Discovery of New Hydrazone-Thiazole Polyphenolic Antioxidants through Computer-Aided Design and In Vitro Experimental Validation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Validation of the Design Hypothesis Using In Silico and Thermodynamic Calculations
2.2. Chemical Synthesis
2.3. In Vitro Antiradical, Electron Transfer and Metal Ions Chelation Assays
2.3.1. Antiradical Assays
2.3.2. Electron Transfer Assays
2.3.3. Assays for Metal Ions Chelation
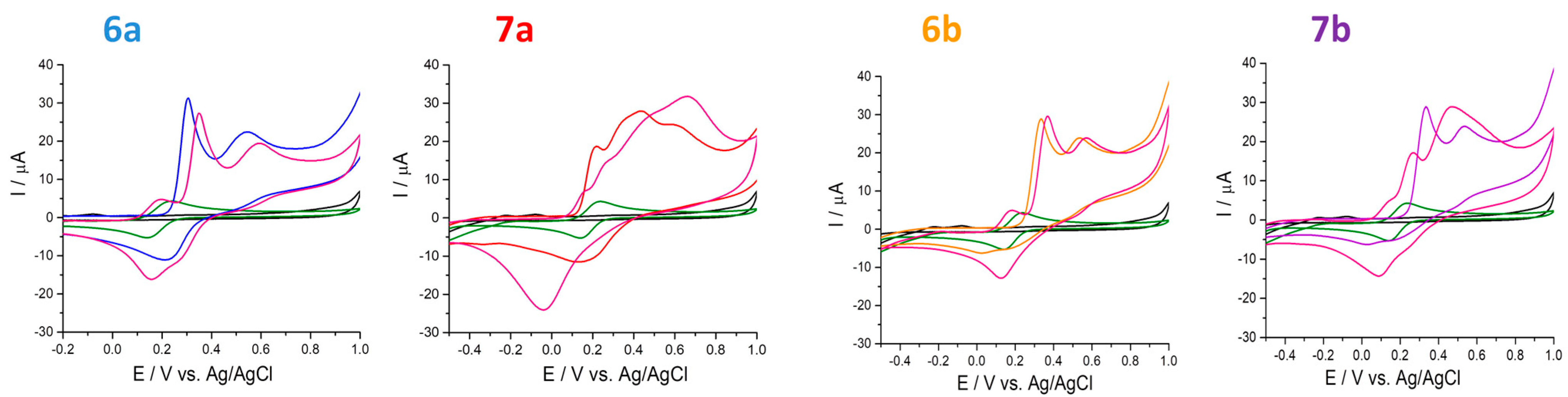
2.4. Electrochemical Behavior of Compounds
2.4.1. Antioxidant Capacity Determined through Ferric Ions (Fe3+)
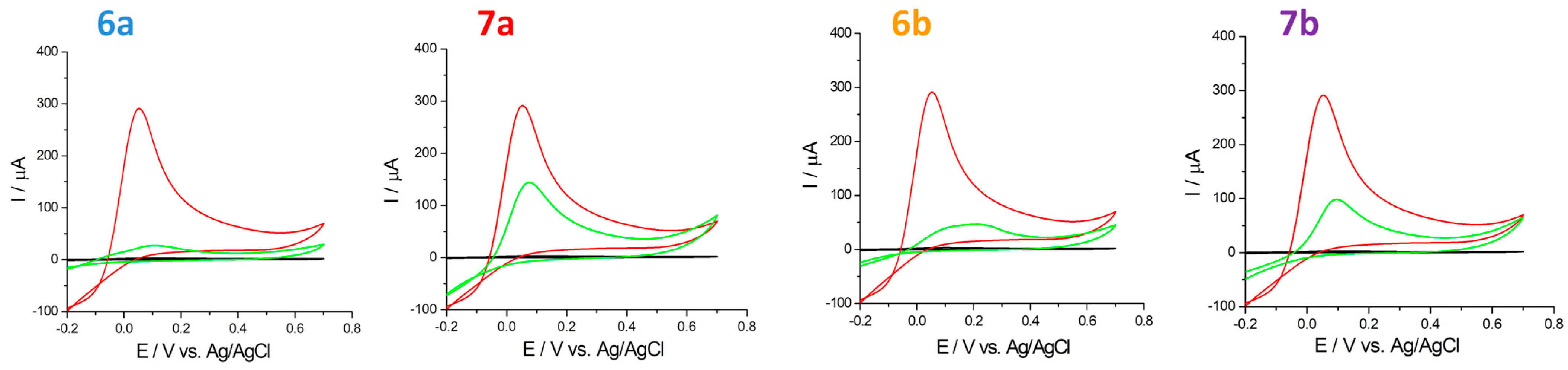
2.4.2. Hydrogen Peroxide Scavenging
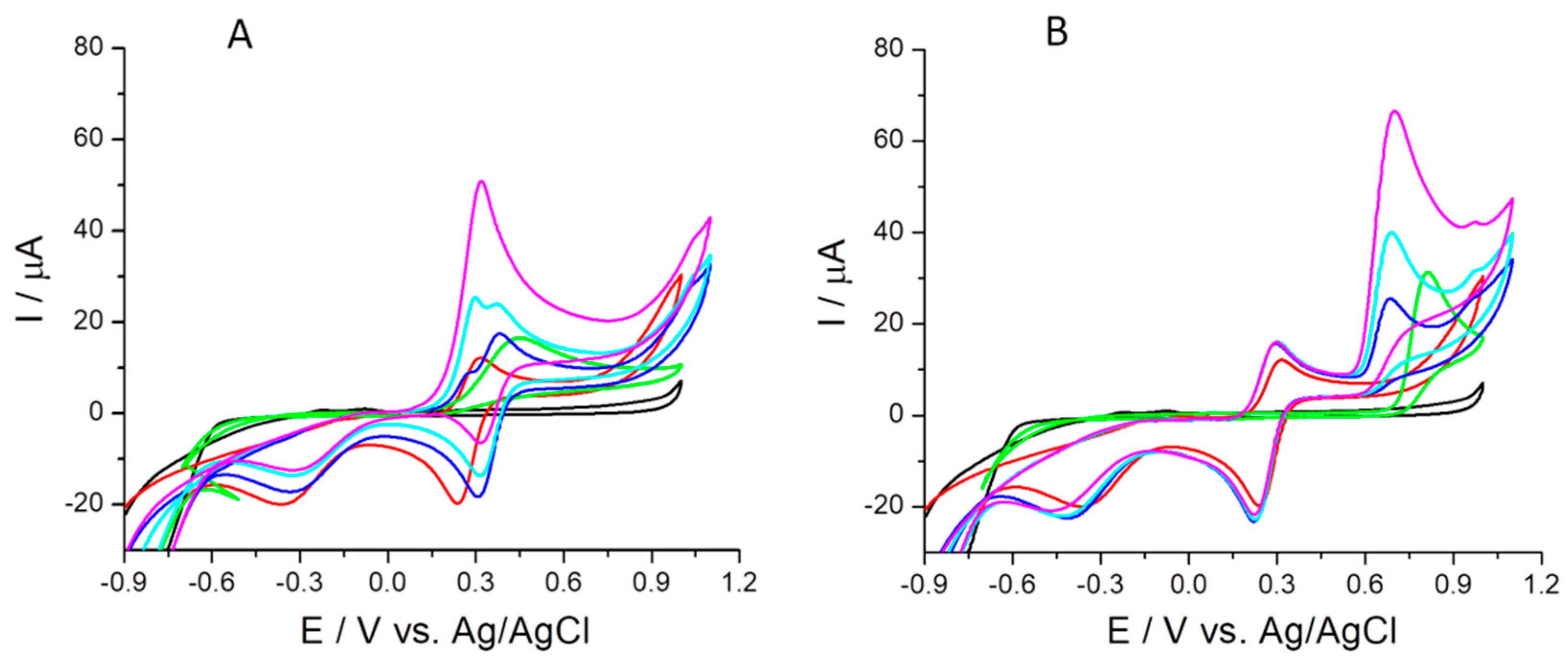
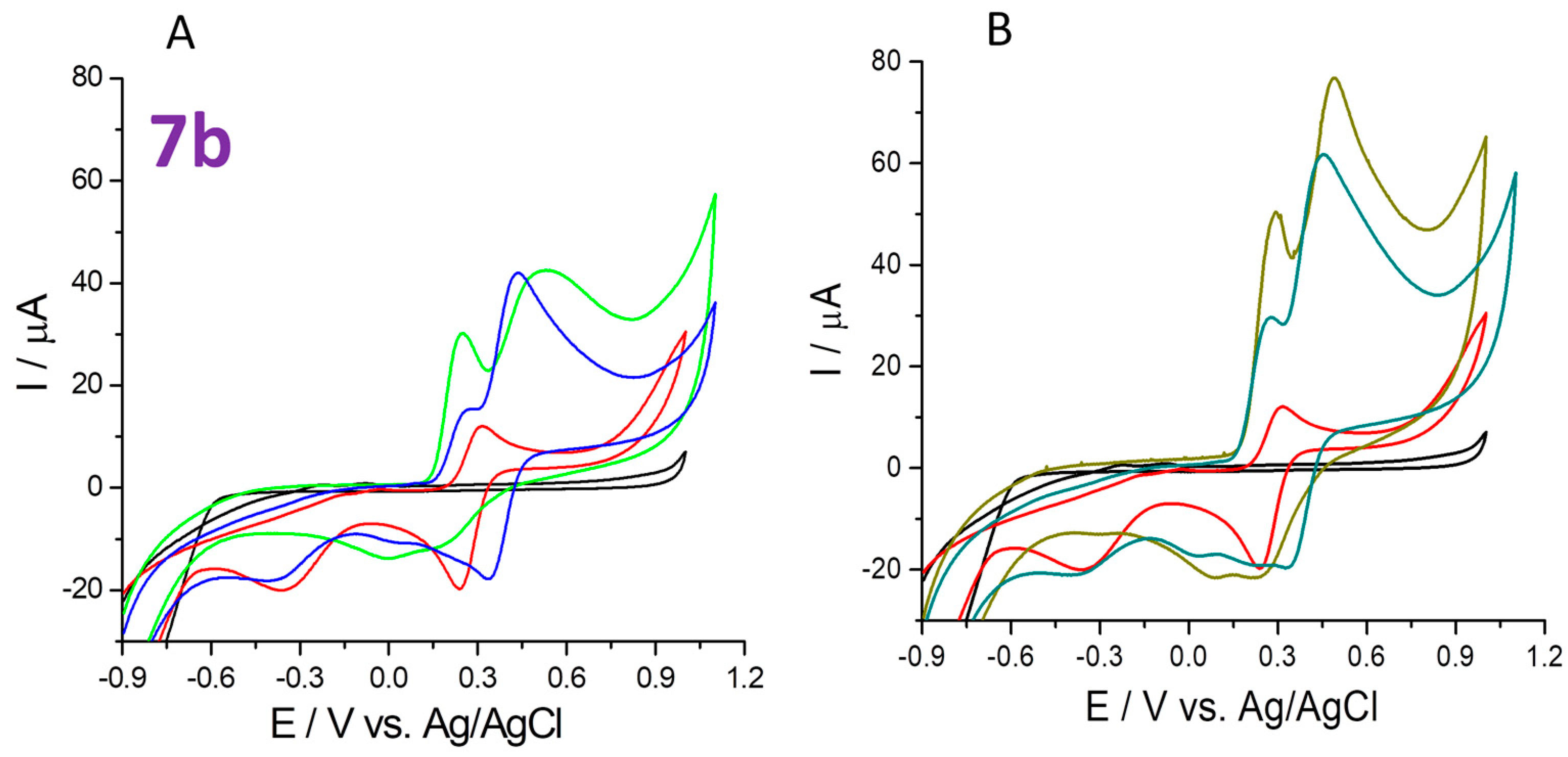
2.4.3. The 1,1-Diphenyl-2-picrylhydrazyl (DPPH•) Free Radical Scavenging
2.4.4. 2,2,6,6-Tetramethylpiperidinyl-N-oxyl (TEMPO) Scavenging
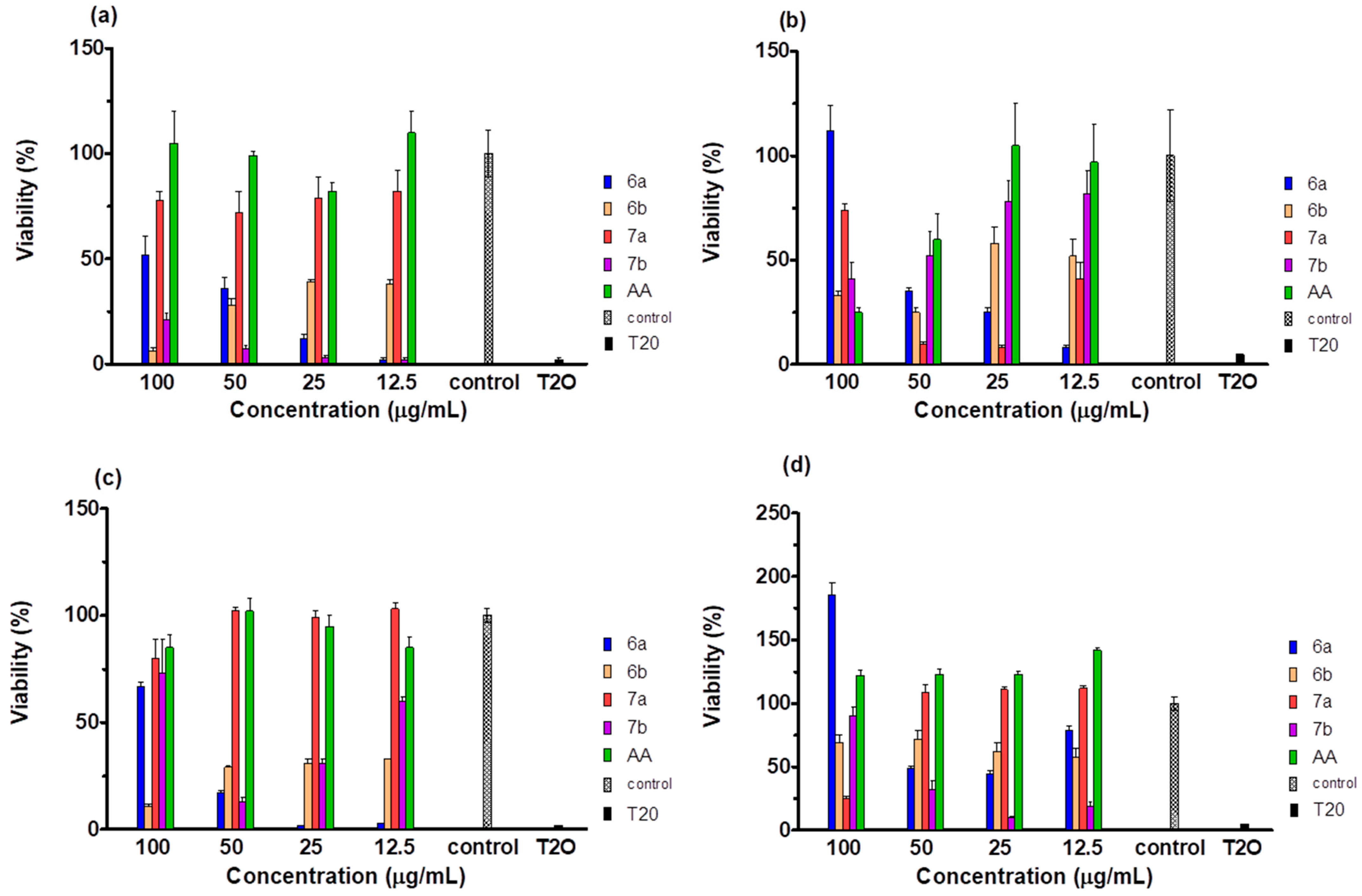
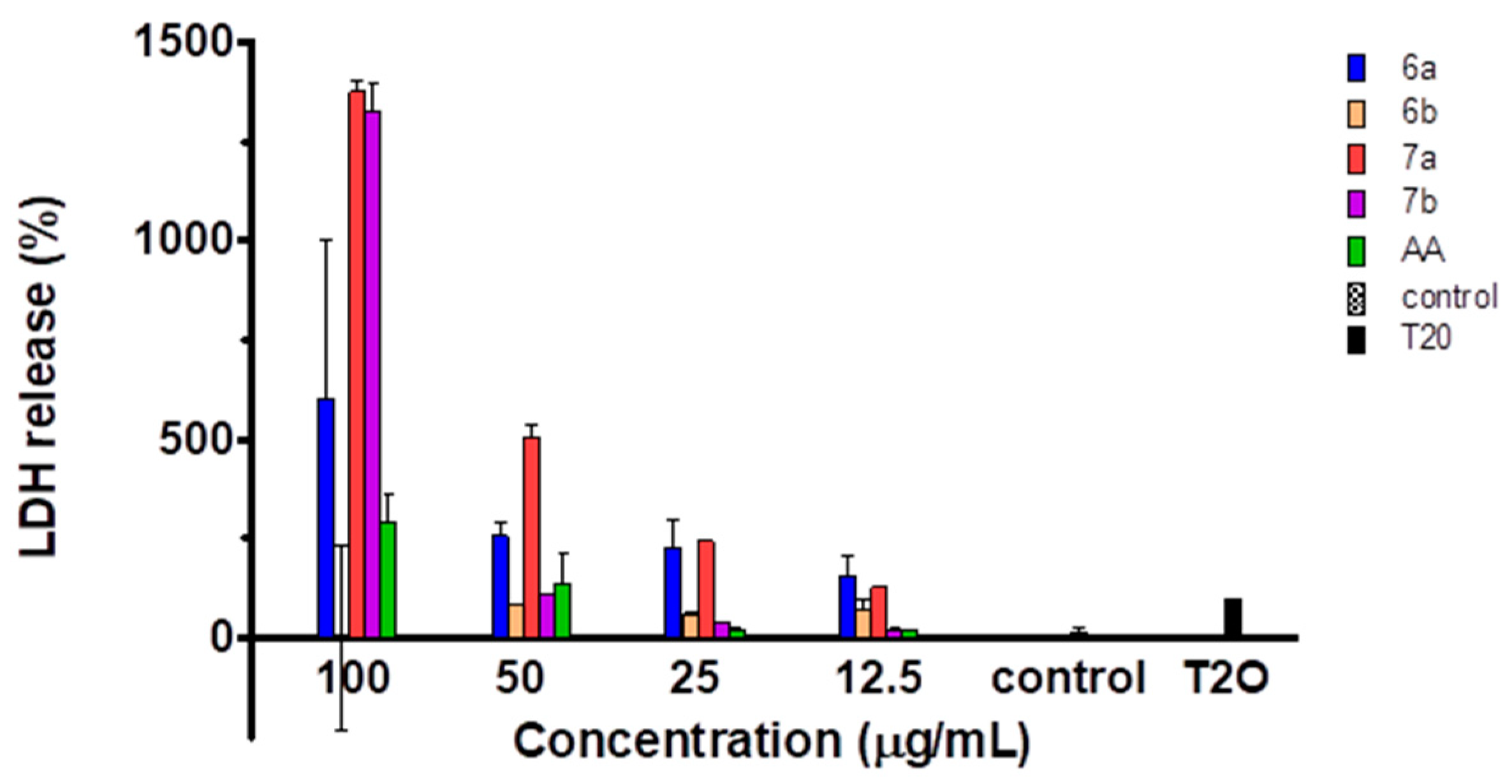
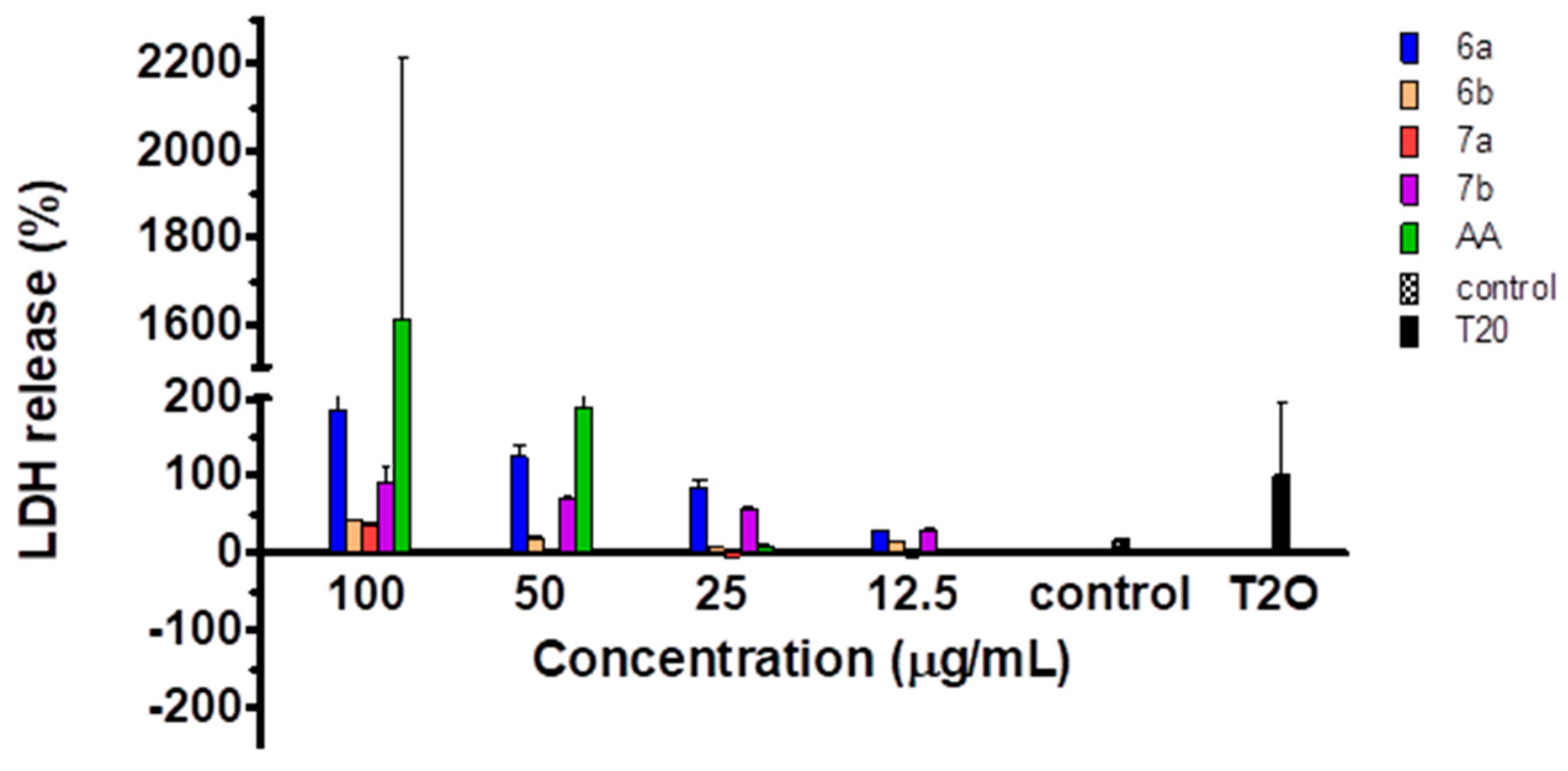
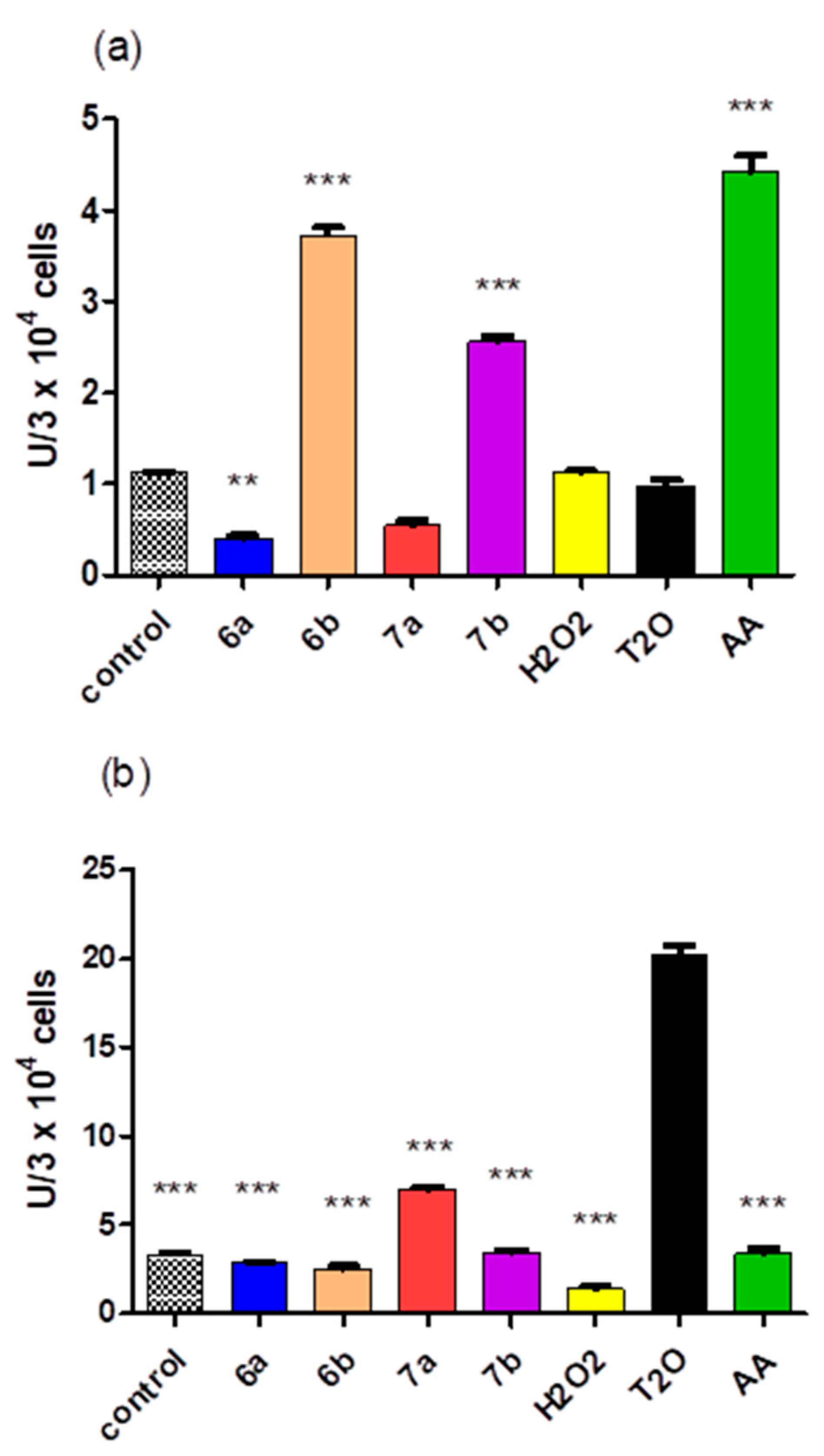
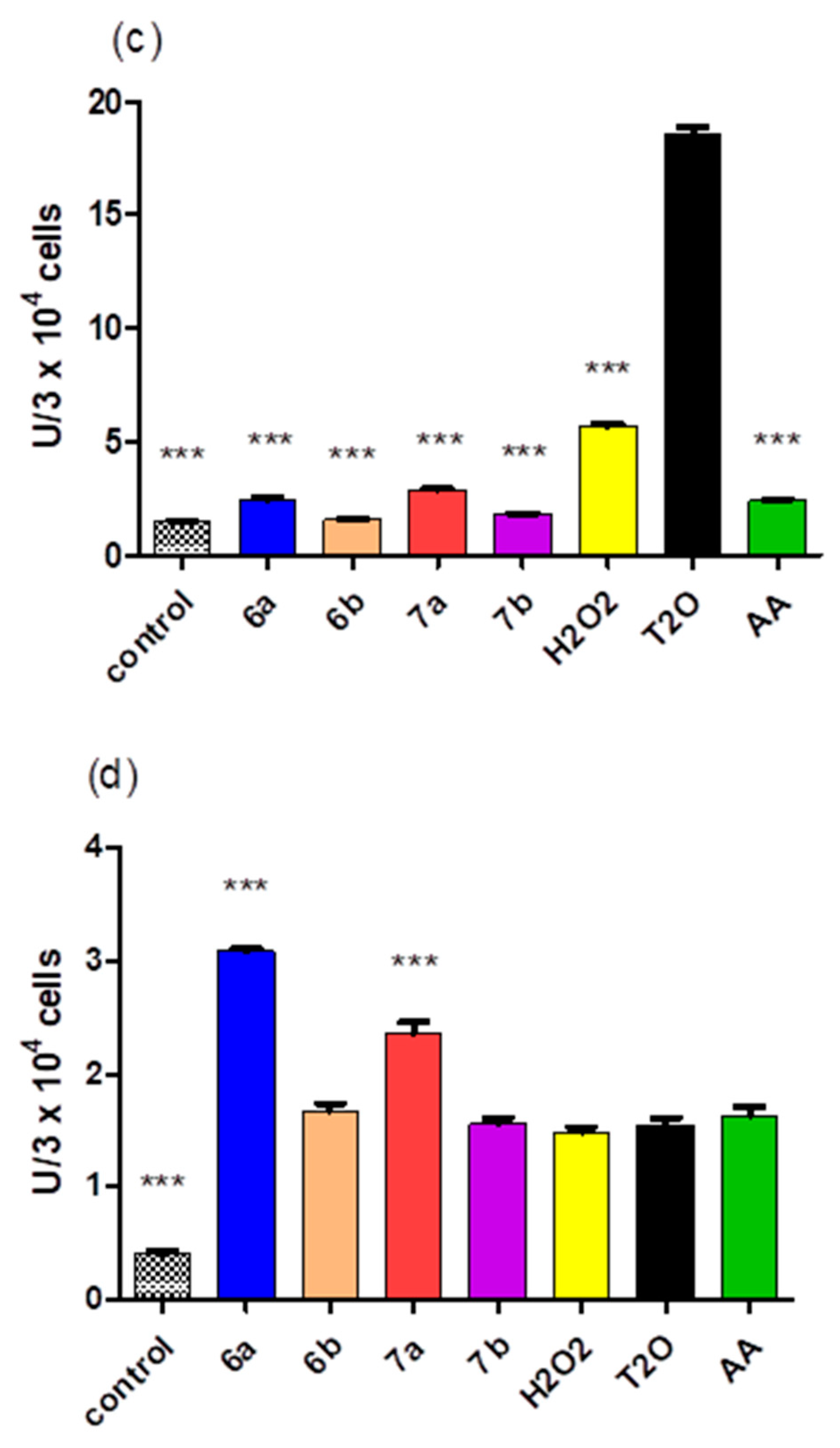
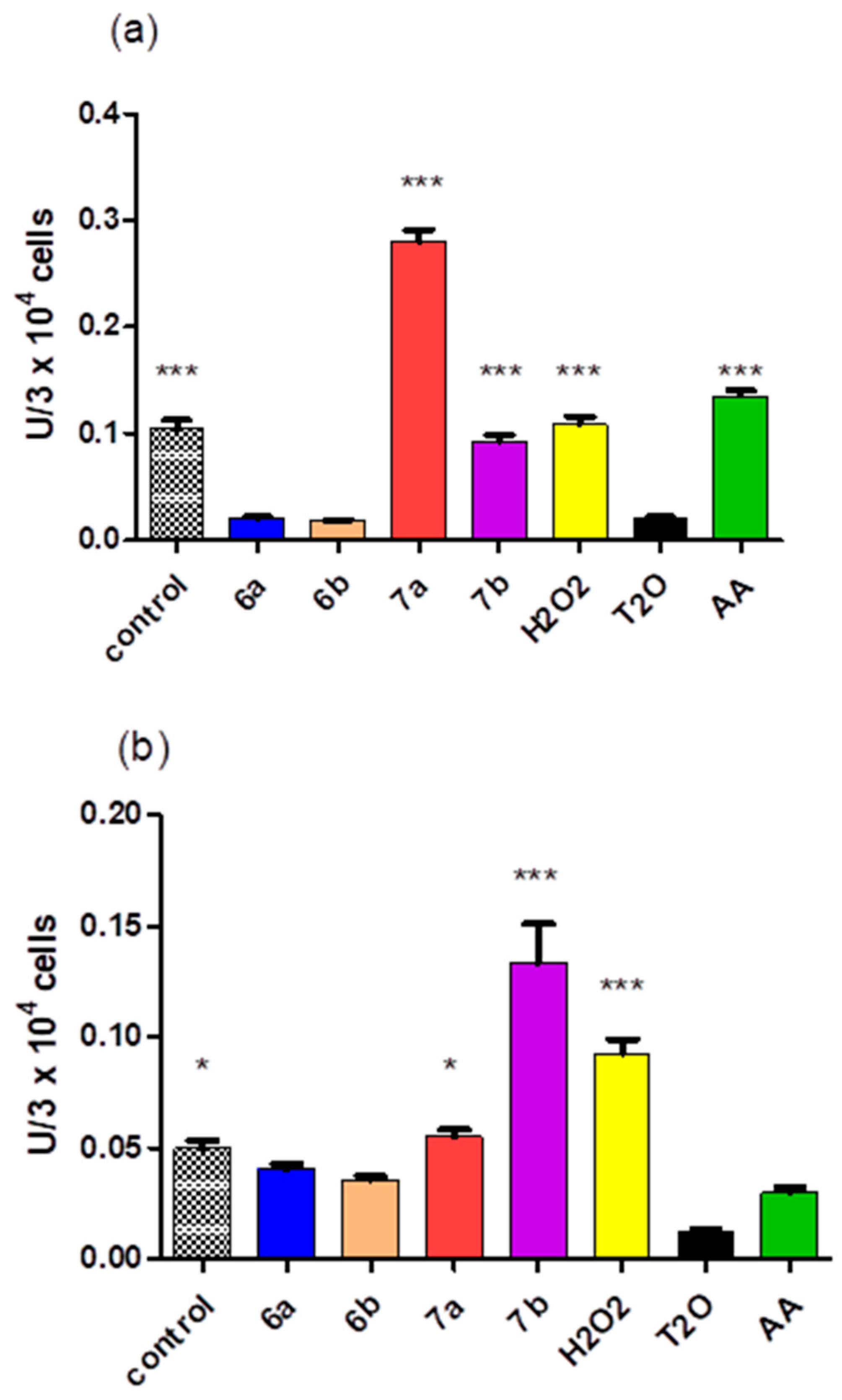
2.5. Cytotoxicity of the Compounds
3. Materials and Methods
3.1. Validation of the Design Hypothesis Using In Silico Thermodynamic Calculations
3.2. Chemical Synthesis
3.3. In Vitro Antiradical, Electron Transfer and Metal Ions Chelation Assays
3.3.1. Antiradical Assays
3.3.2. Electron Transfer Assays
3.3.3. Metal Ions Chelation Assays
3.4. Electrochemical Behavior of Compounds
- ferric ions (Fe3+) reducing antioxidant power (cyclic voltammetry by scanning the potential range between −0.5 V to 1 V with a scan rate of 100 mV s−1);
- hydrogen peroxide (H2O2) scavenging (cyclic voltammetry by scanning the potential range between −0.2 V to 0.7 V with a scan rate of 100 mV s−1);
- the 1,1-diphenyl-2-picrylhydrazyl (DPPH•) free radical scavenging (cyclic voltammetry test in the potential range of −0.5 to 1 V after 30 min, and 120 min reaction of each compound and DPPH• at 20 °C room temperature in the dark);
- 2,2,6,6-tetramethylpiperidinyl-1-oxy (TEMPO) scavenging (cyclic voltammetry test in the potential range of −1 to +1 V after 60 min reaction of each compound and TEMPO at 20 °C room temperature in the dark).
3.5. Cytotoxicity of the Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The Role of Oxidative Stress in Physiopathology and Pharmacological Treatment with Pro- and Antioxidant Properties in Chronic Diseases. Oxid. Med. Cell. Longev. 2020, 2020, 2082145. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef]
- Cutrim, C.S.; Cortez, M.A.S. A review on polyphenols: Classification, beneficial effects and their application in dairy products. Int. J. Dairy Technol. 2018, 71, 564–578. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Mic, M.; Pîrnău, A.; Floare, C.G.; Borlan, R.; Focsan, M.; Oniga, O.; Bogdan, M.; Vlase, L.; Oniga, I.; Marc, G. Antioxidant Activity Evaluation and Assessment of the Binding Affinity to HSA of a New Catechol Hydrazinyl-Thiazole Derivative. Antioxidants 2022, 11, 1245. [Google Scholar] [CrossRef]
- Tayade, K.; Yeom, G.-S.; Sahoo, S.K.; Puschmann, H.; Nimse, S.B.; Kuwar, A. Exploration of Molecular Structure, DFT Calculations, and Antioxidant Activity of a Hydrazone Derivative. Antioxidants 2022, 11, 2138. [Google Scholar] [CrossRef] [PubMed]
- Grozav, A.; Porumb, I.-D.; Găină, L.; Filip, L.; Hanganu, D. Cytotoxicity and Antioxidant Potential of Novel 2-(2-((1H-indol-5yl)methylene)-hydrazinyl)-thiazole Derivatives. Molecules 2017, 22, 260. [Google Scholar] [CrossRef] [PubMed]
- Swesi, A.T.; Farina, Y.; Kassim, M.; Ng, S.W. 2,3-Dihydroxybenzaldehyde thiosemicarbazone hemihydrate. Acta Crystallogr. Sect. E Struct. Reports Online 2006, 62, o5457–o5458. [Google Scholar] [CrossRef]
- Palanimuthu, D.; Poon, R.; Sahni, S.; Anjum, R.; Hibbs, D.; Lin, H.-Y.; Bernhardt, P.V.; Kalinowski, D.S.; Richardson, D.R. A novel class of thiosemicarbazones show multi-functional activity for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2017, 139, 612–632. [Google Scholar] [CrossRef] [PubMed]
- Gatti, A.; Habtemariam, A.; Romero-Canelón, I.; Song, J.-I.; Heer, B.; Clarkson, G.J.; Rogolino, D.; Sadler, P.J.; Carcelli, M. Half-Sandwich Arene Ruthenium(II) and Osmium(II) Thiosemicarbazone Complexes: Solution Behavior and Antiproliferative Activity. Organometallics 2018, 37, 891–899. [Google Scholar] [CrossRef]
- Marc, G.; Stana, A.; Oniga, S.D.; Pîrnău, A.; Vlase, L.; Oniga, O. New Phenolic Derivatives of Thiazolidine-2,4-dione with Antioxidant and Antiradical Properties: Synthesis, Characterization, In Vitro Evaluation, and Quantum Studies. Molecules 2019, 24, 2060. [Google Scholar] [CrossRef]
- Marc, G.; Stana, A.; Franchini, A.H.; Vodnar, D.C.; Barta, G.; Tertiş, M.; Şanta, I.; Cristea, C.; Pîrnău, A.; Ciorîţă, A.; et al. Phenolic Thiazoles with Antioxidant and Antiradical Activity. Synthesis, In Vitro Evaluation, Toxicity, Electrochemical Behavior, Quantum Studies and Antimicrobial Screening. Antioxidants 2021, 10, 1707. [Google Scholar] [CrossRef]
- Pele, R.; Marc, G.; Stana, A.; Ionuț, I.; Nastasă, C.; Tiperciuc, B.; Oniga, I.; Pîrnău, A.; Vlase, L.; Oniga, O. Synthesis of New Phenolic Derivatives of Quinazolin-4(3H)-One as Potential Antioxidant Agents—In Vitro Evaluation and Quantum Studies. Molecules 2022, 27, 2599. [Google Scholar] [CrossRef]
- Pele, R.; Marc, G.; Ionuț, I.; Nastasă, C.; Fizeșan, I.; Pîrnău, A.; Vlase, L.; Palage, M.; Oniga, S.; Oniga, O. Antioxidant and Cytotoxic Activity of New Polyphenolic Derivatives of Quinazolin-4(3H)-one: Synthesis and In Vitro Activities Evaluation. Pharmaceutics 2022, 15, 136. [Google Scholar] [CrossRef]
- Guan, S.; Wang, L.; Xu, S.-M.; Yang, D.; Waterhouse, G.I.N.; Qu, X.; Zhou, S. Vacancy-enhanced generation of singlet oxygen for photodynamic therapy. Chem. Sci. 2019, 10, 2336–2341. [Google Scholar] [CrossRef]
- Carp, O.E.; Moraru, A.; Pinteala, M.; Arvinte, A. Electrochemical behaviour of piperine. Comparison with control antioxidants. Food Chem. 2021, 339, 128110. [Google Scholar] [CrossRef] [PubMed]
- Gorjanović, S.Z.; Novaković, M.M.; Potkonjak, N.I.; LeskoŠek-Čukalović, I.; Sužnjević, D.Z. Application of a Novel Antioxidative Assay in Beer Analysis and Brewing Process Monitoring. J. Agric. Food Chem. 2010, 58, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Sužnjević, D.Ž.; Pastor, F.T.; Gorjanović, S.Ž. Polarographic study of hydrogen peroxide anodic current and its application to antioxidant activity determination. Talanta 2011, 85, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Pandithavidana, D.R.; Jayawardana, S.B. Comparative Study of Antioxidant Potential of Selected Dietary Vitamins; Computational Insights. Molecules 2019, 24, 1646. [Google Scholar] [CrossRef] [PubMed]
- Antonijević, M.R.; Simijonović, D.M.; Avdović, E.H.; Ćirić, A.; Petrović, Z.D.; Marković, J.D.; Stepanić, V.; Marković, Z.S. Green One-Pot Synthesis of Coumarin-Hydroxybenzohydrazide Hybrids and Their Antioxidant Potency. Antioxidants 2021, 10, 1106. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-S.; Zhang, G.-Y.; Hou, Y. Theoretical and Experimental Investigation of the Antioxidation Mechanism of Loureirin C by Radical Scavenging for Treatment of Stroke. Molecules 2023, 28, 380. [Google Scholar] [CrossRef] [PubMed]
- Nantasenamat, C.; Isarankura-Na-Ayudhya, C.; Naenna, T.; Prachayasittikul, V. Prediction of bond dissociation enthalpy of antioxidant phenols by support vector machine. J. Mol. Graph. Model. 2008, 27, 188–196. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, X.-M.; Fry, A.J. Bond dissociation energies of antioxidants. Polym. Degrad. Stab. 1997, 57, 43–50. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, H.; Zheng, J.; Liang, G. Structure-Thermodynamics-Antioxidant Activity Relationships of Selected Natural Phenolic Acids and Derivatives: An Experimental and Theoretical Evaluation. PLoS ONE 2015, 10, e0121276. [Google Scholar] [CrossRef]
- Vo, Q.V.; Nam, P.C.; Thong, N.M.; Trung, N.T.; Phan, C.-T.D.; Mechler, A. Antioxidant Motifs in Flavonoids: O–H versus C–H Bond Dissociation. ACS Omega 2019, 4, 8935–8942. [Google Scholar] [CrossRef]
- Amić, A.; Mastiľák Cagardová, D. DFT Study of the Direct Radical Scavenging Potency of Two Natural Catecholic Compounds. Int. J. Mol. Sci. 2022, 23, 14497. [Google Scholar] [CrossRef] [PubMed]
- Linciano, P.; Moraes, C.B.; Alcantara, L.M.; Franco, C.H.; Pascoalino, B.; Freitas-Junior, L.H.; Macedo, S.; Santarem, N.; Cordeiro-da-Silva, A.; Gul, S.; et al. Aryl thiosemicarbazones for the treatment of trypanosomatidic infections. Eur. J. Med. Chem. 2018, 146, 423–434. [Google Scholar] [CrossRef]
- Bernstein, J.; Yale, H.L.; Losee, K.; Holsing, M.; Martins, J.; Lott, W.A. The Chemotherapy of Experimental Tuberculosis. III. The Synthesis of Thiosemicarbazones and Related Compounds 1,2. J. Am. Chem. Soc. 1951, 73, 906–912. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In Methods in Enzymology; Packer, L., Ed.; Academic Press: Cambridge, MA, USA, 1999; pp. 15–27. [Google Scholar]
- Ganea, I.-V.; Nan, A.; Ciorîță, A.; Turcu, R.; Baciu, C. Responsiveness assessment of cell cultures exposed to poly(tartaric acid) and its corresponding magnetic nanostructures. J. Mol. Struct. 2022, 1248, 131459. [Google Scholar] [CrossRef]
- De Jong, N.W.; Ploscariu, N.T.; Ramyar, K.X.; Garcia, B.L.; Herrera, A.I.; Prakash, O.; Katz, B.B.; Leidal, K.G.; Nauseef, W.M.; van Kessel, K.P.; et al. A structurally dynamic N-terminal region drives function of the staphylococcal peroxidase inhibitor (SPIN). J. Biol. Chem. 2018, 293, 2260–2271. [Google Scholar] [CrossRef]
- Shangari, N.; O’Brien, P.J. Catalase Activity Assays. Curr. Protoc. Toxicol. 2006, 27, 7.7.1–7.7.16. [Google Scholar] [CrossRef] [PubMed]

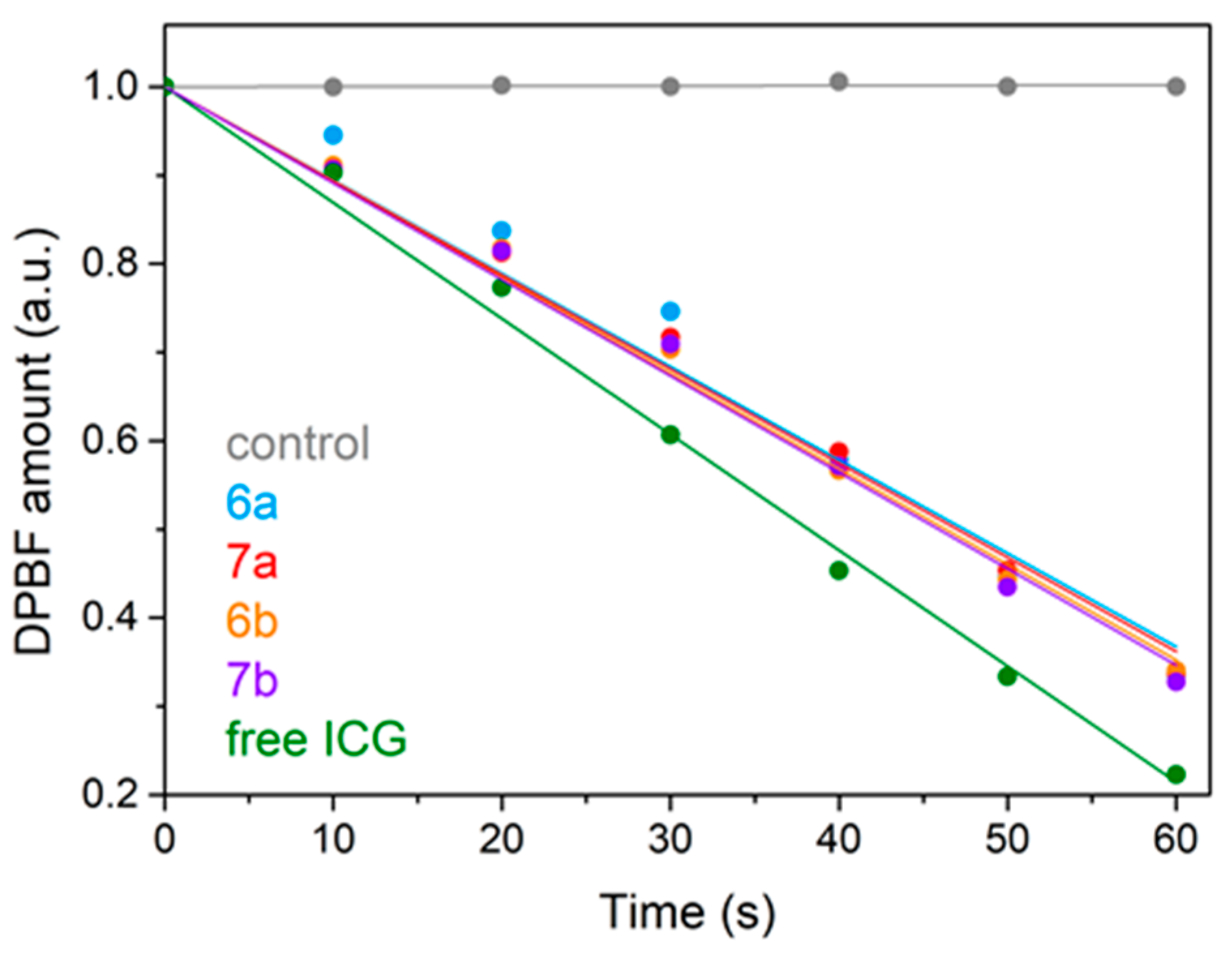
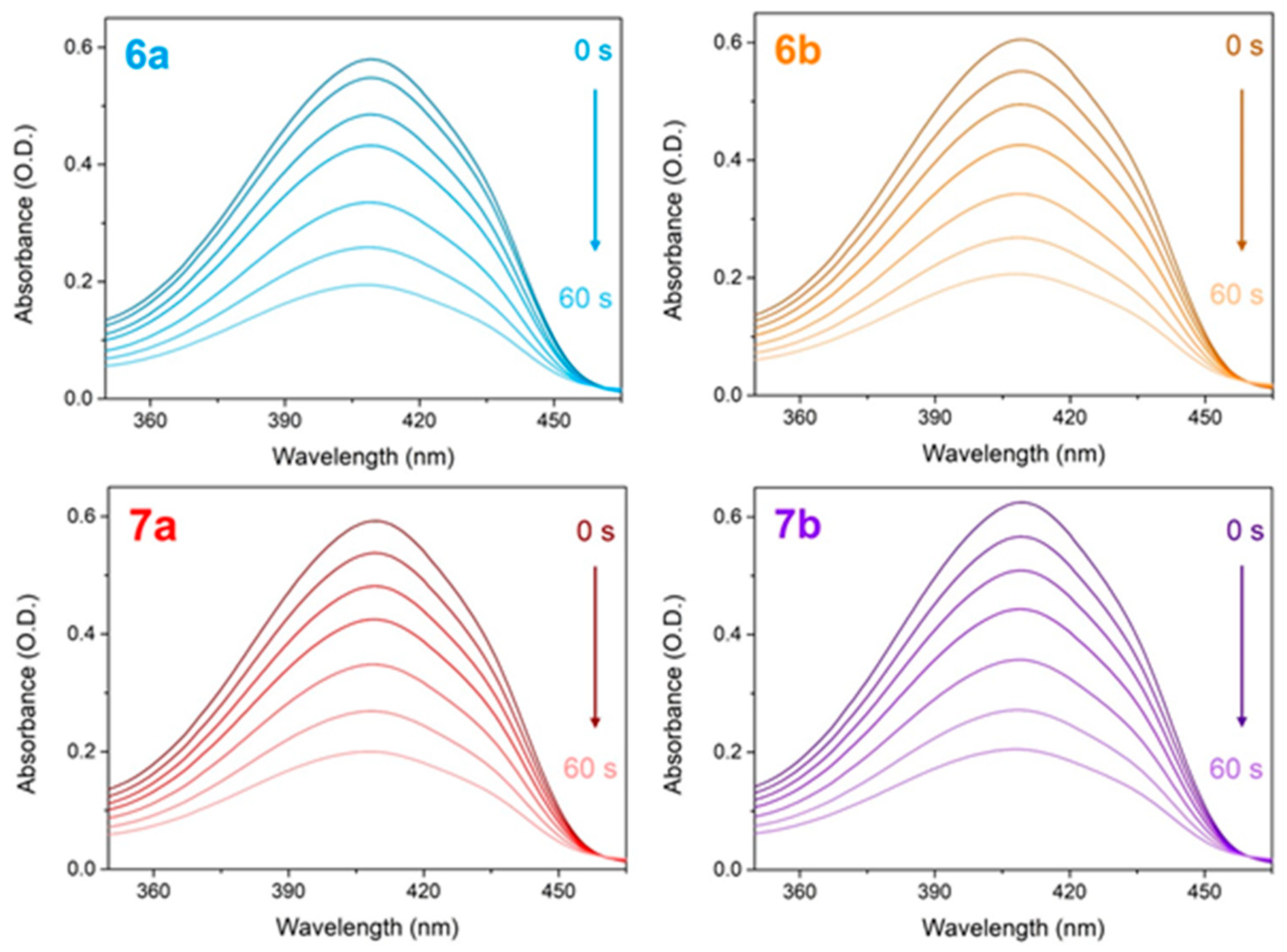
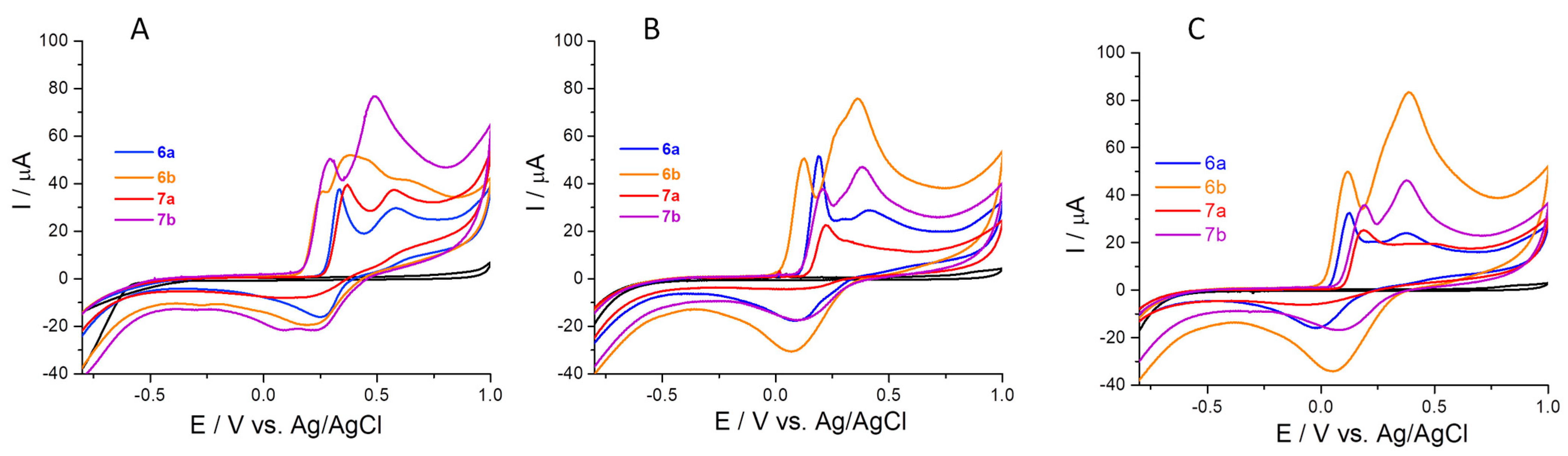
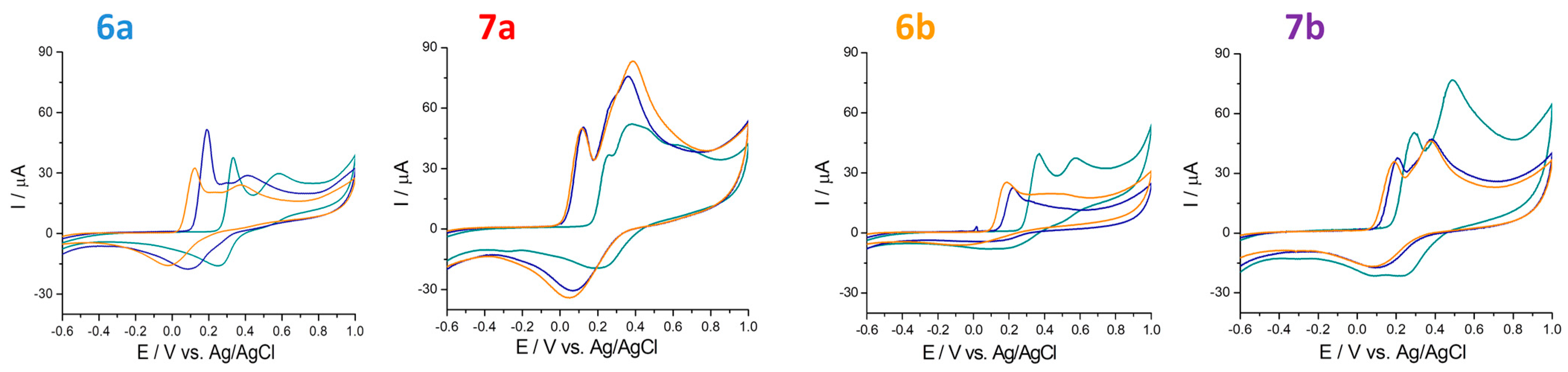









| Substitution |  | 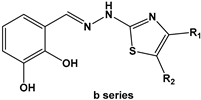 | |||||
|---|---|---|---|---|---|---|---|
| R1 | R2 | O-H BDE * (kcal/mol) | N-H BDE (kcal/mol) | Influence | O-H BDE * (kcal/mol) | N-H BDE (kcal/mol) | Influence |
| -H | -H | 67.70 | 68.77 | medium | 62.78 | 65.12 | negative |
| -CH3 | -H | 67.50 | 68.71 | medium | 62.74 | 65.73 | medium |
| -CH3 | -CH3 | 67.26 | 68.04 | positive | 62.61 | 71.75 | negative |
| -C(CH3)3 | -H | 67.62 | 68.86 | medium | 62.78 | 73.57 | negative |
| -CO-CH3 | -H | 68.38 | 69.63 | negative | 62.74 | 65.55 | negative |
| -COO-CH3 | -H | 68.72 | 69.72 | negative | 62.70 | 65.49 | negative |
| -H | -CO-CH3 | 68.93 | 70.69 | negative | 62.66 | 66.47 | negative |
| -H | -COO-CH3 | 68.70 | 70.29 | negative | 62.56 | 66.06 | medium |
| -C6H5 | -H | 67.82 | 68.83 | medium | 62.58 | 64.62 | positive |
| -C6H5 | -C6H5 | 67.63 | 68.38 | medium | 62.32 | 71.47 | negative |
| -C6H5 | -CH3 | 67.54 | 68.33 | positive | 62.51 | 71.80 | negative |
| -C6H3-3,4-diOH | -H | 67.68 | 68.64 | medium | 62.43 | 64.19 | positive |
| Compound | N-H1 | O-H2 | O-H3 | O-H4 | O-H5 | O-H6 |
|---|---|---|---|---|---|---|
| 6a | 68.71 | 67.50 | 70.17 | |||
| 6b | 65.73 | 81.05 | 62.74 | |||
| 7a | 68.64 | 67.68 | 70.29 | 78.45 | 66.22 | |
| 7b | 64.19 | 81.11 | 62.43 | 78.91 | 67.10 |
| Compound | N-H1 | O-H2 | O-H3 | O-H4 | O-H5 | O-H6 |
|---|---|---|---|---|---|---|
| 6a | 67.99 | 67.01 | 69.53 | |||
| 6b | 64.46 | 78.58 | 62.25 | |||
| 7a | 67.85 | 67.09 | 69.48 | 76.35 | 65.45 | |
| 7b | 63.55 | 78.61 | 62.10 | 76.84 | 66.33 |
| Compound | N-H1 | O-H2 | O-H3 | O-H4 | O-H5 | O-H6 |
|---|---|---|---|---|---|---|
| 6a | 69.80 | 66.78 | 73.29 | |||
| 6b | 66.45 | 79.04 | 64.98 | |||
| 7a | 69.72 | 66.83 | 73.31 | 77.41 | 69.33 | |
| 7b | 65.69 | 79.12 | 64.82 | 77.52 | 69.82 |
| Compound | N-H1 | O-H2 | O-H3 | O-H4 | O-H5 | O-H6 |
|---|---|---|---|---|---|---|
| 6a | 69.76 | 66.25 | 73.43 | |||
| 6b | 66.53 | 76.98 | 65.32 | |||
| 7a | 69.75 | 66.34 | 73.54 | 77.18 | 69.43 | |
| 7b | 65.69 | 73.62 | 65.20 | 77.34 | 69.96 |
| Paper | Lowest BDE (kcal/mol) | Type of Bond |
|---|---|---|
| Marc et al., 2019 [16] | 71.56 | Phenol O-H |
| Marc et al., 2021 [17] | 62.30 | Hydrazone N-H |
| Pele et al., 2022 [18] | 72.00 | Phenol O-H |
| Pele et al., 2022 [19] | 71.97 | Phenol O-H |
| Compound | Scavenging Effect (%) at Specific Concentration (µM) | IC50 (µM) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.5 | 5 | 7.5 | 10 | 12.5 | 20 | 30 | 50 | 60 | 70 | 80 | ||
| 6a * | 20.40 | 28.40 | 37.22 | 48.79 | 57.48 | 81.57 | + | + | + | + | + | 10.80 |
| 6b | 21.99 | 33.55 | 42.15 | 52.88 | 60.98 | 85.12 | + | + | + | + | + | 9.74 |
| 7a | 35.10 | 48.08 | 54.81 | 66.30 | 74.31 | + | + | + | + | + | + | 6.02 |
| 7b | 39.15 | 51.69 | 60.89 | 72.89 | 81.10 | + | + | + | + | + | + | 4.85 |
| Trolox | − | 8.33 | 13.10 | 14.67 | 23.22 | 31.77 | 39.67 | 68.38 | 83.38 | + | + | 35.50 |
| Ascorbic acid | − | − | − | 9.75 | 12.40 | 20.04 | 30.05 | 46.18 | 56.35 | 66.41 | 73.05 | 53.39 |
| Compound | Scavenging Activity (%) at Given Concentration (µM) | IC50 (µM) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1.5 | 2.5 | 3.75 | 5 | 7.5 | 10 | 17.5 | 25 | ||
| 6a * | 22.55 | 28.93 | 41.32 | 49.70 | 70.72 | 87.20 | + | + | 5.03 |
| 6b | 24.55 | 34.74 | 43.27 | 58.42 | 77.85 | 94.57 | + | + | 4.37 |
| 7a | 22.15 | 33.84 | 58.37 | 79.67 | + | + | + | + | 3.28 |
| 7b | 35.16 | 50.60 | 64.44 | 86.46 | + | + | + | + | 2.55 |
| Trolox | − | − | 13.10 | 20.45 | 25.99 | 31.72 | 53.26 | 77.62 | 15.90 |
| Compound | % of Activity Compared to Ascorbic Acid | % of Activity Compared to Trolox | ||||
|---|---|---|---|---|---|---|
| FRAP | RP | TAC | FRAP | RP | TAC | |
| 6a * | 201.55 | 138.54 | 106.46 | 171.35 | 253.86 | 80.10 |
| 6b | 255.09 | 223.28 | 214.42 | 216.87 | 409.14 | 161.33 |
| 7a | 228.88 | 175.10 | 185.38 | 194.58 | 320.86 | 139.47 |
| 7b | 312.67 | 314.45 | 270.42 | 265.81 | 576.19 | 203.46 |
| Compound | 58 nM | 116 nM | 162.4 nM | 185.6 nM | 208.8 nM | 232 nM | 464 nM |
|---|---|---|---|---|---|---|---|
| 6a * | − | − | − | − | − | − | − |
| 6b | − | − | − | − | − | − | 13.51 |
| 7a | − | − | − | − | − | 5.75 | 14.99 |
| 7b | − | − | − | − | − | 9.25 | 17.80 |
| EDTA-Na2 | 11.71 | 20.00 | 36.37 | 61.98 | 86.53 | + | + |
| Compound | 66.66 µM | 99.99 µM | 133.32 µM | 166.65 µM | 199.98 µM |
|---|---|---|---|---|---|
| 6a * | 15.04 | 18.99 | 21.56 | 26.05 | 31.19 |
| 6b | 18.52 | 25.11 | 29.73 | 33.33 | 38.03 |
| 7a | 16.69 | 23.15 | 29.01 | 34.25 | 37.12 |
| 7b | 17.18 | 25.14 | 30.67 | 35.14 | 39.55 |
| EDTA-Na2 | 18.54 | 23.89 | 29.21 | 38.01 | 44.48 |
| Compound | Concentration (μM) | Eox (V) | Iox (μA) | Eox (V) | Iox (μA) | ERed (V) | IRed (μA) |
|---|---|---|---|---|---|---|---|
| 6a | 250 | 0.300 | 23.85 | 0.54 | 5.94 | 0.223 | −9.45 |
| 500 | 0.330 | 28.84 | 0.57 | 8.28 | 0.255 | −14.72 | |
| 6b | 250 | 0.330 | 19.03 | 0.533 | 4.17 | 0.165 | −4.24 |
| 500 | 0.360 | 24.96 | 0.57 | 7.58 | 0.200 | −6.71 | |
| 7a | 250 | 0.201 | 7.50 | 0.418 | 20.04 | 0.157 | −9.11 |
| 500 | 0.241 | 11.16 | 0.364 | 39.95 | 0.223 | −16.12 | |
| 7b | 250 | 0.235 | 16.92 | 0.487 | 24.50 | 0.020 | −9.45 |
| 500 | 0.274 | 22.75 | 0.487 | 33.72 | 0.244 | −17.73 |
| Sample | Electrochemical Oxidation | Electrochemical Reduction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Eox (V) | Iox (μA) | Eox (V) | Iox (μA) | Eox (V) | Iox (μA) | Ered (V) | Ired (μA) | Ered (V) | Ired (μA) | |
| FC * 500 μM | 0.235 | 4.43 | - | - | - | - | 0.140 | −4.69 | - | - |
| 6a 250 μM | - | - | 0.301 | 23.85 | 0.540 | 5.95 | - | - | 0.223 | −9.45 |
| 6a 250 μM + FC * 500 μM | 0.182 | 2.46 | 0.348 | 19.94 | 0.589 | 5.72 | 0.159 | −15.55 | 0.250 | −2.22 |
| 6b 250 μM | - | - | 0.331 | 19.03 | 0.533 | 4.17 | - | - | 0.165 | −4.24 |
| 6b 250 μM + FC * 500 μM | 0.172 | 2.82 | 0.359 | 20.64 | 0.570 | 3.91 | 0.130 | −11.12 | - | - |
| 7a 250 μM | - | - | 0.201 | 7.51 | 0.437 | 10.13 | - | - | 0.157 | −9.11 |
| 7a 250 μM + FC * 500 μM | 0.147 | 1.07 | 0.255 | 1.55 | 0.653 | 13.58 | −0.035 | −21.03 | - | - |
| 7b 250 μM | - | - | 0.235 | 16.92 | 0.499 | 15.61 | - | - | 0.020 | −9.45 |
| 7b 250 μM + FC * 500 μM | 0.130 | 0.39 | 0.252 | 5.34 | 0.467 | 12.72 | 0.093 | −12.58 | - | - |
| Sample | Eox (V) | Iox (μA) | ∆Iox (μA) |
|---|---|---|---|
| H2O2 500 μM | 0.052 | 339.50 | - |
| H2O2 500 μM + 6a 250 μM | 0.091 | 45.21 | 294.29 |
| H2O2 500 μM + 6b 250 μM | 0.147 | 43.67 | 295.83 |
| H2O2 500 μM + 7a 250 μM | 0.071 | 176.21 | 163.29 |
| H2O2 500 μM + 7b 250 μM | 0.093 | 105.24 | 234.26 |
| Sample | Oxidation Signal of TEMPO | Reduction Signal of TEMPO | ||
|---|---|---|---|---|
| Eox (V) | Iox (μA) | Ered (V) | Ired (μA) | |
| TEMPO 2 mM | 0.301 | 13.81 | 0.232 | −22.09 |
| TEMPO 2 mM + ascorbic acid 250 μM | 0.267 | 1.44 | 0.301 | −20.01 |
| TEMPO 2 mM + tyrosine 250 μM (60 min) | 0.289 | 13.28 | 0.223 | −23.21 |
| TEMPO 2 mM + 6a 250 μM (60 min) | 0.299 | 1.59 | 0.334 | −20.66 |
| TEMPO 2 mM + 6b 250 μM (60 min) | 0.108 | 0.10 | 0.333 | −11.45 |
| TEMPO 2 mM + 7a 250 μM (60 min) | 0.120 | 0.24 | 0.325 | −22.12 |
| TEMPO 2 mM + 7b 250 μM (60 min) | 0.242 | 4.50 | 0.343 | −19.75 |
| Cell Line | 6a | 6b | 7a | 7b | Ascorbic Acid |
|---|---|---|---|---|---|
| HaCaT | 158.29 | 26.1 | 219.26 | 23.75 | 1091.63 |
| BJ | 26.98 | 42.37 | 104.42 | 72.86 | 68.22 |
| A375 | 229.27 | 23.11 | 263.46 | 82.92 | 538.48 |
| A549 | 5.45 | 206.42 | 88.62 | 42.14 | 1597.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marc, G.; Stana, A.; Tertiş, M.; Cristea, C.; Ciorîţă, A.; Drăgan, Ș.-M.; Toma, V.-A.; Borlan, R.; Focșan, M.; Pîrnău, A.; et al. Discovery of New Hydrazone-Thiazole Polyphenolic Antioxidants through Computer-Aided Design and In Vitro Experimental Validation. Int. J. Mol. Sci. 2023, 24, 13277. https://doi.org/10.3390/ijms241713277
Marc G, Stana A, Tertiş M, Cristea C, Ciorîţă A, Drăgan Ș-M, Toma V-A, Borlan R, Focșan M, Pîrnău A, et al. Discovery of New Hydrazone-Thiazole Polyphenolic Antioxidants through Computer-Aided Design and In Vitro Experimental Validation. International Journal of Molecular Sciences. 2023; 24(17):13277. https://doi.org/10.3390/ijms241713277
Chicago/Turabian StyleMarc, Gabriel, Anca Stana, Mihaela Tertiş, Cecilia Cristea, Alexandra Ciorîţă, Ștefan-Mihai Drăgan, Vlad-Alexandru Toma, Raluca Borlan, Monica Focșan, Adrian Pîrnău, and et al. 2023. "Discovery of New Hydrazone-Thiazole Polyphenolic Antioxidants through Computer-Aided Design and In Vitro Experimental Validation" International Journal of Molecular Sciences 24, no. 17: 13277. https://doi.org/10.3390/ijms241713277







