miRNAs Epigenetic Tuning of Wall Remodeling in the Early Phase after Myocardial Infarction: A Novel Epidrug Approach
Abstract
1. Introduction
Epigenetic Regulation
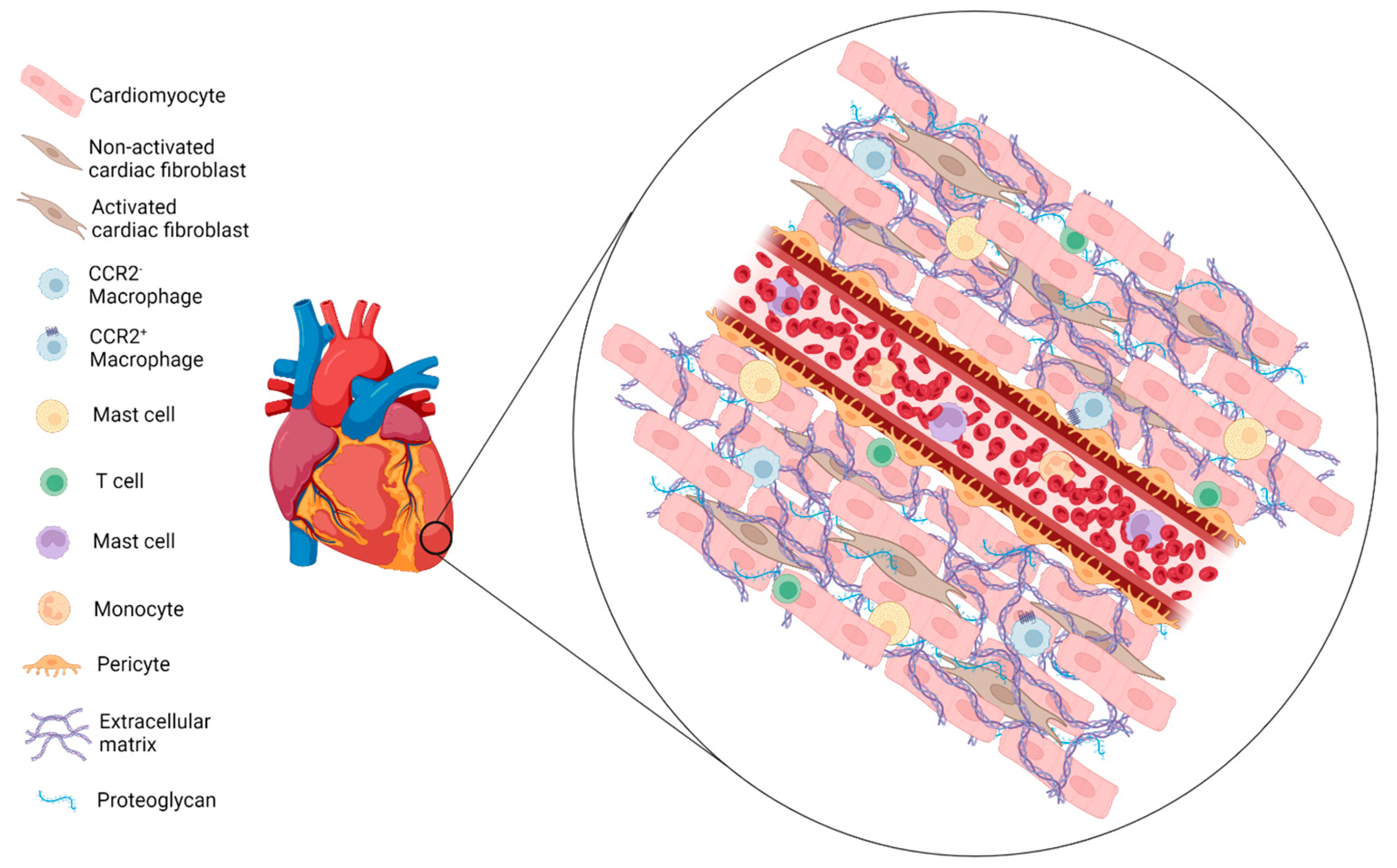
2. Myocardial Infarction
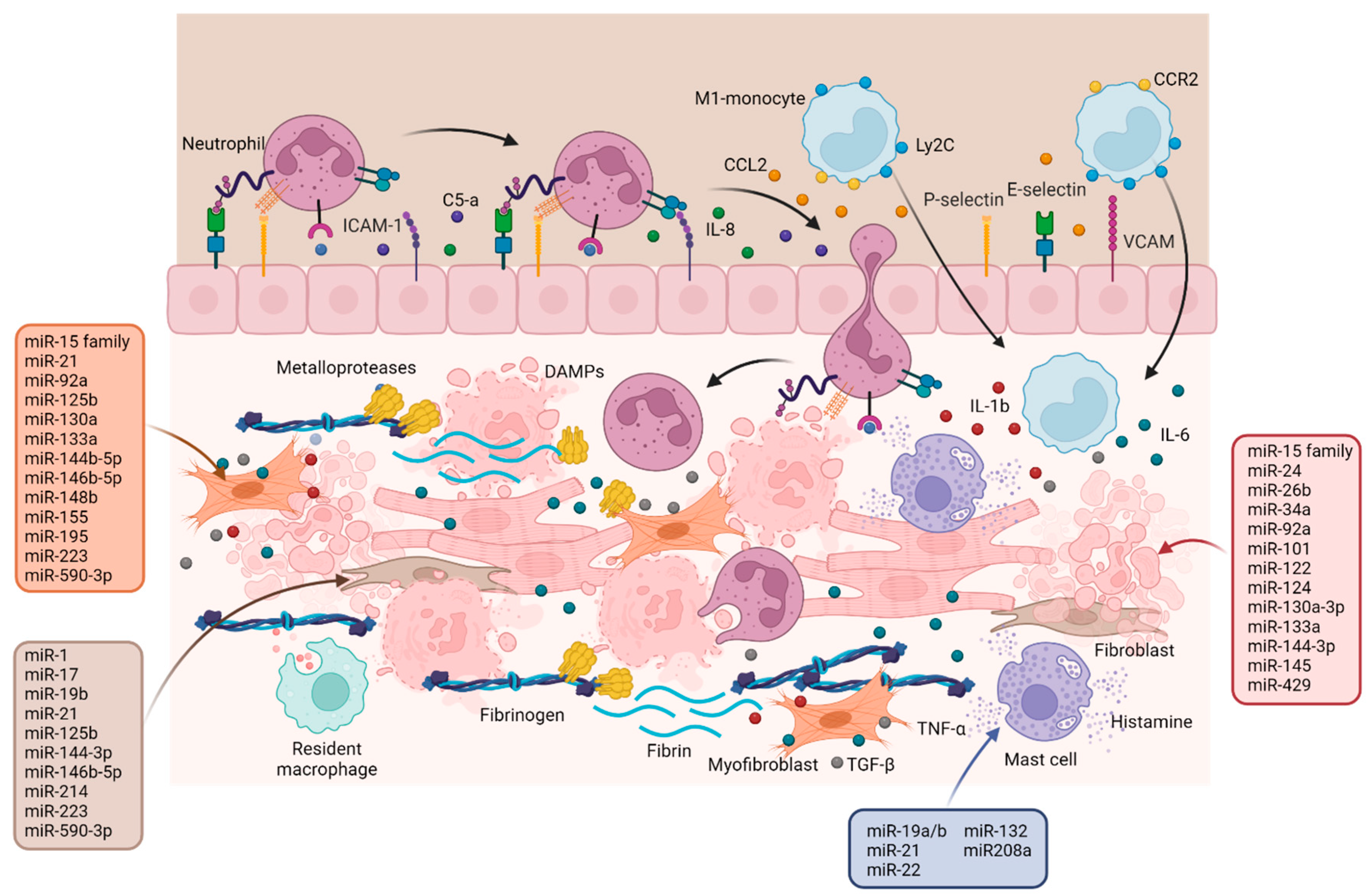
2.1. Inflammation Phase
2.2. Epigenetic Regulation
3. Resolution Phase
Epigenetics Regulation
4. Maturation Phase
Epigenetics Regulation
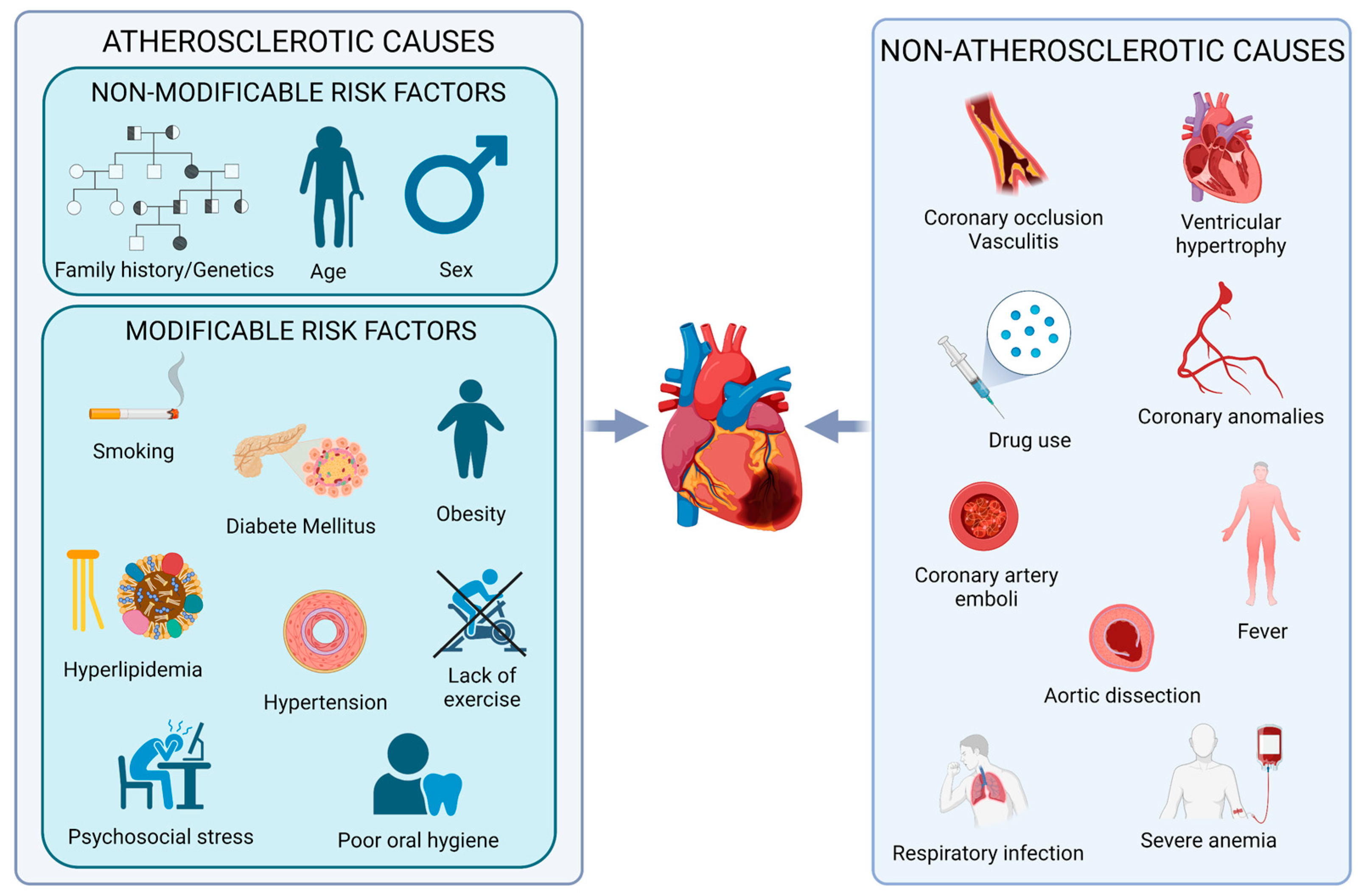
5. Sex-Related Differences in Risk Factors and Long-Term Outcomes for MI
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALDH2 | Aldehyde Dehydrogenase 2 |
| bFGF | Basic Fibroblast Growth Factor |
| CCL2 | Monocyte Chemoattractant Protein-1 (CCL2/MCP1) |
| CCR2 | CC chemokine Receptor 2 |
| CFs | Cardiac Fibroblasts |
| Check1 | Checkpoint Kinase 1 |
| CMs | Cardiomyocytes |
| COL1 | Collagen Type 1 |
| CTGF | Connective Tissue Growth Factor |
| CV | Cardiovascular |
| DAMPs | Damage-Associated Molecular Patterns |
| DCs | Dendritic Cells |
| DMRs | Differently Methylated Regions |
| ECM | Extracellular Matrix |
| ECs | Endothelial Cells |
| eNOS | Endothelial Nitric Oxide Synthase |
| ESCs | Embryonic Stem Cells |
| FGF9 | Fibroblast Growth Factor-9 |
| FPR1 | Formyl Peptide Receptor 1 |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| HDACs | Histone deacetylases |
| HIF-1α | Hypoxia-Inducible Factor 1-alpha |
| I/R | Ischemia/Reperfusion |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| IGF2 | Insulin-like Growth Factor-2 |
| IRA | IL-1 Receptor Antagonist |
| Klf2 | Krupple-like factor 2 |
| lncRNAs | Long Non-coding RNAs |
| LV | Left Ventricular |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MCPs | Mast Cell-Committed Progenitors |
| MCs | Mast Cells |
| M-CSF | Macrophage-Colony Stimulating Factor |
| MCTC | Trypase positive, chymase positive mast cells |
| MI | Myocardial Infarction |
| MIP-1α | Macrophage Inflammatory Protein 1 Alpha |
| miRNAs | MicroRNAs |
| MMPs | Metalloproteinases |
| ncRNAs | Non-coding RNAs |
| NKIRAS2 | NF-kB Inhibitor Interacting Ras like 2 |
| NOMO1 | Nodal Modulator 1 |
| PDGF | Platelet-Derived Growth Factor |
| PI3K | Phosphoinositide 3-kinase |
| PRR | Pathogen Recognition Receptors |
| ROS | Reactive Oxygen Species |
| SIRT | Sirtuin |
| SOCS3 | Suppressor Of Cytokine Signaling 3 |
| SPARC | Secreted Protein Acidic and Rich in Cysteine |
| SPIN1 | Spindlin-1 |
| TGF-β | Transforming Growth Factor-beta |
| TIMP-1 | Tissue metalloproteinase inhibitor-1 |
| TLRs | Toll-Like Receptors |
| TNF-α | Tumor Necrosis Factor-alpha |
| Treg | Regulatory T cells |
| TSP-1 | Thrombospondin-1 |
| TSP-2 | Thrombospondin-2 |
| VCAM-1 | Vascular Cell Adhesion Molecule 1 |
| VEGF | Vascular-Endothelial Growth Factor |
| VLA-4 | Very Late Antigen-4 |
| VSMCs | Vascular Smooth Muscle Cells |
| WAT | White Adipose Tissue |
References
- Tucker, N.R.; Chaffin, M.; Fleming, S.J.; Hall, A.W.; Parsons, V.A.; Bedi, K.C., Jr.; Akkad, A.D.; Herndon, C.N.; Arduini, A.; Papangeli, I.; et al. Transcriptional and Cellular Diversity of the Human Heart. Circulation 2020, 142, 466–482. [Google Scholar] [CrossRef] [PubMed]
- Dostal, D.; Glaser, S.; Baudino, T.A. Cardiac fibroblast physiology and pathology. Compr. Physiol. 2015, 5, 887–909. [Google Scholar] [CrossRef]
- Sun, K.; Li, Y.Y.; Jin, J. A double-edged sword of immuno-microenvironment in cardiac homeostasis and injury repair. Signal Transduct. Target. Ther. 2021, 6, 79. [Google Scholar] [CrossRef] [PubMed]
- Koc, A.; Cagavi, E. Cardiac Immunology: A New Era for Immune Cells in the Heart. Adv. Exp. Med. Biol. 2021, 1312, 75–95. [Google Scholar] [CrossRef]
- Janicki, J.S.; Brower, G.L.; Levick, S.P. The emerging prominence of the cardiac mast cell as a potent mediator of adverse myocardial remodeling. Methods Mol. Biol. 2015, 1220, 121–139. [Google Scholar] [CrossRef] [PubMed]
- Swirski, F.K.; Nahrendorf, M. Cardioimmunology: The immune system in cardiac homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 733–744. [Google Scholar] [CrossRef]
- Kumar, V.; Rosenzweig, R.; Asalla, S.; Nehra, S.; Prabhu, S.D.; Bansal, S.S. TNFR1 Contributes to Activation-Induced Cell Death of Pathological CD4(+) T Lymphocytes During Ischemic Heart Failure. JACC Basic. Transl. Sci. 2022, 7, 1038–1049. [Google Scholar] [CrossRef]
- Kumar, V.; Prabhu, S.D.; Bansal, S.S. CD4(+) T-lymphocytes exhibit biphasic kinetics post-myocardial infarction. Front. Cardiovasc. Med. 2022, 9, 992653. [Google Scholar] [CrossRef]
- Alvarez-Argote, S.; O’Meara, C.C. The Evolving Roles of Cardiac Macrophages in Homeostasis, Regeneration, and Repair. Int. J. Mol. Sci. 2021, 22, 7923. [Google Scholar] [CrossRef]
- Bajpai, G.; Schneider, C.; Wong, N.; Bredemeyer, A.; Hulsmans, M.; Nahrendorf, M.; Epelman, S.; Kreisel, D.; Liu, Y.; Itoh, A.; et al. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat. Med. 2018, 24, 1234–1245. [Google Scholar] [CrossRef]
- Molawi, K.; Wolf, Y.; Kandalla, P.K.; Favret, J.; Hagemeyer, N.; Frenzel, K.; Pinto, A.R.; Klapproth, K.; Henri, S.; Malissen, B.; et al. Progressive replacement of embryo-derived cardiac macrophages with age. J. Exp. Med. 2014, 211, 2151–2158. [Google Scholar] [CrossRef]
- Epelman, S.; Lavine, K.J.; Beaudin, A.E.; Sojka, D.K.; Carrero, J.A.; Calderon, B.; Brija, T.; Gautier, E.L.; Ivanov, S.; Satpathy, A.T.; et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 2014, 40, 91–104. [Google Scholar] [CrossRef]
- Dick, S.A.; Macklin, J.A.; Nejat, S.; Momen, A.; Clemente-Casares, X.; Althagafi, M.G.; Chen, J.; Kantores, C.; Hosseinzadeh, S.; Aronoff, L.; et al. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat. Immunol. 2019, 20, 29–39. [Google Scholar] [CrossRef]
- Ma, Y.; Mouton, A.J.; Lindsey, M.L. Cardiac macrophage biology in the steady-state heart, the aging heart, and following myocardial infarction. Transl. Res. 2018, 191, 15–28. [Google Scholar] [CrossRef]
- Leid, J.; Carrelha, J.; Boukarabila, H.; Epelman, S.; Jacobsen, S.E.; Lavine, K.J. Primitive Embryonic Macrophages are Required for Coronary Development and Maturation. Circ. Res. 2016, 118, 1498–1511. [Google Scholar] [CrossRef] [PubMed]
- Fantin, A.; Vieira, J.M.; Gestri, G.; Denti, L.; Schwarz, Q.; Prykhozhij, S.; Peri, F.; Wilson, S.W.; Ruhrberg, C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 2010, 116, 829–840. [Google Scholar] [CrossRef]
- Cahill, T.J.; Sun, X.; Ravaud, C.; Villa Del Campo, C.; Klaourakis, K.; Lupu, I.E.; Lord, A.M.; Browne, C.; Jacobsen, S.E.W.; Greaves, D.R.; et al. Tissue-resident macrophages regulate lymphatic vessel growth and patterning in the developing heart. Development 2021, 148, dev194563. [Google Scholar] [CrossRef] [PubMed]
- Hulsmans, M.; Clauss, S.; Xiao, L.; Aguirre, A.D.; King, K.R.; Hanley, A.; Hucker, W.J.; Wulfers, E.M.; Seemann, G.; Courties, G.; et al. Macrophages Facilitate Electrical Conduction in the Heart. Cell 2017, 169, 510–522. [Google Scholar] [CrossRef]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabe-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for cardiomyocyte renewal in humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef]
- Gilsbach, R.; Preissl, S.; Gruning, B.A.; Schnick, T.; Burger, L.; Benes, V.; Wurch, A.; Bonisch, U.; Gunther, S.; Backofen, R.; et al. Dynamic DNA methylation orchestrates cardiomyocyte development, maturation and disease. Nat. Commun. 2014, 5, 5288. [Google Scholar] [CrossRef] [PubMed]
- May, D.; Blow, M.J.; Kaplan, T.; McCulley, D.J.; Jensen, B.C.; Akiyama, J.A.; Holt, A.; Plajzer-Frick, I.; Shoukry, M.; Wright, C.; et al. Large-scale discovery of enhancers from human heart tissue. Nat. Genet. 2011, 44, 89–93. [Google Scholar] [CrossRef]
- Yuan, X.; Braun, T. Multimodal Regulation of Cardiac Myocyte Proliferation. Circ. Res. 2017, 121, 293–309. [Google Scholar] [CrossRef]
- Ghosh, A.K. Acetyltransferase p300 Is a Putative Epidrug Target for Amelioration of Cellular Aging-Related Cardiovascular Disease. Cells 2021, 10, 2839. [Google Scholar] [CrossRef]
- Chew, N.W.S.; Loong, S.S.E.; Foo, R. Epigenetics in cardiovascular health and disease. Prog. Mol. Biol. Transl. Sci. 2023, 197, 105–134. [Google Scholar] [CrossRef]
- Gorica, E.; Mohammed, S.A.; Ambrosini, S.; Calderone, V.; Costantino, S.; Paneni, F. Epi-Drugs in Heart Failure. Front. Cardiovasc. Med. 2022, 9, 923014. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, C.M.; Zhu, W.; Wang, Q.; Jia, C.; Kee, H.J.; Li, L.; Hannenhalli, S.; Epstein, J.A. Hopx and Hdac2 interact to modulate Gata4 acetylation and embryonic cardiac myocyte proliferation. Dev. Cell 2010, 19, 450–459. [Google Scholar] [CrossRef]
- Chang, S.; McKinsey, T.A.; Zhang, C.L.; Richardson, J.A.; Hill, J.A.; Olson, E.N. Histone deacetylases 5 and 9 govern responsiveness of the heart to a subset of stress signals and play redundant roles in heart development. Mol. Cell Biol. 2004, 24, 8467–8476. [Google Scholar] [CrossRef] [PubMed]
- Kansakar, U.; Varzideh, F.; Mone, P.; Jankauskas, S.S.; Santulli, G. Functional Role of microRNAs in Regulating Cardiomyocyte Death. Cells 2022, 11, 983. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Jia, Z.; Cui, Q.; Zhang, C.; Wang, W.; Chen, P.; Ma, K.; Zhou, C. MiR-499 regulates cell proliferation and apoptosis during late-stage cardiac differentiation via Sox6 and cyclin D1. PLoS ONE 2013, 8, e74504. [Google Scholar] [CrossRef] [PubMed]
- Porrello, E.R.; Johnson, B.A.; Aurora, A.B.; Simpson, E.; Nam, Y.J.; Matkovich, S.J.; Dorn, G.W., 2nd; van Rooij, E.; Olson, E.N. MiR-15 family regulates postnatal mitotic arrest of cardiomyocytes. Circ. Res. 2011, 109, 670–679. [Google Scholar] [CrossRef]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Johnson, B.A.; Grinsfelder, D.; Canseco, D.; Mammen, P.P.; Rothermel, B.A.; Olson, E.N.; Sadek, H.A. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc. Natl. Acad. Sci. USA 2013, 110, 187–192. [Google Scholar] [CrossRef]
- Patil, M.; Pabla, N.; Dong, Z. Checkpoint kinase 1 in DNA damage response and cell cycle regulation. Cell Mol. Life Sci. 2013, 70, 4009–4021. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.P.; Seok, H.Y.; Ding, J.; Kataoka, M.; Zhang, Z.; Hu, X.; Wang, G.; Lin, Z.; Wang, S.; et al. mir-17-92 cluster is required for and sufficient to induce cardiomyocyte proliferation in postnatal and adult hearts. Circ. Res. 2013, 112, 1557–1566. [Google Scholar] [CrossRef]
- Gao, F.; Kataoka, M.; Liu, N.; Liang, T.; Huang, Z.P.; Gu, F.; Ding, J.; Liu, J.; Zhang, F.; Ma, Q.; et al. Therapeutic role of miR-19a/19b in cardiac regeneration and protection from myocardial infarction. Nat. Commun. 2019, 10, 1802. [Google Scholar] [CrossRef]
- Sun, L.; Zhu, W.; Zhao, P.; Zhang, J.; Lu, Y.; Zhu, Y.; Zhao, W.; Liu, Y.; Chen, Q.; Zhang, F. Down-Regulated Exosomal MicroRNA-221-3p Derived From Senescent Mesenchymal Stem Cells Impairs Heart Repair. Front. Cell Dev. Biol. 2020, 8, 263. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.H.; Wang, Z.Q.; Zhang, S. MiR-208a participates with sevoflurane post-conditioning in protecting neonatal rat cardiomyocytes with simulated ischemia-reperfusion injury via PI3K/AKT signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 943–955. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, S.; Zhang, X.S.; Wang, D.M.; Qian, C.Y. MicroRNA-489 promotes cardiomyocyte apoptosis induced by myocardial ischemia-reperfusion injury through inhibiting SPIN1. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6683–6690. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Chen, X.; Han, J.; Zhong, Y.; Li, Q.; An, Y. MiR-324/SOCS3 Axis Protects Against Hypoxia/Reoxygenation-Induced Cardiomyocyte Injury and Regulates Myocardial Ischemia via TNF/NF-kappaB Signaling Pathway. Int. Heart J. 2020, 61, 1258–1269. [Google Scholar] [CrossRef]
- Ding, Y.; Bi, L.; Wang, J. MiR-1180 promotes cardiomyocyte cell cycle re-entry after injury through the NKIRAS2-NFkappaB pathway. Biochem. Cell Biol. 2020, 98, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.M. Nodal signaling: Developmental roles and regulation. Development 2007, 134, 1023–1034. [Google Scholar] [CrossRef]
- Xing, W.; Li, T.; Wang, Y.; Qiang, Y.; Ai, C.; Tang, H. MiR-33a-5p targets NOMO1 to modulate human cardiomyocyte progenitor cells proliferation and differentiation and apoptosis. J. Recept. Signal Transduct. Res. 2021, 41, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Li, J.; Wu, Y.; Zhen, L.; Li, C.; Qi, M.; Wang, L.; Deng, F.; Huang, J.; Lv, F.; et al. miRNA-204 drives cardiomyocyte proliferation via targeting Jarid2. Int. J. Cardiol. 2015, 201, 38–48. [Google Scholar] [CrossRef]
- Crippa, S.; Nemir, M.; Ounzain, S.; Ibberson, M.; Berthonneche, C.; Sarre, A.; Boisset, G.; Maison, D.; Harshman, K.; Xenarios, I.; et al. Comparative transcriptome profiling of the injured zebrafish and mouse hearts identifies miRNA-dependent repair pathways. Cardiovasc. Res. 2016, 110, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Song, G.; Pettit, S.M.; Li, Q.; Song, X.; Cai, C.L.; Kaushal, S.; Li, D. Epicardial HDAC3 Promotes Myocardial Growth Through a Novel MicroRNA Pathway. Circ. Res. 2022, 131, 151–164. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, Q.; Gao, N.; Wu, F.; Lan, F.; Zhang, F.; Jin, L.; Huang, Z.; Ge, J.; Wang, H.; et al. MircroRNA-10b Promotes Human Embryonic Stem Cell-Derived Cardiomyocyte Proliferation via Novel Target Gene LATS1. Mol. Ther. Nucleic Acids 2020, 19, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Hu, S.; Liu, S.; Zhang, H.; Ma, H.; Huang, K.; Li, Z.; Su, T.; Vandergriff, A.; Tang, J.; et al. microRNA-21-5p dysregulation in exosomes derived from heart failure patients impairs regenerative potential. J. Clin. Investig. 2019, 129, 2237–2250. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, M.; Liu, R.F.; Zhang, J.S.; Xu, M. Cardiac Hypertrophy is Positively Regulated by MicroRNA-24 in Rats. Chin. Med. J. 2018, 131, 1333–1341. [Google Scholar] [CrossRef]
- Wang, B.; Xu, M.; Li, M.; Wu, F.; Hu, S.; Chen, X.; Zhao, L.; Huang, Z.; Lan, F.; Liu, D.; et al. miR-25 Promotes Cardiomyocyte Proliferation by Targeting FBXW7. Mol. Ther. Nucleic Acids 2020, 19, 1299–1308. [Google Scholar] [CrossRef]
- Wu, Y.; Fan, Z.; Chen, Z.; Hu, J.; Cui, J.; Liu, Y.; Wang, Y.; Guo, B.; Shen, J.; Xie, L. Astragaloside IV protects human cardiomyocytes from hypoxia/reoxygenation injury by regulating miR-101a. Mol. Cell Biochem. 2020, 470, 41–51. [Google Scholar] [CrossRef]
- Liang, C.; Wang, S.; Zhao, L.; Han, Y.; Zhang, M. Effects of miR-145-5p on cardiomyocyte proliferation and apoptosis, GIGYF1 expression and oxidative stress response in rats with myocardial ischemia-reperfusion. Cell. Mol. Biol. 2022, 68, 147–159. [Google Scholar] [CrossRef]
- Wang, X.; Ha, T.; Liu, L.; Hu, Y.; Kao, R.; Kalbfleisch, J.; Williams, D.; Li, C. TLR3 Mediates Repair and Regeneration of Damaged Neonatal Heart through Glycolysis Dependent YAP1 Regulated miR-152 Expression. Cell Death Differ. 2018, 25, 966–982. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, H.; Huang, S.; Pei, L.; Feng, M.; Zhao, X.; Ouyang, Z.; Yao, S.; Jiang, R.; Wei, K. miR-199a-3p promotes cardiomyocyte proliferation by inhibiting Cd151 expression. Biochem. Biophys. Res. Commun. 2019, 516, 28–36. [Google Scholar] [CrossRef]
- Torrini, C.; Cubero, R.J.; Dirkx, E.; Braga, L.; Ali, H.; Prosdocimo, G.; Gutierrez, M.I.; Collesi, C.; Licastro, D.; Zentilin, L.; et al. Common Regulatory Pathways Mediate Activity of MicroRNAs Inducing Cardiomyocyte Proliferation. Cell Rep. 2019, 27, 2759–2771.e2755. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, L. Long non-coding RNA MALAT1 regulates cardiomyocytes apoptosis after hypoxia/reperfusion injury via modulating miR-200a-3p/PDCD4 axis. Biomed. Pharmacother. 2019, 111, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Pandey, R.; Alam, P.; Jiang, S.; Sadayappan, S.; Paul, A.; Ahmed, R.P.H. MicroRNA-210-mediated proliferation, survival, and angiogenesis promote cardiac repair post myocardial infarction in rodents. J. Mol. Med. 2017, 95, 1369–1385. [Google Scholar] [CrossRef]
- Borden, A.; Kurian, J.; Nickoloff, E.; Yang, Y.; Troupes, C.D.; Ibetti, J.; Lucchese, A.M.; Gao, E.; Mohsin, S.; Koch, W.J.; et al. Transient Introduction of miR-294 in the Heart Promotes Cardiomyocyte Cell Cycle Reentry After Injury. Circ. Res. 2019, 125, 14–25. [Google Scholar] [CrossRef]
- Zhen, L.; Zhao, Q.; Lu, J.; Deng, S.; Xu, Z.; Zhang, L.; Zhang, Y.; Fan, H.; Chen, X.; Liu, Z.; et al. miR-301a-PTEN-AKT Signaling Induces Cardiomyocyte Proliferation and Promotes Cardiac Repair Post-MI. Mol. Ther. Nucleic Acids 2020, 22, 251–262. [Google Scholar] [CrossRef]
- Xu, F.; Yang, J.; Shang, J.; Lan, F.; Li, M.; Shi, L.; Shen, L.; Wang, Y.; Ge, J. MicroRNA-302d promotes the proliferation of human pluripotent stem cell-derived cardiomyocytes by inhibiting LATS2 in the Hippo pathway. Clin. Sci. 2019, 133, 1387–1399. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Qin, S.; Wang, R.; Zhang, Z.; Li, H.; Hao, C. MicroRNA-362-3p Implicated in Cardioprotection Against Hypoxia/Reoxygenation-Induced Cardiomyocyte Apoptosis by Repressing TP53INP2 and Regulating SDF-1/CXCR4 Pathway. Altern. Ther. Health Med. 2023, 29, 254–261. [Google Scholar]
- Zhao, Z.; Zhao, Y.; Ying-Chun, L.; Zhao, L.; Zhang, W.; Yang, J.G. Protective role of microRNA-374 against myocardial ischemia-reperfusion injury in mice following thoracic epidural anesthesia by downregulating dystrobrevin alpha-mediated Notch1 axis. J. Cell Physiol. 2019, 234, 10726–10740. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.L.; Naya, F.J. MicroRNAs in the Myocyte Enhancer Factor 2 (MEF2)-regulated Gtl2-Dio3 Noncoding RNA Locus Promote Cardiomyocyte Proliferation by Targeting the Transcriptional Coactivator Cited2. J. Biol. Chem. 2015, 290, 23162–23172. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.L.; Ren, F.; Xu, H.; Song, H.J. Overexpression of miRNA-410-3p protects hypoxia-induced cardiomyocyte injury via targeting TRAF5. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9050–9057. [Google Scholar] [CrossRef]
- Jin, Y.; Ni, S. miR-496 remedies hypoxia reoxygenation-induced H9c2 cardiomyocyte apoptosis via Hook3-targeted PI3k/Akt/mTOR signaling pathway activation. J. Cell Biochem. 2020, 121, 698–712. [Google Scholar] [CrossRef]
- Deng, S.; Zhao, Q.; Zhen, L.; Zhang, C.; Liu, C.; Wang, G.; Zhang, L.; Bao, L.; Lu, Y.; Meng, L.; et al. Neonatal Heart-Enriched miR-708 Promotes Proliferation and Stress Resistance of Cardiomyocytes in Rodents. Theranostics 2017, 7, 1953–1965. [Google Scholar] [CrossRef]
- Gao, X.; Li, H.; Wang, X.; Ren, Z.; Tian, Y.; Zhao, J.; Qi, W.; Wang, H.; Yu, Y.; Gong, R.; et al. Light Emitting Diodes Irradiation Regulates miRNA-877-3p to Promote Cardiomyocyte Proliferation. Int. J. Med. Sci. 2022, 19, 1254–1264. [Google Scholar] [CrossRef]
- Hu, Y.; Jin, G.; Li, B.; Chen, Y.; Zhong, L.; Chen, G.; Chen, X.; Zhong, J.; Liao, W.; Liao, Y.; et al. Suppression of miRNA let-7i-5p promotes cardiomyocyte proliferation and repairs heart function post injury by targetting CCND2 and E2F2. Clin. Sci. 2019, 133, 425–441. [Google Scholar] [CrossRef]
- Gan, J.; Tang, F.M.K.; Su, X.; Lu, G.; Xu, J.; Lee, H.S.S.; Lee, K.K.H. microRNA-1 inhibits cardiomyocyte proliferation in mouse neonatal hearts by repressing CCND1 expression. Ann. Transl. Med. 2019, 7, 455. [Google Scholar] [CrossRef]
- Xu, J.; Cao, D.; Zhang, D.; Zhang, Y.; Yue, Y. MicroRNA-1 facilitates hypoxia-induced injury by targeting NOTCH3. J. Cell Biochem. 2020, 121, 4458–4469. [Google Scholar] [CrossRef]
- Zheng, J.; Peng, B.; Zhang, Y.; Ai, F.; Hu, X. miR-9 knockdown inhibits hypoxia-induced cardiomyocyte apoptosis by targeting Yap1. Life Sci. 2019, 219, 129–135. [Google Scholar] [CrossRef]
- Cao, X.; Wang, J.; Wang, Z.; Du, J.; Yuan, X.; Huang, W.; Meng, J.; Gu, H.; Nie, Y.; Ji, B.; et al. MicroRNA profiling during rat ventricular maturation: A role for miR-29a in regulating cardiomyocyte cell cycle re-entry. FEBS Lett. 2013, 587, 1548–1555. [Google Scholar] [CrossRef]
- Zhang, Y.; Matsushita, N.; Eigler, T.; Marban, E. Targeted MicroRNA Interference Promotes Postnatal Cardiac Cell Cycle Re-Entry. J. Regen. Med. 2013, 2, 2. [Google Scholar] [CrossRef]
- Yang, Q.; Wu, F.; Mi, Y.; Wang, F.; Cai, K.; Yang, X.; Zhang, R.; Liu, L.; Zhang, Y.; Wang, Y.; et al. Aberrant expression of miR-29b-3p influences heart development and cardiomyocyte proliferation by targeting NOTCH2. Cell Prolif. 2020, 53, e12764. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, H.W.; Qiu, Y.; Dupee, D.; Noonan, M.; Lin, Y.D.; Fisch, S.; Unno, K.; Sereti, K.I.; Liao, R. MicroRNA-34a Plays a Key Role in Cardiac Repair and Regeneration Following Myocardial Infarction. Circ. Res. 2015, 117, 450–459. [Google Scholar] [CrossRef]
- Huang, W.; Feng, Y.; Liang, J.; Yu, H.; Wang, C.; Wang, B.; Wang, M.; Jiang, L.; Meng, W.; Cai, W.; et al. Loss of microRNA-128 promotes cardiomyocyte proliferation and heart regeneration. Nat. Commun. 2018, 9, 700. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Bezprozvannaya, S.; Williams, A.H.; Qi, X.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes. Dev. 2008, 22, 3242–3254. [Google Scholar] [CrossRef]
- Ma, W.Y.; Song, R.J.; Xu, B.B.; Xu, Y.; Wang, X.X.; Sun, H.Y.; Li, S.N.; Liu, S.Z.; Yu, M.X.; Yang, F.; et al. Melatonin promotes cardiomyocyte proliferation and heart repair in mice with myocardial infarction via miR-143-3p/Yap/Ctnnd1 signaling pathway. Acta Pharmacol. Sin. 2021, 42, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.L.; Zhu, B.L.; Sun, Y.Y.; Qiu, G.R.; Fu, W.N.; Jiang, H.K. MicroRNA-144 Regulates Cardiomyocyte Proliferation and Apoptosis by Targeting TBX1 through the JAK2/STAT1 Pathway. Cytogenet. Genome Res. 2019, 159, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lu, X. MiR-423-5p inhibition alleviates cardiomyocyte apoptosis and mitochondrial dysfunction caused by hypoxia/reoxygenation through activation of the wnt/beta-catenin signaling pathway via targeting MYBL2. J. Cell Physiol. 2019, 234, 22034–22043. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, X.Y.; Song, L.; Tao, C.; Fang, J.; Tao, L. miR-484 targeting of Yap1-induced LPS-inhibited proliferation, and promoted apoptosis and inflammation in cardiomyocyte. Biosci. Biotechnol. Biochem. 2021, 85, 378–385. [Google Scholar] [CrossRef]
- Zhang, J.S.; Zhao, Y.; Lv, Y.; Liu, P.Y.; Ruan, J.X.; Sun, Y.L.; Gong, T.X.; Wan, N.; Qiu, G.R. miR-873 suppresses H9C2 cardiomyocyte proliferation by targeting GLI1. Gene 2017, 626, 426–432. [Google Scholar] [CrossRef]
- Chapman, A.R.; Adamson, P.D.; Mills, N.L. Assessment and classification of patients with myocardial injury and infarction in clinical practice. Heart 2017, 103, 10–18. [Google Scholar] [CrossRef]
- Smyth, A.; O’Donnell, M.; Lamelas, P.; Teo, K.; Rangarajan, S.; Yusuf, S.; Investigators, I. Physical Activity and Anger or Emotional Upset as Triggers of Acute Myocardial Infarction: The INTERHEART Study. Circulation 2016, 134, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Ghiasmand, M.; Moghadamnia, M.T.; Pourshaikhian, M.; Kazemnejad Lili, E. Acute triggers of myocardial infarction: A case-crossover study. Egypt. Heart J. 2017, 69, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Claeys, M.J.; Rajagopalan, S.; Nawrot, T.S.; Brook, R.D. Climate and environmental triggers of acute myocardial infarction. Eur. Heart J. 2017, 38, 955–960. [Google Scholar] [CrossRef]
- Fathima, S.N. An Update on Myocardial Infarction. Curr. Res. Trends Med. Sci. Technol. 2021, 1, 216. [Google Scholar]
- Gemmati, D.; Varani, K.; Bramanti, B.; Piva, R.; Bonaccorsi, G.; Trentini, A.; Manfrinato, M.C.; Tisato, V.; Care, A.; Bellini, T. “Bridging the Gap” Everything that Could Have Been Avoided If We Had Applied Gender Medicine, Pharmacogenetics and Personalized Medicine in the Gender-Omics and Sex-Omics Era. Int. J. Mol. Sci. 2019, 21, 296. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Pathophysiology of Myocardial Infarction. Compr. Physiol. 2015, 5, 1841–1875. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Group, E.S.C.S.D. Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef]
- Girelli, D.; Russo, C.; Ferraresi, P.; Olivieri, O.; Pinotti, M.; Friso, S.; Manzato, F.; Mazzucco, A.; Bernardi, F.; Corrocher, R. Polymorphisms in the factor VII gene and the risk of myocardial infarction in patients with coronary artery disease. N. Engl. J. Med. 2000, 343, 774–780. [Google Scholar] [CrossRef]
- Hoekema, L.; Castoldi, E.; Tans, G.; Girelli, D.; Gemmati, D.; Bernardi, F.; Rosing, J. Functional properties of factor V and factor Va encoded by the R2-gene. Thromb. Haemost. 2001, 85, 75–81. [Google Scholar] [CrossRef]
- Gemmati, D.; Federici, F.; Campo, G.; Tognazzo, S.; Serino, M.L.; De Mattei, M.; Valgimigli, M.; Malagutti, P.; Guardigli, G.; Ferraresi, P.; et al. Factor XIIIA-V34L and factor XIIIB-H95R gene variants: Effects on survival in myocardial infarction patients. Mol. Med. 2007, 13, 112–120. [Google Scholar] [CrossRef]
- Gemmati, D.; Vigliano, M.; Burini, F.; Mari, R.; El Mohsein, H.H.; Parmeggiani, F.; Serino, M.L. Coagulation Factor XIIIA (F13A1): Novel Perspectives in Treatment and Pharmacogenetics. Curr. Pharm. Des. 2016, 22, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.O.S.; Losada, D.M.; Jordani, M.C.; Evora, P.; Castro, E.S.O. Ischemia/Reperfusion Injury Revisited: An Overview of the Latest Pharmacological Strategies. Int. J. Mol. Sci. 2019, 20, 5034. [Google Scholar] [CrossRef] [PubMed]
- Epelman, S.; Liu, P.P.; Mann, D.L. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat. Rev. Immunol. 2015, 15, 117–129. [Google Scholar] [CrossRef]
- Igney, F.H.; Krammer, P.H. Death and anti-death: Tumour resistance to apoptosis. Nat. Rev. Cancer 2002, 2, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.; Azimpouran, M. Necrosis. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2023. [Google Scholar]
- Lodrini, A.M.; Goumans, M.J. Cardiomyocytes Cellular Phenotypes After Myocardial Infarction. Front. Cardiovasc. Med. 2021, 8, 750510. [Google Scholar] [CrossRef]
- Oka, T.; Hikoso, S.; Yamaguchi, O.; Taneike, M.; Takeda, T.; Tamai, T.; Oyabu, J.; Murakawa, T.; Nakayama, H.; Nishida, K.; et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 2012, 485, 251–255. [Google Scholar] [CrossRef]
- Frangogiannis, N.G.; Lindsey, M.L.; Michael, L.H.; Youker, K.A.; Bressler, R.B.; Mendoza, L.H.; Spengler, R.N.; Smith, C.W.; Entman, M.L. Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation 1998, 98, 699–710. [Google Scholar] [CrossRef]
- Frangogiannis, N.G.; Smith, C.W.; Entman, M.L. The inflammatory response in myocardial infarction. Cardiovasc. Res. 2002, 53, 31–47. [Google Scholar] [CrossRef]
- Nahrendorf, M.; Hu, K.; Frantz, S.; Jaffer, F.A.; Tung, C.H.; Hiller, K.H.; Voll, S.; Nordbeck, P.; Sosnovik, D.; Gattenlohner, S.; et al. Factor XIII deficiency causes cardiac rupture, impairs wound healing, and aggravates cardiac remodeling in mice with myocardial infarction. Circulation 2006, 113, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Heymans, S.; Luttun, A.; Nuyens, D.; Theilmeier, G.; Creemers, E.; Moons, L.; Dyspersin, G.D.; Cleutjens, J.P.; Shipley, M.; Angellilo, A.; et al. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat. Med. 1999, 5, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Gemmati, D.; Zeri, G.; Orioli, E.; Mari, R.; Moratelli, S.; Vigliano, M.; Marchesini, J.; Grossi, M.E.; Pecoraro, A.; Cuneo, A.; et al. Factor XIII-A dynamics in acute myocardial infarction: A novel prognostic biomarker? Thromb. Haemost. 2015, 114, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, D.; Heymans, S. Factor XIII: The cement of the heart after myocardial infarction? Eur. Heart J. 2008, 29, 427–428. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Weissleder, R.; Ertl, G. Does FXIII deficiency impair wound healing after myocardial infarction? PLoS ONE 2006, 1, e48. [Google Scholar] [CrossRef]
- Ansani, L.; Marchesini, J.; Pestelli, G.; Luisi, G.A.; Scillitani, G.; Longo, G.; Milani, D.; Serino, M.L.; Tisato, V.; Gemmati, D. F13A1 Gene Variant (V34L) and Residual Circulating FXIIIA Levels Predict Short- and Long-Term Mortality in Acute Myocardial Infarction after Coronary Angioplasty. Int. J. Mol. Sci. 2018, 19, 2766. [Google Scholar] [CrossRef]
- Zamboni, P.; Gemmati, D. Clinical implications of gene polymorphisms in venous leg ulcer: A model in tissue injury and reparative process. Thromb. Haemost. 2007, 98, 131–137. [Google Scholar]
- Zamboni, P.; De Mattei, M.; Ongaro, A.; Fogato, L.; Carandina, S.; De Palma, M.; Tognazzo, S.; Scapoli, G.L.; Serino, M.L.; Caruso, A.; et al. Factor XIII contrasts the effects of metalloproteinases in human dermal fibroblast cultured cells. Vasc. Endovasc. Surg. 2004, 38, 431–438. [Google Scholar] [CrossRef]
- Gemmati, D.; Tognazzo, S.; Serino, M.L.; Fogato, L.; Carandina, S.; De Palma, M.; Izzo, M.; De Mattei, M.; Ongaro, A.; Scapoli, G.L.; et al. Factor XIII V34L polymorphism modulates the risk of chronic venous leg ulcer progression and extension. Wound Repair. Regen. 2004, 12, 512–517. [Google Scholar] [CrossRef]
- Ngkelo, A.; Richart, A.; Kirk, J.A.; Bonnin, P.; Vilar, J.; Lemitre, M.; Marck, P.; Branchereau, M.; Le Gall, S.; Renault, N.; et al. Mast cells regulate myofilament calcium sensitization and heart function after myocardial infarction. J. Exp. Med. 2016, 213, 1353–1374. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, K.; Chen, F.; Liu, Q.; Ni, J.; Cao, W.; Hua, Y.; He, F.; Liu, Z.; Li, L.; et al. Role of the CCL2-CCR2 axis in cardiovascular disease: Pathogenesis and clinical implications. Front. Immunol. 2022, 13, 975367. [Google Scholar] [CrossRef]
- Chen, B.; Frangogiannis, N.G. Chemokines in Myocardial Infarction. J. Cardiovasc. Transl. Res. 2021, 14, 35–52. [Google Scholar] [CrossRef]
- van der Laan, A.M.; Nahrendorf, M.; Piek, J.J. Healing and adverse remodelling after acute myocardial infarction: Role of the cellular immune response. Heart 2012, 98, 1384–1390. [Google Scholar] [CrossRef]
- Nahrendorf, M.; Pittet, M.J.; Swirski, F.K. Monocytes: Protagonists of infarct inflammation and repair after myocardial infarction. Circulation 2010, 121, 2437–2445. [Google Scholar] [CrossRef]
- Frangogiannis, N.G.; Mendoza, L.H.; Ren, G.; Akrivakis, S.; Jackson, P.L.; Michael, L.H.; Smith, C.W.; Entman, M.L. MCSF expression is induced in healing myocardial infarcts and may regulate monocyte and endothelial cell phenotype. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H483–H492. [Google Scholar] [CrossRef]
- Bazzan, E.; Casara, A.; Radu, C.M.; Tine, M.; Biondini, D.; Faccioli, E.; Pezzuto, F.; Bernardinello, N.; Conti, M.; Balestro, E.; et al. Macrophages-derived Factor XIII links coagulation to inflammation in COPD. Front. Immunol. 2023, 14, 1131292. [Google Scholar] [CrossRef]
- Anzai, A.; Choi, J.L.; He, S.; Fenn, A.M.; Nairz, M.; Rattik, S.; McAlpine, C.S.; Mindur, J.E.; Chan, C.T.; Iwamoto, Y.; et al. The infarcted myocardium solicits GM-CSF for the detrimental oversupply of inflammatory leukocytes. J. Exp. Med. 2017, 214, 3293–3310. [Google Scholar] [CrossRef] [PubMed]
- Matthia, E.L.; Setteducato, M.L.; Elzeneini, M.; Vernace, N.; Salerno, M.; Kramer, C.M.; Keeley, E.C. Circulating Biomarkers in Hypertrophic Cardiomyopathy. J. Am. Heart Assoc. 2022, 11, e027618. [Google Scholar] [CrossRef]
- Lillo, R.; Graziani, F.; Franceschi, F.; Iannaccone, G.; Massetti, M.; Olivotto, I.; Crea, F.; Liuzzo, G. Inflammation across the spectrum of hypertrophic cardiac phenotypes. Heart Fail. Rev. 2023, 28, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.C.; Owens, A.P., 3rd; Sadayappan, S. Tissue-level inflammation and ventricular remodeling in hypertrophic cardiomyopathy. J. Thromb. Thrombolysis 2020, 49, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Weirather, J.; Hofmann, U.D.; Beyersdorf, N.; Ramos, G.C.; Vogel, B.; Frey, A.; Ertl, G.; Kerkau, T.; Frantz, S. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ. Res. 2014, 115, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Swirski, F.K. Regulating repair: Regulatory T cells in myocardial infarction. Circ. Res. 2014, 115, 7–9. [Google Scholar] [CrossRef]
- Xia, N.; Lu, Y.; Gu, M.; Li, N.; Liu, M.; Jiao, J.; Zhu, Z.; Li, J.; Li, D.; Tang, T.; et al. A Unique Population of Regulatory T Cells in Heart Potentiates Cardiac Protection From Myocardial Infarction. Circulation 2020, 142, 1956–1973. [Google Scholar] [CrossRef]
- Lu, Y.; Xia, N.; Cheng, X. Regulatory T Cells in Chronic Heart Failure. Front. Immunol. 2021, 12, 732794. [Google Scholar] [CrossRef]
- Delgobo, M.; Weiss, E.; Ashour, D.; Richter, L.; Popiolkowski, L.; Arampatzi, P.; Stangl, V.; Arias-Loza, P.; Mariotti-Ferrandiz, E.; Rainer, P.P.; et al. Myocardial Milieu Favors Local Differentiation of Regulatory T Cells. Circ. Res. 2023, 132, 565–582. [Google Scholar] [CrossRef]
- Wang, J.; Lin, B.; Zhang, Y.; Ni, L.; Hu, L.; Yang, J.; Xu, L.; Shi, D.; Chen, Y.H. The Regulatory Role of Histone Modification on Gene Expression in the Early Stage of Myocardial Infarction. Front. Cardiovasc. Med. 2020, 7, 594325. [Google Scholar] [CrossRef]
- Lei, I.; Tian, S.; Gao, W.; Liu, L.; Guo, Y.; Tang, P.; Chen, E.; Wang, Z. Acetyl-CoA production by specific metabolites promotes cardiac repair after myocardial infarction via histone acetylation. Elife 2021, 10, e60311. [Google Scholar] [CrossRef]
- Cohen, H.Y.; Miller, C.; Bitterman, K.J.; Wall, N.R.; Hekking, B.; Kessler, B.; Howitz, K.T.; Gorospe, M.; de Cabo, R.; Sinclair, D.A. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 2004, 305, 390–392. [Google Scholar] [CrossRef]
- Motta, M.C.; Divecha, N.; Lemieux, M.; Kamel, C.; Chen, D.; Gu, W.; Bultsma, Y.; McBurney, M.; Guarente, L. Mammalian SIRT1 represses forkhead transcription factors. Cell 2004, 116, 551–563. [Google Scholar] [CrossRef]
- Mattagajasingh, I.; Kim, C.S.; Naqvi, A.; Yamamori, T.; Hoffman, T.A.; Jung, S.B.; DeRicco, J.; Kasuno, K.; Irani, K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2007, 104, 14855–14860. [Google Scholar] [CrossRef]
- Shinozaki, S.; Chang, K.; Sakai, M.; Shimizu, N.; Yamada, M.; Tanaka, T.; Nakazawa, H.; Ichinose, F.; Yamada, Y.; Ishigami, A.; et al. Inflammatory stimuli induce inhibitory S-nitrosylation of the deacetylase SIRT1 to increase acetylation and activation of p53 and p65. Sci. Signal 2014, 7, ra106. [Google Scholar] [CrossRef]
- Hsu, C.P.; Zhai, P.; Yamamoto, T.; Maejima, Y.; Matsushima, S.; Hariharan, N.; Shao, D.; Takagi, H.; Oka, S.; Sadoshima, J. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation 2010, 122, 2170–2182. [Google Scholar] [CrossRef]
- Izarra, A.; Moscoso, I.; Levent, E.; Canon, S.; Cerrada, I.; Diez-Juan, A.; Blanca, V.; Nunez-Gil, I.J.; Valiente, I.; Ruiz-Sauri, A.; et al. miR-133a enhances the protective capacity of cardiac progenitors cells after myocardial infarction. Stem Cell Rep. 2014, 3, 1029–1042. [Google Scholar] [CrossRef]
- Han, F.; Chen, Q.; Su, J.; Zheng, A.; Chen, K.; Sun, S.; Wu, H.; Jiang, L.; Xu, X.; Yang, M.; et al. MicroRNA-124 regulates cardiomyocyte apoptosis and myocardial infarction through targeting Dhcr24. J. Mol. Cell. Cardiol. 2019, 132, 178–188. [Google Scholar] [CrossRef]
- Fan, F.; Sun, A.; Zhao, H.; Liu, X.; Zhang, W.; Jin, X.; Wang, C.; Ma, X.; Shen, C.; Zou, Y.; et al. MicroRNA-34a promotes cardiomyocyte apoptosis post myocardial infarction through down-regulating aldehyde dehydrogenase 2. Curr. Pharm. Des. 2013, 19, 4865–4873. [Google Scholar] [CrossRef]
- Xu, H.; Jin, L.; Chen, Y.; Li, J. Downregulation of microRNA-429 protects cardiomyocytes against hypoxia-induced apoptosis by increasing Notch1 expression. Int. J. Mol. Med. 2016, 37, 1677–1685. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Wa, M.; Liu, Z.; Hu, S. MicroRNA-101 Protects Against Cardiac Remodeling Following Myocardial Infarction via Downregulation of Runt-Related Transcription Factor 1. J. Am. Heart Assoc. 2019, 8, e013112. [Google Scholar] [CrossRef]
- Li, Q.; Gao, Y.; Zhu, J.; Jia, Q. MiR-101 Attenuates Myocardial Infarction-induced Injury by Targeting DDIT4 to Regulate Autophagy. Curr. Neurovascular Res. 2020, 17, 123–130. [Google Scholar] [CrossRef]
- Ding, S.; Liu, D.; Wang, L.; Wang, G.; Zhu, Y. Inhibiting MicroRNA-29a Protects Myocardial Ischemia-Reperfusion Injury by Targeting SIRT1 and Suppressing Oxidative Stress and NLRP3-Mediated Pyroptosis Pathway. J. Pharmacol. Exp. Ther. 2020, 372, 128–135. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, K.S.; Liu, L.; Li, S.L. MicroRNA-132 promotes oxidative stress-induced pyroptosis by targeting sirtuin 1 in myocardial ischaemia-reperfusion injury. Int. J. Mol. Med. 2020, 45, 1942–1950. [Google Scholar] [CrossRef]
- Huang, G.; Hao, F.; Hu, X. Downregulation of microRNA-155 stimulates sevoflurane-mediated cardioprotection against myocardial ischemia/reperfusion injury by binding to SIRT1 in mice. J. Cell Biochem. 2019, 120, 15494–15505. [Google Scholar] [CrossRef]
- Feng, Y.; Bao, Y.; Ding, J.; Li, H.; Liu, W.; Wang, X.; Guan, H.; Chen, Z. MicroRNA-130a attenuates cardiac fibrosis after myocardial infarction through TGF-beta/Smad signaling by directly targeting TGF-beta receptor 1. Bioengineered 2022, 13, 5779–5791. [Google Scholar] [CrossRef]
- Yu, B.T.; Yu, N.; Wang, Y.; Zhang, H.; Wan, K.; Sun, X.; Zhang, C.S. Role of miR-133a in regulating TGF-beta1 signaling pathway in myocardial fibrosis after acute myocardial infarction in rats. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8588–8597. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, S.; Wang, Y.; Jin, P.; Lu, F. lncRNA SRA1 Promotes the Activation of Cardiac Myofibroblasts Through Negative Regulation of miR-148b. DNA Cell Biol. 2019, 38, 385–394. [Google Scholar] [CrossRef]
- Yuan, X.; Pan, J.; Wen, L.; Gong, B.; Li, J.; Gao, H.; Tan, W.; Liang, S.; Zhang, H.; Wang, X. MiR-590-3p regulates proliferation, migration and collagen synthesis of cardiac fibroblast by targeting ZEB1. J. Cell Mol. Med. 2020, 24, 227–237. [Google Scholar] [CrossRef]
- Wang, X.; Morelli, M.B.; Matarese, A.; Sardu, C.; Santulli, G. Cardiomyocyte-derived exosomal microRNA-92a mediates post-ischemic myofibroblast activation both in vitro and ex vivo. ESC Heart Fail. 2020, 7, 284–288. [Google Scholar] [CrossRef]
- Morelli, M.B.; Shu, J.; Sardu, C.; Matarese, A.; Santulli, G. Cardiosomal microRNAs Are Essential in Post-Infarction Myofibroblast Phenoconversion. Int. J. Mol. Sci. 2019, 21, 201. [Google Scholar] [CrossRef]
- Tzavlaki, K.; Moustakas, A. TGF-β Signaling. Biomolecules 2020, 10, 487. [Google Scholar] [CrossRef]
- Dardik, R.; Loscalzo, J.; Eskaraev, R.; Inbal, A. Molecular mechanisms underlying the proangiogenic effect of factor XIII. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 526–532. [Google Scholar] [CrossRef]
- Dardik, R.; Solomon, A.; Loscalzo, J.; Eskaraev, R.; Bialik, A.; Goldberg, I.; Schiby, G.; Inbal, A. Novel proangiogenic effect of factor XIII associated with suppression of thrombospondin 1 expression. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1472–1477. [Google Scholar] [CrossRef]
- Bournazou, I.; Pound, J.D.; Duffin, R.; Bournazos, S.; Melville, L.A.; Brown, S.B.; Rossi, A.G.; Gregory, C.D. Apoptotic human cells inhibit migration of granulocytes via release of lactoferrin. J. Clin. Investig. 2009, 119, 20–32. [Google Scholar] [CrossRef]
- Soehnlein, O.; Lindbom, L. Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 2010, 10, 427–439. [Google Scholar] [CrossRef]
- Morimoto, H.; Takahashi, M. Role of monocyte chemoattractant protein-1 in myocardial infarction. Int. J. Biomed. Sci. 2007, 3, 159–167. [Google Scholar]
- Hanna, A.; Frangogiannis, N.G. The Role of the TGF-beta Superfamily in Myocardial Infarction. Front. Cardiovasc. Med. 2019, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. The mechanistic basis of infarct healing. Antioxid. Redox Signal. 2006, 8, 1907–1939. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Matricellular proteins in cardiac adaptation and disease. Physiol. Rev. 2012, 92, 635–688. [Google Scholar] [CrossRef]
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’Antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.A.; et al. Revisiting Cardiac Cellular Composition. Circ. Res. 2016, 118, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Tombor, L.S.; Dimmeler, S. Why is endothelial resilience key to maintain cardiac health? Basic. Res. Cardiol. 2022, 117, 35. [Google Scholar] [CrossRef]
- Ren, G.; Michael, L.H.; Entman, M.L.; Frangogiannis, N.G. Morphological characteristics of the microvasculature in healing myocardial infarcts. J. Histochem. Cytochem. 2002, 50, 71–79. [Google Scholar] [CrossRef]
- Tisato, V.; Zauli, G.; Rimondi, E.; Gianesini, S.; Brunelli, L.; Menegatti, E.; Zamboni, P.; Secchiero, P. Inhibitory effect of natural anti-inflammatory compounds on cytokines released by chronic venous disease patient-derived endothelial cells. Mediat. Inflamm. 2013, 2013, 423407. [Google Scholar] [CrossRef][Green Version]
- Cervellati, C.; Valacchi, G.; Tisato, V.; Zuliani, G.; Marsillach, J. Evaluating the link between Paraoxonase-1 levels and Alzheimer’s disease development. Minerva Med. 2019, 110, 238–250. [Google Scholar] [CrossRef]
- Gemmati, D.; Occhionorelli, S.; Tisato, V.; Vigliano, M.; Longo, G.; Gonelli, A.; Sibilla, M.G.; Serino, M.L.; Zamboni, P. Inherited genetic predispositions in F13A1 and F13B genes predict abdominal adhesion formation: Identification of gender prognostic indicators. Sci. Rep. 2018, 8, 16916. [Google Scholar] [CrossRef]
- Duong, T.E.; Hagood, J.S. Epigenetic Regulation of Myofibroblast Phenotypes in Fibrosis. Curr. Pathobiol. Rep. 2018, 6, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, V.; De Pascale, M.R.; Zullo, A.; Soricelli, A.; Infante, T.; Mancini, F.P.; Napoli, C. Evidence of epigenetic tags in cardiac fibrosis. J. Cardiol. 2017, 69, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Seok, Y.M.; Song, M.J.; Lee, H.A.; Kurz, T.; Kim, I. Histone deacetylase inhibition attenuates cardiac hypertrophy and fibrosis through acetylation of mineralocorticoid receptor in spontaneously hypertensive rats. Mol. Pharmacol. 2015, 87, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Shiojima, I.; Yefremashvili, M.; Luo, Z.; Kureishi, Y.; Takahashi, A.; Tao, J.; Rosenzweig, A.; Kahn, C.R.; Abel, E.D.; Walsh, K. Akt signaling mediates postnatal heart growth in response to insulin and nutritional status. J. Biol. Chem. 2002, 277, 37670–37677. [Google Scholar] [CrossRef] [PubMed]
- Catalucci, D.; Condorelli, G. Effects of Akt on cardiac myocytes: Location counts. Circ. Res. 2006, 99, 339–341. [Google Scholar] [CrossRef]
- Shen, T.; Ding, L.; Ruan, Y.; Qin, W.; Lin, Y.; Xi, C.; Lu, Y.; Dou, L.; Zhu, Y.; Cao, Y.; et al. SIRT1 functions as an important regulator of estrogen-mediated cardiomyocyte protection in angiotensin II-induced heart hypertrophy. Oxid. Med. Cell Longev. 2014, 2014, 713894. [Google Scholar] [CrossRef]
- Sundaresan, N.R.; Gupta, M.; Kim, G.; Rajamohan, S.B.; Isbatan, A.; Gupta, M.P. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J. Clin. Investig. 2009, 119, 2758–2771. [Google Scholar] [CrossRef]
- Tian, K.; Liu, Z.; Wang, J.; Xu, S.; You, T.; Liu, P. Sirtuin-6 inhibits cardiac fibroblasts differentiation into myofibroblasts via inactivation of nuclear factor kappaB signaling. Transl. Res. 2015, 165, 374–386. [Google Scholar] [CrossRef]
- Alique, M.; Bodega, G.; Giannarelli, C.; Carracedo, J.; Ramirez, R. MicroRNA-126 regulates Hypoxia-Inducible Factor-1alpha which inhibited migration, proliferation, and angiogenesis in replicative endothelial senescence. Sci. Rep. 2019, 9, 7381. [Google Scholar] [CrossRef]
- Soufi-Zomorrod, M.; Hajifathali, A.; Kouhkan, F.; Mehdizadeh, M.; Rad, S.M.; Soleimani, M. MicroRNAs modulating angiogenesis: miR-129-1 and miR-133 act as angio-miR in HUVECs. Tumour Biol. 2016, 37, 9527–9534. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, D.; Xu, C.; Duan, H. MicroRNA-139-5p inhibits vascular endothelial cell viability and serves as a diagnostic biomarker in acute myocardial infarction patients. Exp. Gerontol. 2021, 152, 111453. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Margariti, A.; Kelaini, S.; Cochrane, A.; Guha, S.T.; Hu, Y.; Stitt, A.W.; Zhang, L.; Xu, Q. MicroRNA-199b Modulates Vascular Cell Fate During iPS Cell Differentiation by Targeting the Notch Ligand Jagged1 and Enhancing VEGF Signaling. Stem Cells 2015, 33, 1405–1418. [Google Scholar] [CrossRef]
- Ranjan, P.; Kumari, R.; Goswami, S.K.; Li, J.; Pal, H.; Suleiman, Z.; Cheng, Z.; Krishnamurthy, P.; Kishore, R.; Verma, S.K. Myofibroblast-Derived Exosome Induce Cardiac Endothelial Cell Dysfunction. Front. Cardiovasc. Med. 2021, 8, 676267. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wang, X.; Ha, T.; Hu, Y.; Liu, L.; Zhang, X.; Yu, H.; Miao, J.; Kao, R.; Kalbfleisch, J.; et al. Attenuation of cardiac dysfunction and remodeling of myocardial infarction by microRNA-130a are mediated by suppression of PTEN and activation of PI3K dependent signaling. J. Mol. Cell. Cardiol. 2015, 89, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-T.; Xu, M.-G. Potential link between microRNA-208 and cardiovascular diseases. J. Xiangya Med. 2021, 6, 10–21037. [Google Scholar] [CrossRef]
- Wei, C.; Kim, I.K.; Kumar, S.; Jayasinghe, S.; Hong, N.; Castoldi, G.; Catalucci, D.; Jones, W.K.; Gupta, S. NF-kappaB mediated miR-26a regulation in cardiac fibrosis. J. Cell. Physiol. 2013, 228, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, B.J.; Chen, Q.; Yan, B.J.; Liu, Z.L. MicroRNA-29b upregulation improves myocardial fibrosis and cardiac function in myocardial infarction rats through targeting SH2B3. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10115–10122. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, R.; Wu, F.; Li, X. MicroRNA-208a Regulates H9c2 Cells Simulated Ischemia-Reperfusion Myocardial Injury via Targeting CHD9 through Notch/NF-kappa B Signal Pathways. Int. Heart J. 2018, 59, 580–588. [Google Scholar] [CrossRef]
- Zhu, J.N.; Chen, R.; Fu, Y.H.; Lin, Q.X.; Huang, S.; Guo, L.L.; Zhang, M.Z.; Deng, C.Y.; Zou, X.; Zhong, S.L.; et al. Smad3 inactivation and MiR-29b upregulation mediate the effect of carvedilol on attenuating the acute myocardium infarction-induced myocardial fibrosis in rat. PLoS ONE 2013, 8, e75557. [Google Scholar] [CrossRef]
- Shen, D.; He, Z. Mesenchymal stem cell-derived exosomes regulate the polarization and inflammatory response of macrophages via miR-21-5p to promote repair after myocardial reperfusion injury. Ann. Transl. Med. 2021, 9, 1323. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Ma, J.; Zhang, J.; Guo, X.; Liu, Q.; Yang, Z. MicroRNA-150 deficiency accelerates intimal hyperplasia by acting as a novel regulator of macrophage polarization. Life Sci. 2020, 240, 116985. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, X.; Hu, J.; Chen, F.; Qiao, S.; Sun, X.; Gao, L.; Xie, J.; Xu, B. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc. Res. 2019, 115, 1205–1216. [Google Scholar] [CrossRef]
- Zhu, F.; Chen, Y.; Li, J.; Yang, Z.; Lin, Y.; Jiang, B.; Shao, L.; Hu, S.; Shen, Z. Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Attenuate Myocardial Infarction Injury via miR-24-3p-Promoted M2 Macrophage Polarization. Adv. Biol. 2022, 6, e2200074. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Ikeda, G.; Tada, Y.; von Bornstadt, D.; Santoso, M.R.; Wahlquist, C.; Rhee, S.; Jeon, Y.J.; Yu, A.C.; O’Brien, C.G.; et al. miR-106a-363 cluster in extracellular vesicles promotes endogenous myocardial repair via Notch3 pathway in ischemic heart injury. Basic. Res. Cardiol. 2021, 116, 19. [Google Scholar] [CrossRef]
- Garikipati, V.N.S.; Verma, S.K.; Jolardarashi, D.; Cheng, Z.; Ibetti, J.; Cimini, M.; Tang, Y.; Khan, M.; Yue, Y.; Benedict, C.; et al. Therapeutic inhibition of miR-375 attenuates post-myocardial infarction inflammatory response and left ventricular dysfunction via PDK-1-AKT signalling axis. Cardiovasc. Res. 2017, 113, 938–949. [Google Scholar] [CrossRef]
- Zimmerman, S.D.; Thomas, D.P.; Velleman, S.G.; Li, X.; Hansen, T.R.; McCormick, R.J. Time course of collagen and decorin changes in rat cardiac and skeletal muscle post-MI. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H1816–H1822. [Google Scholar] [CrossRef]
- Alex, L.; Frangogiannis, N.G. Pericytes in the infarcted heart. Vasc. Biol. 2019, 1, H23–H31. [Google Scholar] [CrossRef][Green Version]
- Hinz, B. Formation and function of the myofibroblast during tissue repair. J. Investig. Dermatol. 2007, 127, 526–537. [Google Scholar] [CrossRef]
- Fu, X.; Khalil, H.; Kanisicak, O.; Boyer, J.G.; Vagnozzi, R.J.; Maliken, B.D.; Sargent, M.A.; Prasad, V.; Valiente-Alandi, I.; Blaxall, B.C.; et al. Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J. Clin. Investig. 2018, 128, 2127–2143. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The extracellular matrix in myocardial injury, repair, and remodeling. J. Clin. Investig. 2017, 127, 1600–1612. [Google Scholar] [CrossRef]
- Zymek, P.; Bujak, M.; Chatila, K.; Cieslak, A.; Thakker, G.; Entman, M.L.; Frangogiannis, N.G. The role of platelet-derived growth factor signaling in healing myocardial infarcts. J. Am. Coll. Cardiol. 2006, 48, 2315–2323. [Google Scholar] [CrossRef]
- Hellstrom, M.; Kalen, M.; Lindahl, P.; Abramsson, A.; Betsholtz, C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 1999, 126, 3047–3055. [Google Scholar] [CrossRef]
- Felician, G.; Collesi, C.; Lusic, M.; Martinelli, V.; Ferro, M.D.; Zentilin, L.; Zacchigna, S.; Giacca, M. Epigenetic modification at Notch responsive promoters blunts efficacy of inducing notch pathway reactivation after myocardial infarction. Circ. Res. 2014, 115, 636–649. [Google Scholar] [CrossRef]
- Kraus, L. Targeting Epigenetic Regulation of Cardiomyocytes through Development for Therapeutic Cardiac Regeneration after Heart Failure. Int. J. Mol. Sci. 2022, 23, 11878. [Google Scholar] [CrossRef] [PubMed]
- Boulet, J.; Mehra, M.R. Left Ventricular Reverse Remodeling in Heart Failure: Remission to Recovery. Struct. Heart 2021, 5, 466–481. [Google Scholar] [CrossRef]
- Millett, E.R.C.; Peters, S.A.E.; Woodward, M. Sex differences in risk factors for myocardial infarction: Cohort study of UK Biobank participants. BMJ 2018, 363, k4247. [Google Scholar] [CrossRef]
- Vaccarezza, M.; Papa, V.; Milani, D.; Gonelli, A.; Secchiero, P.; Zauli, G.; Gemmati, D.; Tisato, V. Sex/Gender-Specific Imbalance in CVD: Could Physical Activity Help to Improve Clinical Outcome Targeting CVD Molecular Mechanisms in Women? Int. J. Mol. Sci. 2020, 21, 1477. [Google Scholar] [CrossRef]
- Stehli, J.; Duffy, S.J.; Burgess, S.; Kuhn, L.; Gulati, M.; Chow, C.; Zaman, S. Sex Disparities in Myocardial Infarction: Biology or Bias? Heart Lung Circ. 2021, 30, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, C.; Bonaccorsi, G.; Bergamini, C.M.; Fila, E.; Greco, P.; Valacchi, G.; Massari, L.; Gonelli, A.; Tisato, V. Association between circulatory levels of adipokines and bone mineral density in postmenopausal women. Menopause 2016, 23, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, C.; Bulla, R.; Tisato, V.; De Seta, F.; Alberico, S.; Secchiero, P.; Zauli, G. Soluble TRAIL is elevated in recurrent miscarriage and inhibits the in vitro adhesion and migration of HTR8 trophoblastic cells. Hum. Reprod. 2012, 27, 2941–2947. [Google Scholar] [CrossRef]
- Bertelsen, D.M.; Neergaard, J.S.; Bager, C.L.; Nielsen, S.H.; Secher, N.H.; Svendsen, J.H.; Bihlet, A.R.; Andersen, J.R.; Karsdal, M.A.; Christiansen, C.; et al. Matrix Metalloproteinase Mediated Type I Collagen Degradation is an Independent Predictor of Increased Risk of Acute Myocardial Infarction in Postmenopausal Women. Sci. Rep. 2018, 8, 5371. [Google Scholar] [CrossRef]
- Haug, E.B.; Horn, J.; Markovitz, A.R.; Fraser, A.; Klykken, B.; Dalen, H.; Vatten, L.J.; Romundstad, P.R.; Rich-Edwards, J.W.; Asvold, B.O. Association of Conventional Cardiovascular Risk Factors With Cardiovascular Disease After Hypertensive Disorders of Pregnancy: Analysis of the Nord-Trondelag Health Study. JAMA Cardiol. 2019, 4, 628–635. [Google Scholar] [CrossRef]
- Retnakaran, R.; Shah, B.R. Glucose screening in pregnancy and future risk of cardiovascular disease in women: A retrospective, population-based cohort study. Lancet Diabetes Endocrinol. 2019, 7, 378–384. [Google Scholar] [CrossRef]
- Brush, J.E., Jr.; Krumholz, H.M.; Greene, E.J.; Dreyer, R.P. Sex Differences in Symptom Phenotypes Among Patients With Acute Myocardial Infarction. Circ. Cardiovasc. Qual. Outcomes 2020, 13, e005948. [Google Scholar] [CrossRef]
- Crea, F.; Battipaglia, I.; Andreotti, F. Sex differences in mechanisms, presentation and management of ischaemic heart disease. Atherosclerosis 2015, 241, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Asleh, R.; Manemann, S.M.; Weston, S.A.; Bielinski, S.J.; Chamberlain, A.M.; Jiang, R.; Gerber, Y.; Roger, V.L. Sex Differences in Outcomes After Myocardial Infarction in the Community. Am. J. Med. 2021, 134, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Gemmati, D.; Tisato, V. Chapter 24—Genomic and epigenomic signature at the branch-point among genome, phenome, and sexome in health and disease: A multiomics approach. In Principles of Gender-Specific Medicine, 4th ed.; Legato, M.J., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 393–408. [Google Scholar] [CrossRef]
- Gemmati, D.; Tisato, V. Chapter 15—Genetics and epigenetics of the one-carbon metabolism pathway in autism spectrum disorder: Role of a sex-specific brain epigenome. In Sex, Gender, and Epigenetics; Legato, M.J., Feldberg, D., Glezerman, M., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 181–191. [Google Scholar] [CrossRef]
- Tisato, V.; Silva, J.A.; Longo, G.; Gallo, I.; Singh, A.V.; Milani, D.; Gemmati, D. Genetics and Epigenetics of One-Carbon Metabolism Pathway in Autism Spectrum Disorder: A Sex-Specific Brain Epigenome? Genes 2021, 12, 782. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Doss, C.G.P.; Lee, S.S. Therapeutic miRNA and siRNA: Moving from Bench to Clinic as Next Generation Medicine. Mol. Ther. Nucleic Acids 2017, 8, 132–143. [Google Scholar] [CrossRef]
- Dasgupta, I.; Chatterjee, A. Recent Advances in miRNA Delivery Systems. Methods Protoc. 2021, 4, 10. [Google Scholar] [CrossRef]
- Hullinger, T.G.; Montgomery, R.L.; Seto, A.G.; Dickinson, B.A.; Semus, H.M.; Lynch, J.M.; Dalby, C.M.; Robinson, K.; Stack, C.; Latimer, P.A.; et al. Inhibition of miR-15 protects against cardiac ischemic injury. Circ. Res. 2012, 110, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, W.; Xu, R.; Nie, Y.; Cao, X.; Meng, J.; Xu, X.; Hu, S.; Zheng, Z. MicroRNA-24 regulates cardiac fibrosis after myocardial infarction. J. Cell Mol. Med. 2012, 16, 2150–2160. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Yang, Z.; Sayed, D.; He, M.; Gao, S.; Lin, L.; Yoon, S.; Abdellatif, M. GATA4 expression is primarily regulated via a miR-26b-dependent post-transcriptional mechanism during cardiac hypertrophy. Cardiovasc. Res. 2012, 93, 645–654. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, M.; Li, C.; Zhou, J.; Li, H.; Zhu, D.; Wang, Z.; Chen, A.; Zhao, Q. MicroRNA-92a inhibition attenuates hypoxia/reoxygenation-induced myocardiocyte apoptosis by targeting Smad7. PLoS ONE 2014, 9, e100298. [Google Scholar] [CrossRef]
- Liang, W.; Guo, J.; Li, J.; Bai, C.; Dong, Y. Downregulation of miR-122 attenuates hypoxia/reoxygenation (H/R)-induced myocardial cell apoptosis by upregulating GATA-4. Biochem. Biophys. Res. Commun. 2016, 478, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Qin, G.; Yang, L.; Xiang, D.; Li, S. LncRNA XIST regulates myocardial infarction by targeting miR-130a-3p. J. Cell Physiol. 2019, 234, 8659–8667. [Google Scholar] [CrossRef]
- Zhao, Z.; Du, S.; Shen, S.; Wang, L. microRNA-132 inhibits cardiomyocyte apoptosis and myocardial remodeling in myocardial infarction by targeting IL-1beta. J. Cell Physiol. 2020, 235, 2710–2721. [Google Scholar] [CrossRef]
- Gong, X.; Zhu, Y.; Chang, H.; Li, Y.; Ma, F. Long noncoding RNA MALAT1 promotes cardiomyocyte apoptosis after myocardial infarction via targeting miR-144-3p. Biosci. Rep. 2019, 39, BSR20191103. [Google Scholar] [CrossRef]
- Li, R.; Yan, G.; Li, Q.; Sun, H.; Hu, Y.; Sun, J.; Xu, B. MicroRNA-145 protects cardiomyocytes against hydrogen peroxide (H(2)O(2))-induced apoptosis through targeting the mitochondria apoptotic pathway. PLoS ONE 2012, 7, e44907. [Google Scholar] [CrossRef]
- Yang, L.; Wang, B.; Zhou, Q.; Wang, Y.; Liu, X.; Liu, Z.; Zhan, Z. MicroRNA-21 prevents excessive inflammation and cardiac dysfunction after myocardial infarction through targeting KBTBD7. Cell Death Dis. 2018, 9, 769. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Zhan, H.; Zhou, X.Y.; Yao, L.; Yan, M.; Chen, A.; Liu, J.; Ren, X.; Zhang, X.; Liu, J.X.; et al. MicroRNA-22 regulates inflammation and angiogenesis via targeting VE-cadherin. FEBS Lett. 2017, 591, 513–526. [Google Scholar] [CrossRef]
- Yuan, H.; Du, S.; Deng, Y.; Xu, X.; Zhang, Q.; Wang, M.; Wang, P.; Su, Y.; Liang, X.; Sun, Y.; et al. Effects of microRNA-208a on inflammation and oxidative stress in ketamine-induced cardiotoxicity through Notch/NF-kappaB signal pathways by CHD9. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Valkov, N.; King, M.E.; Moeller, J.; Liu, H.; Li, X.; Zhang, P. MicroRNA-1-Mediated Inhibition of Cardiac Fibroblast Proliferation Through Targeting Cyclin D2 and CDK6. Front. Cardiovasc. Med. 2019, 6, 65. [Google Scholar] [CrossRef]
- Tijsen, A.J.; van der Made, I.; van den Hoogenhof, M.M.; Wijnen, W.J.; van Deel, E.D.; de Groot, N.E.; Alekseev, S.; Fluiter, K.; Schroen, B.; Goumans, M.J.; et al. The microRNA-15 family inhibits the TGFbeta-pathway in the heart. Cardiovasc. Res. 2014, 104, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Wang, K.; Liu, Y.; Lv, D.; Zheng, B.; Zhou, Q.; Sun, Q.; Chen, P.; Ding, S.; Xu, Y.; et al. miR-19b controls cardiac fibroblast proliferation and migration. J. Cell Mol. Med. 2016, 20, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Chen, H.; Ge, D.; Xu, Y.; Xu, H.; Yang, Y.; Gu, M.; Zhou, Y.; Zhu, J.; Ge, T.; et al. miR-21 Promotes Cardiac Fibrosis After Myocardial Infarction Via Targeting Smad7. Cell Physiol. Biochem. 2017, 42, 2207–2219. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Shi, P.; Ge, J.J. miR-21 enhances cardiac fibrotic remodeling and fibroblast proliferation via CADM1/STAT3 pathway. BMC Cardiovasc. Disord. 2017, 17, 88. [Google Scholar] [CrossRef]
- Zhou, X.L.; Xu, H.; Liu, Z.B.; Wu, Q.C.; Zhu, R.R.; Liu, J.C. miR-21 promotes cardiac fibroblast-to-myofibroblast transformation and myocardial fibrosis by targeting Jagged1. J. Cell Mol. Med. 2018, 22, 3816–3824. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, V.; Rai, R.; Place, A.T.; Murphy, S.B.; Verma, S.K.; Ghosh, A.K.; Vaughan, D.E. MiR-125b Is Critical for Fibroblast-to-Myofibroblast Transition and Cardiac Fibrosis. Circulation 2016, 133, 291–301. [Google Scholar] [CrossRef]
- Yuan, X.; Pan, J.; Wen, L.; Gong, B.; Li, J.; Gao, H.; Tan, W.; Liang, S.; Zhang, H.; Wang, X. MiR-144-3p Enhances Cardiac Fibrosis After Myocardial Infarction by Targeting PTEN. Front. Cell Dev. Biol. 2019, 7, 249. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Li, H.; Cao, H.; Dong, Y.; Gao, L.; Liu, Z.; Ge, J.; Zhu, H. Therapeutic silencing miR-146b-5p improves cardiac remodeling in a porcine model of myocardial infarction by modulating the wound reparative phenotype. Protein Cell 2021, 12, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Yan, X.; Yan, L.; Hu, F.; Ma, W.; Wang, Y.; Lu, S.; Zeng, Q.; Wang, Z. Inhibition of microRNA-155 ameliorates cardiac fibrosis in the process of angiotensin II-induced cardiac remodeling. Mol. Med. Rep. 2017, 16, 7287–7296. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Yu, H.; Zhang, Y.; Li, Z.; Gao, W. MicroRNA-214 Mediates Isoproterenol-induced Proliferation and Collagen Synthesis in Cardiac Fibroblasts. Sci. Rep. 2015, 5, 18351. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Deng, Y.; Li, H. MicroRNA-223 Regulates Cardiac Fibrosis After Myocardial Infarction by Targeting RASA1. Cell Physiol. Biochem. 2018, 46, 1439–1454. [Google Scholar] [CrossRef]
- Iaconetti, C.; Polimeni, A.; Sorrentino, S.; Sabatino, J.; Pironti, G.; Esposito, G.; Curcio, A.; Indolfi, C. Inhibition of miR-92a increases endothelial proliferation and migration in vitro as well as reduces neointimal proliferation in vivo after vascular injury. Basic. Res. Cardiol. 2012, 107, 296. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yin, Y.L.; Guo, T.; Sun, X.Y.; Ma, H.; Zhu, M.L.; Zhao, F.R.; Xu, P.; Chen, Y.; Wan, G.R.; et al. Inhibition of Aberrant MicroRNA-133a Expression in Endothelial Cells by Statin Prevents Endothelial Dysfunction by Targeting GTP Cyclohydrolase 1 in Vivo. Circulation 2016, 134, 1752–1765. [Google Scholar] [CrossRef]
- Bernardo, B.C.; Gao, X.M.; Winbanks, C.E.; Boey, E.J.; Tham, Y.K.; Kiriazis, H.; Gregorevic, P.; Obad, S.; Kauppinen, S.; Du, X.J.; et al. Therapeutic inhibition of the miR-34 family attenuates pathological cardiac remodeling and improves heart function. Proc. Natl. Acad. Sci. USA 2012, 109, 17615–17620. [Google Scholar] [CrossRef]
- Huang, Y.; Qi, Y.; Du, J.Q.; Zhang, D.F. MicroRNA-34a regulates cardiac fibrosis after myocardial infarction by targeting Smad4. Expert Opin. Ther. Targets 2014, 18, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Liu, H.; Gao, W.; Zhang, L.; Ye, Y.; Yuan, L.; Ding, Z.; Wu, J.; Kang, L.; Zhang, X.; et al. MicroRNA-378 suppresses myocardial fibrosis through a paracrine mechanism at the early stage of cardiac hypertrophy following mechanical stress. Theranostics 2018, 8, 2565–2582. [Google Scholar] [CrossRef]
- Goto, S.; Ichihara, G.; Katsumata, Y.; Ko, S.; Anzai, A.; Shirakawa, K.; Endo, J.; Kataoka, M.; Moriyama, H.; Hiraide, T.; et al. Time-Series Transcriptome Analysis Reveals the miR-27a-5p-Ppm1l Axis as a New Pathway Regulating Macrophage Alternative Polarization After Myocardial Infarction. Circ. J. 2021, 85, 929–938. [Google Scholar] [CrossRef]
- Chen, C.; Cai, S.; Wu, M.; Wang, R.; Liu, M.; Cao, G.; Dong, M.; Yiu, K.H. Role of Cardiomyocyte-Derived Exosomal MicroRNA-146a-5p in Macrophage Polarization and Activation. Dis. Markers 2022, 2022, 2948578. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, Q.; He, S.; Yang, M.; Maguire, E.M.; An, W.; Afzal, T.A.; Luong, L.A.; Zhang, L.; Xiao, Q. miR-22 Is a Novel Mediator of Vascular Smooth Muscle Cell Phenotypic Modulation and Neointima Formation. Circulation 2018, 137, 1824–1841. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Zhu, L.; Ruan, Z.; Wang, R.; Shen, Y. MicroRNA-122 promotes cardiomyocyte hypertrophy via targeting FoxO3. Biochem. Biophys. Res. Commun. 2019, 519, 682–688. [Google Scholar] [CrossRef]
- Wang, G.; Wang, R.; Ruan, Z.; Liu, L.; Li, Y.; Zhu, L. MicroRNA-132 attenuated cardiac fibrosis in myocardial infarction-induced heart failure rats. Biosci. Rep. 2020, 40, BSR20201696. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, X.; Yang, J.; Lin, Y.; Xu, D.Z.; Lu, Q.; Deitch, E.A.; Huo, Y.; Delphin, E.S.; Zhang, C. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ. Res. 2009, 105, 158–166. [Google Scholar] [CrossRef]
- Seok, H.Y.; Chen, J.; Kataoka, M.; Huang, Z.P.; Ding, J.; Yan, J.; Hu, X.; Wang, D.Z. Loss of MicroRNA-155 protects the heart from pathological cardiac hypertrophy. Circ. Res. 2014, 114, 1585–1595. [Google Scholar] [CrossRef]
- Das, S.; Kohr, M.; Dunkerly-Eyring, B.; Lee, D.I.; Bedja, D.; Kent, O.A.; Leung, A.K.; Henao-Mejia, J.; Flavell, R.A.; Steenbergen, C. Divergent Effects of miR-181 Family Members on Myocardial Function Through Protective Cytosolic and Detrimental Mitochondrial microRNA Targets. J. Am. Heart Assoc. 2017, 6, e004694. [Google Scholar] [CrossRef]
- Ucar, A.; Gupta, S.K.; Fiedler, J.; Erikci, E.; Kardasinski, M.; Batkai, S.; Dangwal, S.; Kumarswamy, R.; Bang, C.; Holzmann, A.; et al. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat. Commun. 2012, 3, 1078. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lv, L.; Liang, R.; Guo, W.; Liao, Z.; Chen, Y.; Zhu, K.; Huang, R.; Zhao, H.; Pu, Q.; et al. miR-486 improves fibrotic activity in myocardial infarction by targeting SRSF3/p21-Mediated cardiac myofibroblast senescence. J. Cell Mol. Med. 2022, 26, 5135–5149. [Google Scholar] [CrossRef]
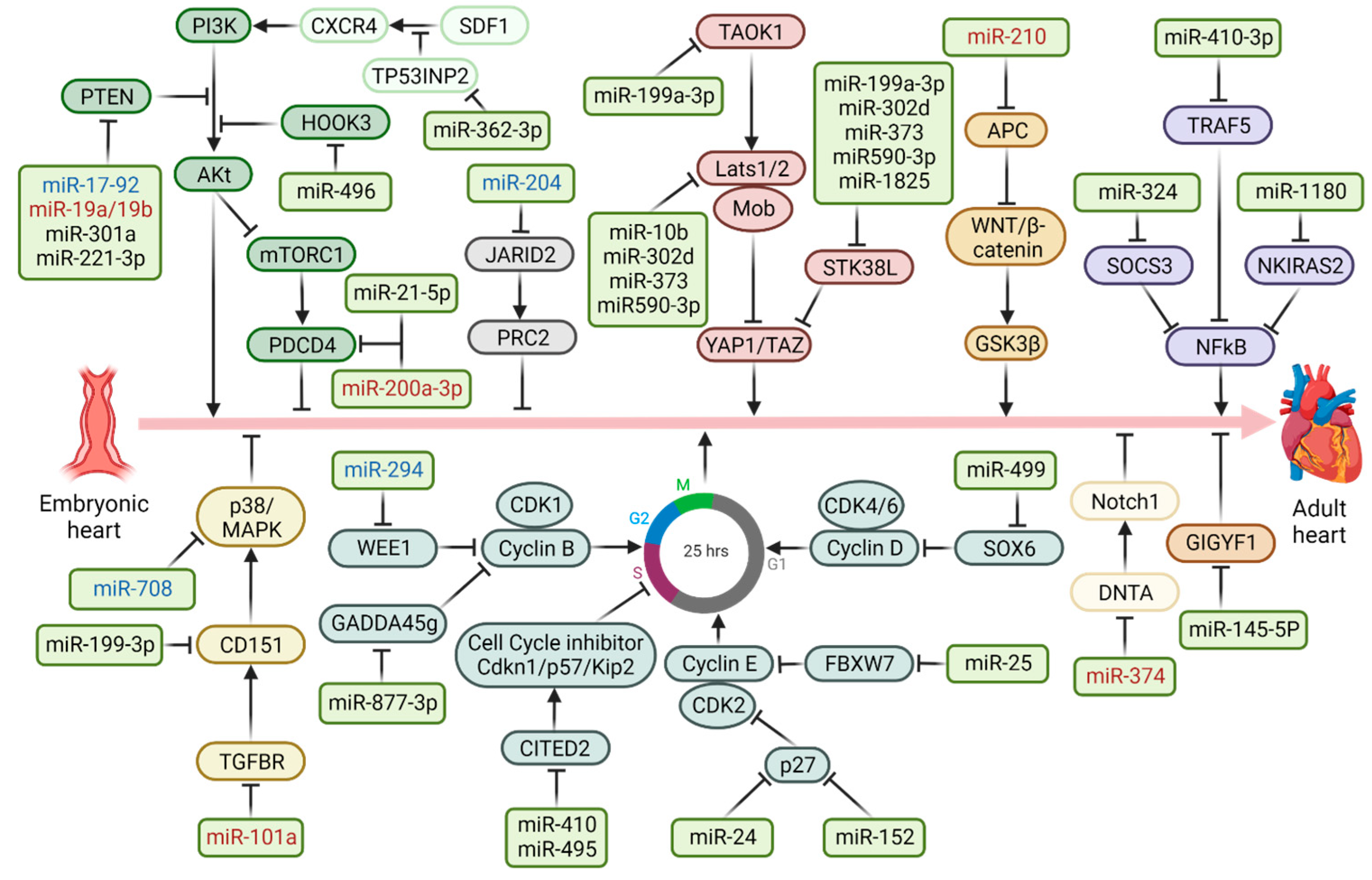
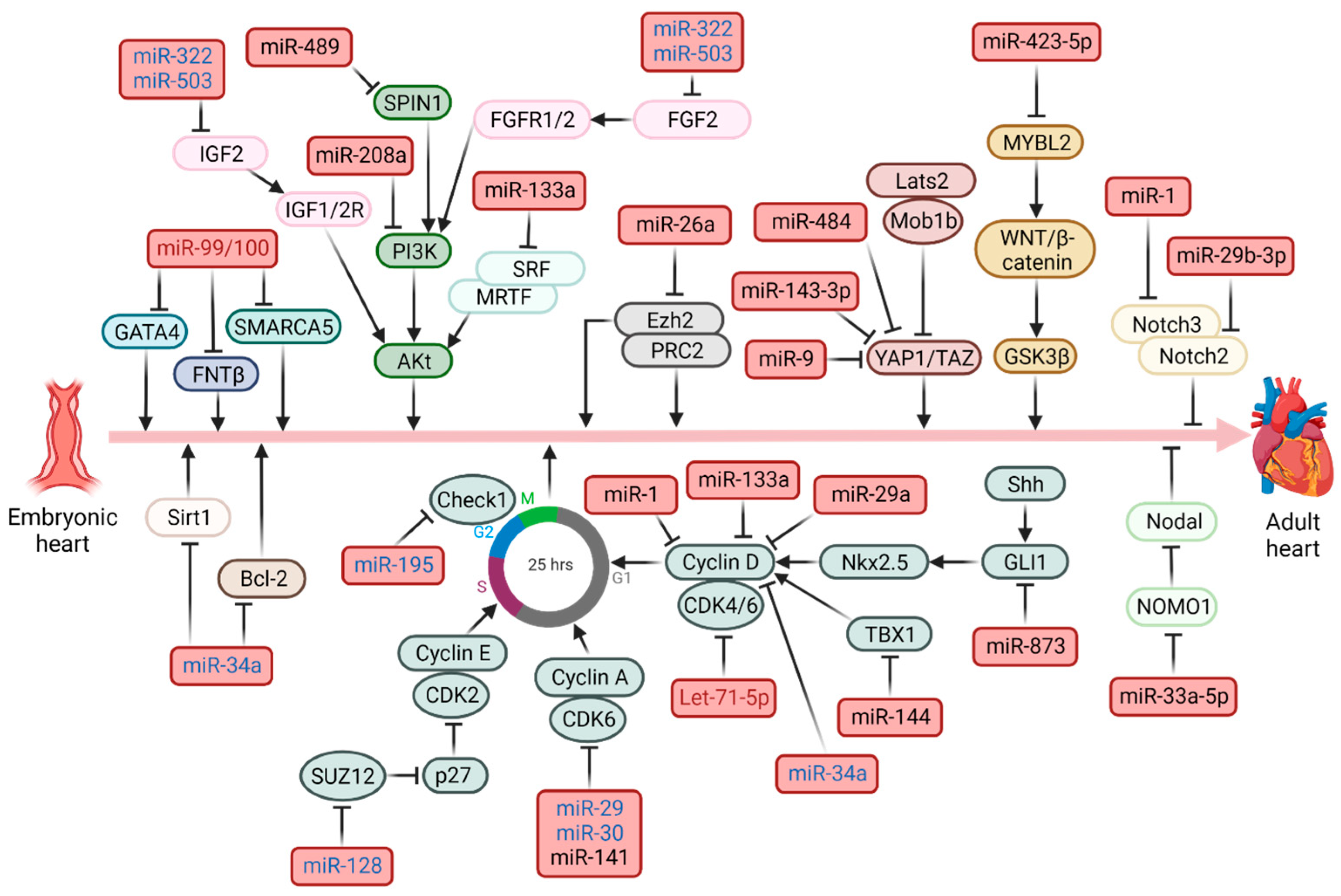


| miRNA | Model | Targets | Pathway | Effects | Mechanism(s) | Ref. |
|---|---|---|---|---|---|---|
| miR-10b | Hu | Lats1 | Hippo | ↓apoptosis | miR-10b blocks the expression of Lats1 and thereby inhibits the Hippo pathway | [45] |
| miR-17-92 | M; R | PTEN | Akt | ↑proliferation | miR-17-92 represses PTEN-inducing cardiomyocyte proliferation mediated by the AKt signaling pathway | [33] |
| miR19a/19b | M | PTEN | Akt | ↑proliferation | miR-19a/19b blocks the expression of PTEN inducing cardiomyocyte proliferation mediated by the AKt signaling | [34] |
| miR-21-5p | Hu | PDCD4/ PTEN | Akt | ↑proliferation | miR-21-5p directly represses PCDN4 e PTEN-inducing cardiomyocyte proliferation mediated by the AKt signaling | [46] |
| miR-24 | R | p27 | Cell cycle | ↑proliferation | miR-24 promotes cells in G0/G1 phase into S phase by repressing p27 expression | [47] |
| miR-25 | Hu; Zb | FBXW7 | Cell cycle | ↑proliferation | miR-25 inhibits FBXW7, a cell-cycle regulatory factor that mediates the proteolysis of positive cell-cycle regulators | [48] |
| miR-101a | Hu | TGFBR1 | MAPK | ↑proliferation | miR-101a blocks the expression of TGFBR1 and thereby activates the MAPK signaling pathway | [49] |
| miR-145-5p | R | GIGYF1 | / | ↓apoptosis | miR-145-5p inhibits GIGYF1, a regulator of mRNA turnover, blocking translation and activating transcript decay | [50] |
| miR-152 | M | p27 | Hippo | / | The increase of miR-152 by the activation of YAP1 represses the expression of p27 | [51] |
| miR-199a-3p | R | CD151 | MAPK | ↑proliferation | miR-199a-3p inhibits the tetraspanin CD151, involved in several cellular process | [52] |
| miR-199a-3p | R | TAOK1 | Hippo | ↓apoptosis | miR-199a-3p represses Serine/threonine-protein kinase TAOK1 involved in the Hippo pathway | [53] |
| miR-199a-3p; -302d; -373; -590-3p; -1825 | R | STK38L | Hippo | ↓apoptosis ↑proliferation | They downregulate STK38L, a direct targeting on the 3’UTR of the related mRNA has not been identified | [53] |
| miR-200a-3p | Hu; M | PDCD4 | Akt | ↑proliferation | miR-200a-3p represses PCDN4 activating AKt signaling | [54] |
| miR-204 | R | Jarid2 | / | ↑proliferation | miR-204 downregulates Jarid2, a non-catalytic member of the polycomb repressive complex 2 | [42] |
| miR-210 | M | APC/β-catenin | WNT | ↑proliferation | miR-210 represses the cell cycle inhibitor APC, involved in the WNT pathway | [55] |
| miR-221-3p | R | PTEN | Akt | ↑proliferation | It enhances Akt kinase activity by inhibiting PTEN | [35] |
| miR-294 | M | Wee1 | Cell cycle | / | miR-294 inhibits Wee1, increasing the activity of the cyclin B1/CDK1 | [56] |
| miR-301a | M; R | PTEN | Akt | ↑proliferation | PTEN is a direct target of miR-301a in regulating cardiomyocyte proliferation | [57] |
| miR-302d; -373; -590-3p | Hu | LATS2 | Hippo | ↓apoptosis ↑proliferation | miR-302d, miR-373 and miR590-3p target LATS2, inhibiting the Hippo pathway | [58] [53] |
| miR-324 | Hu | SOCS3 | NFkB | ↑proliferation ↓apoptosis | miR-32a represses SOCS3, activating the NFkB pathway | [38] |
| miR-362-3p | R | TP53INP2 | SDF1/ CXCR4 | ↑proliferation ↓apoptosis | miR-362-3p downregulates TP53INP2, activating at the same time the SDF-1/CXCR4 pathway | [59] |
| miR-374 | M | DTNA | Notch | ↑viability ↓apoptosis | miR-374 inhibits DTNA and the Notch1 axis | [60] |
| miR-410; -495 | M; R | Cited2 | Cell cycle | ↑proliferation | miR-410 and miR-495 downregulate the transcriptional coactivator Cited2 | [61] |
| miR-410-3p | Hu | TRAF5 | NFkB | ↓apoptosis | miR-410-3p protects CMs from apoptosis by repressing TRAF5 expression | [62] |
| miR-496 | R | HOOK3 | Akt | ↓apoptosis ↑proliferation | miR-496 binds to Hook3 to inhibit the activation of PI3K/Akt/mTOR signalling pathway | [63] |
| miR-499 | M; R | SOX6 | Cell cycle | ↑proliferation ↓apoptosis | miR-499 promotes cell proliferation and inhibits apoptosis in the late stage of cardiac differentiation targeting SOX6 and leading to activation of cyclin D1 | [29] |
| miR-708 | M; R | MAPK14 | MAPK | ↑proliferation | miR-708 inhibits the expression of MAPK14, which arrests the cell cycle in CMs | [64] |
| miR-877-3p | M | GADD45g | Cell cycle | ↑proliferation | LED-Red irradiation increases miR-877-3p expression promoting proliferation of neonatal CMs via GADD45g | [65] |
| miR-1180 | R | NKIRAS2 | NFkB | ↑proliferation ↑viability ↓apoptosis | miR-1180 represses the NF-κB inhibitor interacting with Ras-like 2 (NKIRA2), activating the NFkB pathway | [39] |
| miRNA | Model | Targets | Pathway | Effects | Mechanism(s) | Ref. |
|---|---|---|---|---|---|---|
| Let-71-5p | M | E2F2–CCND2 | Cell cycle | ↓proliferation | Let-7i-5p downregulates the expression of E2F2 and CCND2 and further represses CM proliferation | [66] |
| miR-1 | M | CCND1 | Cell cycle | ↓proliferation | It directly suppresses the cell-cycle regulator CCND1 | [67] |
| miR-1 | R | NOTCH3 | Notch | ↑apoptosis ↓proliferation ↓autophagy | miR-1 suppresses NOTCH3 thereby negatively regulating the Notch pathway | [68] |
| miR-9 | R | YAP1 | Hippo | ↑apoptosis | miR-9 promotes hypoxia-induced cardiomyocyte apoptosis by targeting Yap1 | [69] |
| miR-26a | R, Zb | Ezh2 | / | ↓proliferation | miR-26a targets activators of the cell cycle and Ezh2, a component of PRC2, with repressive functions on negative regulators of the cell cycle | [43] |
| miR29a | R | CCND2 | Cell cycle | ↓proliferation | miE29a inhibits proliferation by acting on cyclin D2 | [70] |
| miR-29a; -30a; -141 | R | CCNA2–CDK6 | Cell cycle | ↓proliferation | miR-29a, miR-30a and miR-141 reduce expression of Cyclin A2, reducing cell proliferation | [71] |
| miR29b-3p | R; Zb | NOTCH2 | Notch | ↓proliferation | miR29b-3p inhibits CMs proliferation by targeting NOTCH2 | [72] |
| miR-33a-5p | Hu | NOMO1 | Nodal | ↓proliferation ↑apoptosis ↓differentiation | miR-33a-5p targets NOMO1, a component of a protein complex involved in Nodal signaling leading to apoptosis | [41] |
| miR-34a | M; R | Bcl2, Sirt1, CCND1 | / | ↓proliferation ↑apoptosis | miR-34a directly regulated cell cycle and death via modulation of its targets such as Bcl2, Cyclin D1, and Sirt1 | [73] |
| miR-128 | R | SUZ12 | Cell cycle | ↓proliferation | miR-128 downregulates SUZ12, which cannot suppress p27 expression and activate the positive cell cycle regulators Cyclin E and CDK2 | [74] |
| miR-133a | M | CCND2 SRF | Cell cycle Akt | ↓proliferation | miR-133 functions as an inhibitor of cardiomyocyte proliferation, targeting cyclin D2 and SRF. | [75] |
| miR-143-3p | M | YAP–Ctnnd1 | Hippo | ↑apoptosis | miR-143-3p inhibited the mitosis of CMs, targeting YAP and Ctnnd1 | [76] |
| miR-144 | R | TBX1 | Jak2/Stat1 | ↑apoptosis | miR-144 leads to proliferation via TBX1/JAK2/STAT1 axis | [77] |
| miR-195 | M | Chek1 | Cell cycle | ↓proliferation | miR-195 regulates the expression of many cell cycle genes, including Chek1 | [30] |
| miR-208a | R | PI3K | Akt | ↑apoptosis ↑autophagy | miR-208a inhibits the PI3K/AKT pathway | [36] |
| miR-322; -503 | M | IGF2 FGF9 | Akt | ↓proliferation | They suppress FGF9 and IGF2, disrupting epicardial signaling and impairing myocardial growth | [44] |
| miR-423 | R | MYBL2 | WNT | ↑apoptosis | miR-423 silences MYBL2 with suppression of wnt/β- | [78] |
| miR-484 | R | YAP1 | Hippo | ↑apoptosis ↑inflammation | Catenin signaling pathway | [79] |
| miR-489 | R | SPIN1 | Akt | ↑apoptosis | It directly targets Yap1mRNA to inhibit cell viability | [37] |
| miR-873 | R | GLI1 | Hedgehog | ↓proliferation | It inhibits SPIN1 thereby inactivating the Akt pathway | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvatori, F.; D’Aversa, E.; Serino, M.L.; Singh, A.V.; Secchiero, P.; Zauli, G.; Tisato, V.; Gemmati, D. miRNAs Epigenetic Tuning of Wall Remodeling in the Early Phase after Myocardial Infarction: A Novel Epidrug Approach. Int. J. Mol. Sci. 2023, 24, 13268. https://doi.org/10.3390/ijms241713268
Salvatori F, D’Aversa E, Serino ML, Singh AV, Secchiero P, Zauli G, Tisato V, Gemmati D. miRNAs Epigenetic Tuning of Wall Remodeling in the Early Phase after Myocardial Infarction: A Novel Epidrug Approach. International Journal of Molecular Sciences. 2023; 24(17):13268. https://doi.org/10.3390/ijms241713268
Chicago/Turabian StyleSalvatori, Francesca, Elisabetta D’Aversa, Maria Luisa Serino, Ajay Vikram Singh, Paola Secchiero, Giorgio Zauli, Veronica Tisato, and Donato Gemmati. 2023. "miRNAs Epigenetic Tuning of Wall Remodeling in the Early Phase after Myocardial Infarction: A Novel Epidrug Approach" International Journal of Molecular Sciences 24, no. 17: 13268. https://doi.org/10.3390/ijms241713268
APA StyleSalvatori, F., D’Aversa, E., Serino, M. L., Singh, A. V., Secchiero, P., Zauli, G., Tisato, V., & Gemmati, D. (2023). miRNAs Epigenetic Tuning of Wall Remodeling in the Early Phase after Myocardial Infarction: A Novel Epidrug Approach. International Journal of Molecular Sciences, 24(17), 13268. https://doi.org/10.3390/ijms241713268








