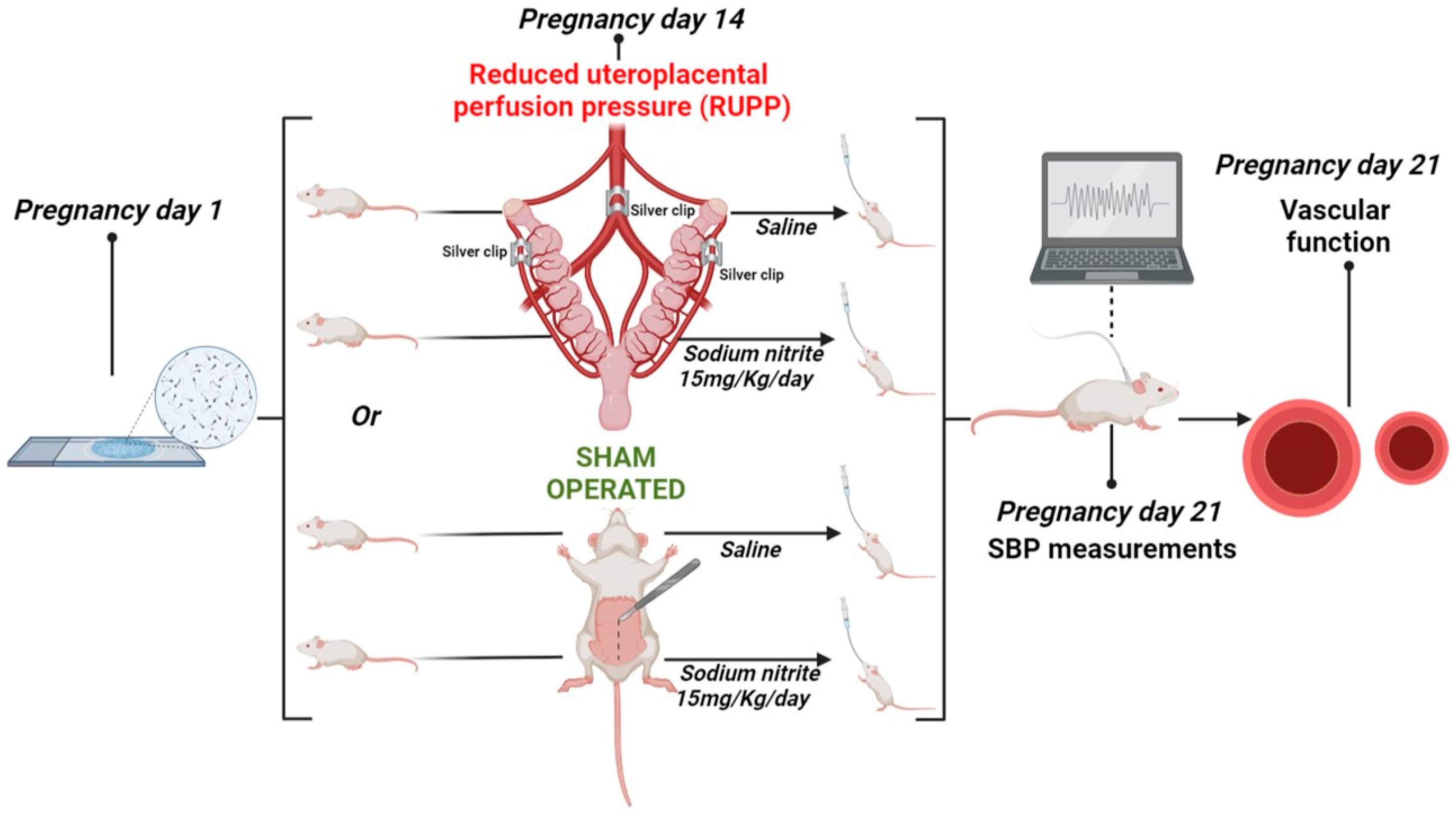
4.1. Animals and Experimental Protocol
4.2. Maternal Blood Pressure Measurements and Blood, Arteries, and Placenta Harvests
4.3. Determination of NO Metabolites, Nitrite/Nitrate (NOx), in Plasma and Placenta
4.4. Vascular Reactivity Experiments
4.5. Gelatin Zymography for Vascular MMP-2 Activity
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. Oxidative Stress in Preeclampsia and Placental Diseases. Int. J. Mol. Sci. 2018, 19, 1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakrania, B.A.; Spradley, F.T.; Drummond, H.A.; LaMarca, B.; Ryan, M.J.; Granger, J.P. Preeclampsia: Linking Placental Is-chemia with Maternal Endothelial and Vascular Dysfunction. In Comprehensive Physiology; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2020; pp. 1315–1349. [Google Scholar] [CrossRef]
- Garovic, V.D.; August, P. Preeclampsia and the Future Risk of Hypertension: The Pregnant Evidence. Curr. Hypertens. Rep. 2013, 15, 114–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodžić, J.; Izetbegović, S.; Muračević, B.; Iriškić, R.; Štimjanin Jović, H. Nitric Oxide Biosynthesis during Normal Pregnancy and Pregnancy Complicated by Preeclampsia. Med. Glas. 2017, 14, 211–217. [Google Scholar] [CrossRef]
- Zhu, M.; Ren, Z.; Possomato-Vieira, J.S.; Khalil, R.A. Restoring Placental Growth Factor-Soluble Fms-like Tyrosine Kinase-1 Balance Reverses Vascular Hyper-Reactivity and Hypertension in Pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R505–R521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves-Rizzi, V.H.; Possomato-Vieira, J.S.; Sales Graça, T.U.; Nascimento, R.A.; Dias-Junior, C.A. Sodium Nitrite Attenuates Hypertension-in-Pregnancy and Blunts Increases in Soluble Fms-like Tyrosine Kinase-1 and in Vascular Endothelial Growth Factor. Nitric Oxide 2016, 57, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Ormesher, L.; Myers, J.E.; Chmiel, C.; Wareing, M.; Greenwood, S.L.; Tropea, T.; Lundberg, J.O.; Weitzberg, E.; Nihlen, C.; Sibley, C.P.; et al. Effects of Dietary Nitrate Supplementation, from Beetroot Juice, on Blood Pressure in Hypertensive Pregnant Women: A Randomised, Double-Blind, Placebo-Controlled Feasibility Trial. Nitric Oxide 2018, 80, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Cindrova-Davies, T.; Sferruzzi-Perri, A.N. Human Placental Development and Function. Semin. Cell Dev. Biol. 2022, 131, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Possomato-Vieira, J.S.; Khalil, R.A. Mechanisms of Endothelial Dysfunction in Hypertensive Pregnancy and Preeclampsia. Adv. Pharmacol. 2016, 77, 361–431. [Google Scholar] [CrossRef] [Green Version]
- Calvert, J.W.; Lefer, D.J. Clinical Translation of Nitrite Therapy for Cardiovascular Diseases. Nitric Oxide 2010, 22, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric Oxide: Physiology, Pathophysiology, and Pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar]
- Lundberg, J.O.; Gladwin, M.T.; Weitzberg, E. Strategies to Increase Nitric Oxide Signalling in Cardiovascular Disease. Nat. Rev. Drug Discov. 2015, 14, 623–641. [Google Scholar] [CrossRef] [PubMed]
- Carlstrom, M.; Montenegro, M.F. Therapeutic Value of Stimulating the Nitrate-Nitrite-Nitric Oxide Pathway to Attenuate Oxidative Stress and Restore Nitric Oxide Bioavailability in Cardiorenal Disease. J. Intern. Med. 2019, 285, 2–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dou, C.; Han, X.; Xie, H.; Liao, H.; Xiao, X.; Huang, Z.; Luo, G.; Zhang, X.; Yao, W. Protective Role of Nitric Oxide Donors on Endothelium in Ischemia-Reperfusion Injury: A Meta-Analysis of Randomized Controlled Trials. BMC Anesthesiol. 2023, 23, 189. [Google Scholar] [CrossRef] [PubMed]
- Tropea, T.; Wareing, M.; Greenwood, S.L.; Feelisch, M.; Sibley, C.P.; Cottrell, E.C. Nitrite Mediated Vasorelaxation in Human Chorionic Plate Vessels Is Enhanced by Hypoxia and Dependent on the NO-SGC-CGMP Pathway. Nitric Oxide 2018, 80, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, M.F.; Sundqvist, M.L.; Larsen, F.J.; Zhuge, Z.; Carlström, M.; Weitzberg, E.; Lundberg, J.O. Blood Pressure–Lowering Effect of Orally Ingested Nitrite Is Abolished by a Proton Pump Inhibitor. Hypertension 2017, 69, 23–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim-Shapiro, D.B.; Gladwin, M.T. Mechanisms of Nitrite Bioactivation. Nitric Oxide 2014, 38, 58–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, A.; Bond, R.; McLean, P.; Uppal, R.; Benjamin, N.; Ahluwalia, A. Reduction of Nitrite to Nitric Oxide during Ischemia Protects against Myocardial Ischemia–Reperfusion Damage. Proc. Natl. Acad. Sci. USA 2004, 101, 13683–13688. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.C.; Murugan, D.D.; Lau, Y.S.; Vanhoutte, P.M.; Mustafa, M.R. Sodium Nitrite Exerts an Antihypertensive Effect and Improves Endothelial Function through Activation of ENOS in the SHR. Sci. Rep. 2016, 6, 33048. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, L.C.; Oliveira-Paula, G.H.; Ferreira, G.C.; Dal-Cin de Paula, T.; Duarte, D.A.; Costa-Neto, C.M.; Tanus-Santos, J.E. Oral Nitrite Treatment Increases S-Nitrosylation of Vascular Protein Kinase C and Attenuates the Responses to Angiotensin II. Redox Biol. 2021, 38, 101769. [Google Scholar] [CrossRef]
- Rossman, M.J.; Gioscia-Ryan, R.A.; Santos-Parker, J.R.; Ziemba, B.P.; Lubieniecki, K.L.; Johnson, L.C.; Poliektov, N.E.; Bispham, N.Z.; Woodward, K.A.; Nagy, E.E.; et al. Inorganic Nitrite Supplementation Improves Endothelial Function With Aging: Translational Evidence for Suppression of Mitochondria-Derived Oxidative Stress. Hypertension 2021, 77, 1212–1222. [Google Scholar] [CrossRef]
- Chimini, J.S.; Possomato-Vieira, J.S.; da Silva, M.L.S.; Dias-Junior, C.A. Placental Nitric Oxide Formation and Endothelium-dependent Vasodilation Underlie Pravastatin Effects against Angiogenic Imbalance, Hypertension in Pregnancy and Intra-uterine Growth Restriction. Basic Clin. Pharmacol. Toxicol. 2019, 124, 385–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias-Junior, C.A.; Chen, J.; Cui, N.; Chiang, C.L.; Zhu, M.; Ren, Z.; Possomato-Vieira, J.S.; Khalil, R.A. Angiogenic Imbalance and Diminished Matrix Metalloproteinase-2 and -9 Underlie Regional Decreases in Uteroplacental Vascularization and Fe-to-Placental Growth in Hypertensive Pregnancy. Biochem. Pharmacol. 2017, 146, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mata, K.M.; Mazzuca, M.Q.; Khalil, R.A. Altered Matrix Metalloproteinase-2 and -9 Expression/Activity Links Placental Ischemia and Anti-Angiogenic SFlt-1 to Uteroplacental and Vascular Remodeling and Collagen Deposition in Hypertensive Pregnancy. Biochem. Pharmacol. 2014, 89, 370–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.; He, H.; Cui, N.; Ren, Z.; Zhu, M.; Khalil, R.A. Decreased Uterine Vascularization and Uterine Arterial Expansive Remodeling with Reduced Matrix Metalloproteinase-2 and -9 in Hypertensive Pregnancy. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H165–H180. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Cui, N.; Zhu, M.; Khalil, R.A. Placental Growth Factor Reverses Decreased Vascular and Uteroplacental MMP-2 and MMP-9 and Increased MMP-1 and MMP-7 and Collagen Types I and IV in Hypertensive Pregnancy. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H33–H47. [Google Scholar] [CrossRef]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Khalil, R.A. Matrix Metalloproteinases in Normal Pregnancy and Preeclampsia. Prog. Mol. Biol. Transl. Sci. 2017, 148, 87–165. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Cui, N.; Zhu, M.; Khalil, R.A. TNFα Blockade Reverses Vascular and Uteroplacental Matrix Metalloproteinases Imbalance and Collagen Accumulation in Hypertensive Pregnant Rats. Biochem. Pharmacol. 2021, 193, 114790. [Google Scholar] [CrossRef]
- Palei, A.C.; Granger, J.P.; Spradley, F.T. Placental Ischemia Says “NO” to Proper NOS-Mediated Control of Vascular Tone and Blood Pressure in Preeclampsia. Int. J. Mol. Sci. 2021, 22, 11261. [Google Scholar] [CrossRef]
- Brennan, L.; Morton, J.S.; Quon, A.; Davidge, S.T. Postpartum Vascular Dysfunction in the Reduced Uteroplacental Perfusion Model of Preeclampsia. PLoS ONE 2016, 11, e0162487. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Chen, J.; Khalil, R.A. Zymography as a Research Tool in the Study of Matrix Metalloproteinase Inhibitors. Methods Mol. Biol. 2017, 1626, 79–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phipps, E.A.; Thadhani, R.; Benzing, T.; Karumanchi, S.A. Pre-Eclampsia: Pathogenesis, Novel Diagnostics and Therapies. Nat. Rev. Nephrol. 2019, 15, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Mannaerts, D.; Faes, E.; Cornette, J.; Gyselaers, W.; Spaanderman, M.; Goovaerts, I.; Stoop, T.; Roelant, E.; Jacquemyn, Y.; Van Craenenbroeck, E.M. Low-Flow Mediated Constriction as a Marker of Endothelial Function in Healthy Pregnancy and Preeclampsia: A Pilot Study. Pregnancy Hypertens. 2019, 17, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Mannaerts, D.; Faes, E.; Goovaerts, I.; Stoop, T.; Cornette, J.; Gyselaers, W.; Spaanderman, M.; Van Craenenbroeck, E.M.; Jacquemyn, Y. Flow-Mediated Dilation and Peripheral Arterial Tonometry Are Disturbed in Preeclampsia and Reflect Dif-ferent Aspects of Endothelial Function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R518–R525. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Paula, G.H.; Batista, R.I.M.; Stransky, S.; Tella, S.C.; Ferreira, G.C.; Portella, R.L.; Pinheiro, L.C.; Damacena-Angelis, C.; Riascos-Bernal, D.F.; Sidoli, S.; et al. Orally Administered Sodium Nitrite Prevents the Increased α-1 Adrenergic Vasoconstriction Induced by Hypertension and Promotes the S-Nitrosylation of Calcium/Calmodulin-Dependent Protein Kinase II. Biochem. Pharmacol. 2023, 212, 115571. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.C.; Mustafa, M.R.; Vanhoutte, P.M.; Murugan, D.D. Chronic Administration of Sodium Nitrite Prevents Hypertension and Protects Arterial Endothelial Function by Reducing Oxidative Stress in Angiotensin II-Infused Mice. Vascul. Pharmacol. 2018, 102, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-Rizzi, V.H.; Nascimento, R.A.; Possomato-Vieira, J.S.; Dias-Junior, C.A. Sodium Nitrite Prevents Both Reductions in Circulating Nitric Oxide and Hypertension in 7-Day Lead-Treated Rats. Basic Clin. Pharmacol. Toxicol. 2016, 118, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Hughan, K.S.; Levine, A.; Helbling, N.; Anthony, S.; DeLany, J.P.; Stefanovic-Racic, M.; Goodpaster, B.H.; Gladwin, M.T. Effects of Oral Sodium Nitrite on Blood Pressure, Insulin Sensitivity, and Intima-Media Arterial Thickening in Adults With Hy-pertension and Metabolic Syndrome. Hypertension 2020, 76, 866–874. [Google Scholar] [CrossRef]
- Oliveira-Paula, G.H.; Tanus-Santos, J.E. Nitrite-Stimulated Gastric Formation of S-Nitrosothiols As An Antihypertensive Therapeutic Strategy. Curr. Drug Targets 2019, 20, 431–443. [Google Scholar] [CrossRef]
- Crews, J.K.; Herrington, J.N.; Granger, J.P.; Khalil, R.A. Decreased Endothelium-Dependent Vascular Relaxation During Reduction of Uterine Perfusion Pressure in Pregnant Rat. Hypertension 2000, 35, 367–372. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The Nitrate–Nitrite–Nitric Oxide Pathway in Physiology and Therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Shiva, S. Nitrite: A Physiological Store of Nitric Oxide and Modulator of Mitochondrial Function. Redox Biol. 2013, 1, 40–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, Y.; Li, W.; Tran, V.; Khalil, R.A. EMMPRIN-Mediated Induction of Uterine and Vascular Matrix Metalloproteinases during Pregnancy and in Response to Estrogen and Progesterone. Biochem. Pharmacol. 2013, 86, 734–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Z.; Sada, A.A.; Reslan, O.M.; Narula, N.; Khalil, R.A. Increased MMPs Expression and Decreased Contraction in the Rat Myometrium during Pregnancy and in Response to Prolonged Stretch and Sex Hormones. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E55–E70. [Google Scholar] [CrossRef] [PubMed]
- Amaral, L.M.; Pinheiro, L.C.; Guimaraes, D.A.; Palei, A.C.T.; Sertório, J.T.; Portella, R.L.; Tanus-Santos, J.E. Antihypertensive Effects of Inducible Nitric Oxide Synthase Inhibition in Experimental Pre-eclampsia. J. Cell. Mol. Med. 2013, 17, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; He, F.; Kuang, L.; Tang, W.; Li, Y.; Chen, D. ENOS/INOS and Endoplasmic Reticulum Stress-Induced Apoptosis in the Placentas of Patients with Preeclampsia. J. Hum. Hypertens. 2017, 31, 49–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krause, B.J.; Hanson, M.A.; Casanello, P. Role of Nitric Oxide in Placental Vascular Development and Function. Placenta 2011, 32, 797–805. [Google Scholar] [CrossRef] [Green Version]
- Cantu-Medellin, N.; Kelley, E.E. Xanthine Oxidoreductase-Catalyzed Reduction of Nitrite to Nitric Oxide: Insights Regarding Where, When and How. Nitric Oxide 2013, 34, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Fantone, S.; Ermini, L.; Piani, F.; Di Simone, N.; Barbaro, G.; Giannubilo, S.R.; Gesuita, R.; Tossetta, G.; Marzioni, D. Downregulation of Argininosuccinate Synthase 1 (ASS1) Is Associated with Hypoxia in Placental Development. Hum. Cell 2023, 36, 1190–1198. [Google Scholar] [CrossRef]
- Cancemi, P.; Aiello, A.; Accardi, G.; Caldarella, R.; Candore, G.; Caruso, C.; Ciaccio, M.; Cristaldi, L.; Di Gaudio, F.; Siino, V.; et al. The Role of Matrix Metalloproteinases (MMP-2 and MMP-9) in Ageing and Longevity: Focus on Sicilian Long-Living Individuals (LLIs). Mediat. Inflamm. 2020, 2020, 8635158. [Google Scholar] [CrossRef]
- Li, J.; LaMarca, B.; Reckelhoff, J.F. A Model of Preeclampsia in Rats: The Reduced Uterine Perfusion Pressure (RUPP) Model. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H1–H8. [Google Scholar] [CrossRef] [Green Version]
- Amaral, J.H.; Ferreira, G.C.; Pinheiro, L.C.; Montenegro, M.F.; Tanus-Santos, J.E. Consistent Antioxidant and Antihypertensive Effects of Oral Sodium Nitrite in DOCA-Salt Hypertension. Redox Biol. 2015, 5, 340–346. [Google Scholar] [CrossRef] [Green Version]
- Classen, H.G.; Stein-Hammer, C.; Thöni, H. Hypothesis: The Effect of Oral Nitrite on Blood Pressure in the Spontaneously Hypertensive Rat. Does Dietary Nitrate Mitigate Hypertension after Conversion to Nitrite? J. Am. Coll. Nutr. 1990, 9, 500–502. [Google Scholar] [CrossRef]
- Nascimento, R.A.; Possomato-Vieira, J.S.; Bonacio, G.F.; Rizzi, E.; Dias-Junior, C.A. Reductions of Circulating Nitric Oxide Are Followed by Hypertension during Pregnancy and Increased Activity of Matrix Metalloproteinases-2 and -9 in Rats. Cells 2019, 8, 1402. [Google Scholar] [CrossRef] [Green Version]
- Rizzi, É.; Castro, M.M.; Prado, C.M.; Silva, C.A.; Fazan, R.; Rossi, M.A.; Tanus-Santos, J.E.; Gerlach, R.F. Matrix Metallopro-teinase Inhibition Improves Cardiac Dysfunction and Remodeling in 2-Kidney, 1-Clip Hypertension. J. Card. Fail. 2010, 16, 599–608. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, L.Z.; da Silva, M.L.S.; Rodrigues, S.D.; Gomes, S.E.B.; Molezini, L.; Rizzi, E.; Montenegro, M.F.; Dias-Junior, C.A. Sodium Nitrite Attenuates Reduced Activity of Vascular Matrix Metalloproteinase-2 and Vascular Hyper-Reactivity and Increased Systolic Blood Pressure Induced by the Placental Ischemia Model of Preeclampsia in Anesthetized Rats. Int. J. Mol. Sci. 2023, 24, 12818. https://doi.org/10.3390/ijms241612818
Martins LZ, da Silva MLS, Rodrigues SD, Gomes SEB, Molezini L, Rizzi E, Montenegro MF, Dias-Junior CA. Sodium Nitrite Attenuates Reduced Activity of Vascular Matrix Metalloproteinase-2 and Vascular Hyper-Reactivity and Increased Systolic Blood Pressure Induced by the Placental Ischemia Model of Preeclampsia in Anesthetized Rats. International Journal of Molecular Sciences. 2023; 24(16):12818. https://doi.org/10.3390/ijms241612818
Chicago/Turabian StyleMartins, Laisla Zanetoni, Maria Luiza Santos da Silva, Serginara David Rodrigues, Sáskia Estela Biasotti Gomes, Laura Molezini, Elen Rizzi, Marcelo Freitas Montenegro, and Carlos Alan Dias-Junior. 2023. "Sodium Nitrite Attenuates Reduced Activity of Vascular Matrix Metalloproteinase-2 and Vascular Hyper-Reactivity and Increased Systolic Blood Pressure Induced by the Placental Ischemia Model of Preeclampsia in Anesthetized Rats" International Journal of Molecular Sciences 24, no. 16: 12818. https://doi.org/10.3390/ijms241612818





