Significance of Intra-plaque Hemorrhage for the Development of High-Risk Vulnerable Plaque: Current Understanding from Basic to Clinical Points of View
Abstract
:1. Introduction
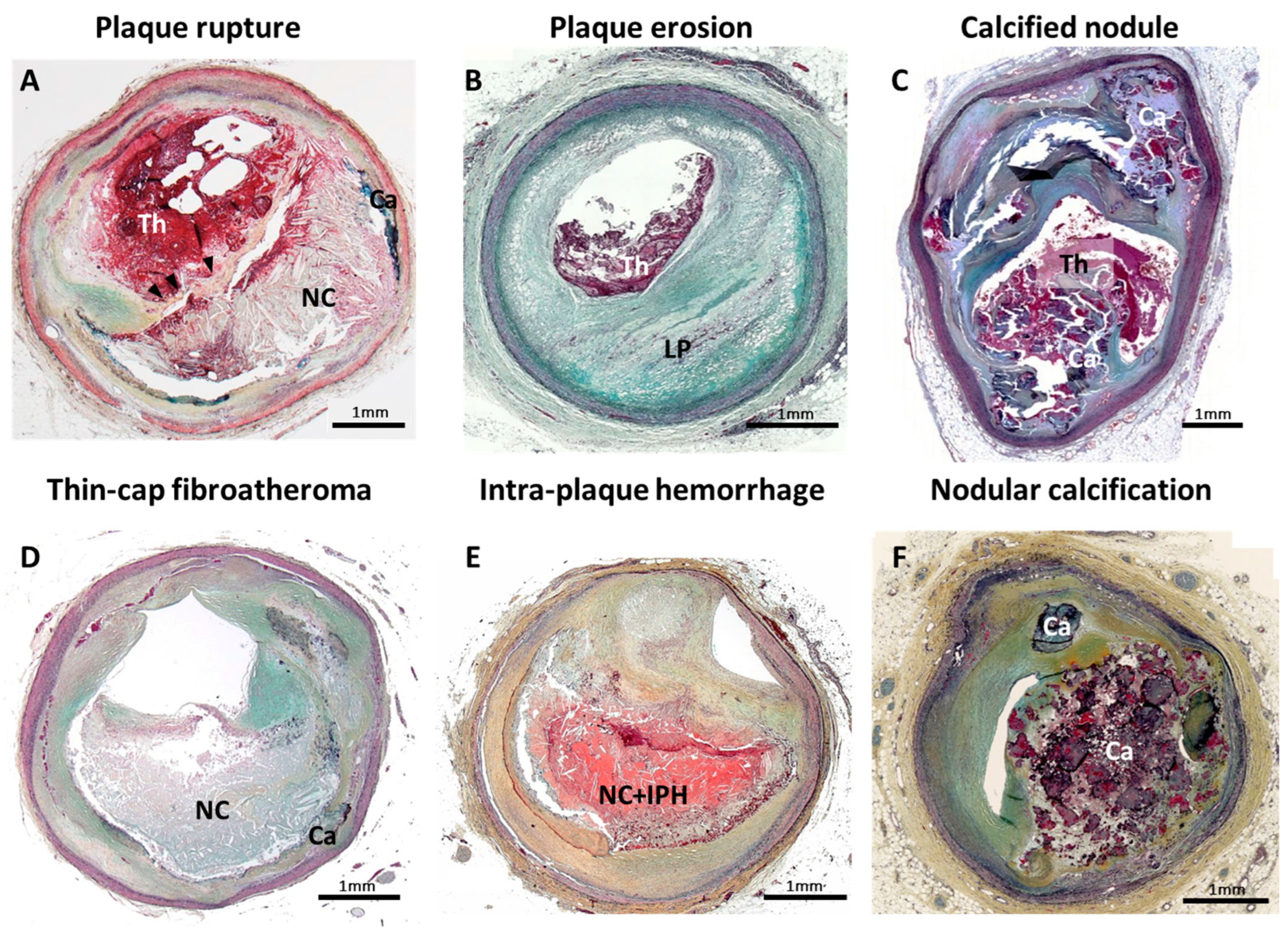
2. Intraplaque Hemorrhage: One of the Critical Features of Unstable Plaque
3. Neovascularization as an Origin of Intraplaque Hemorrhage
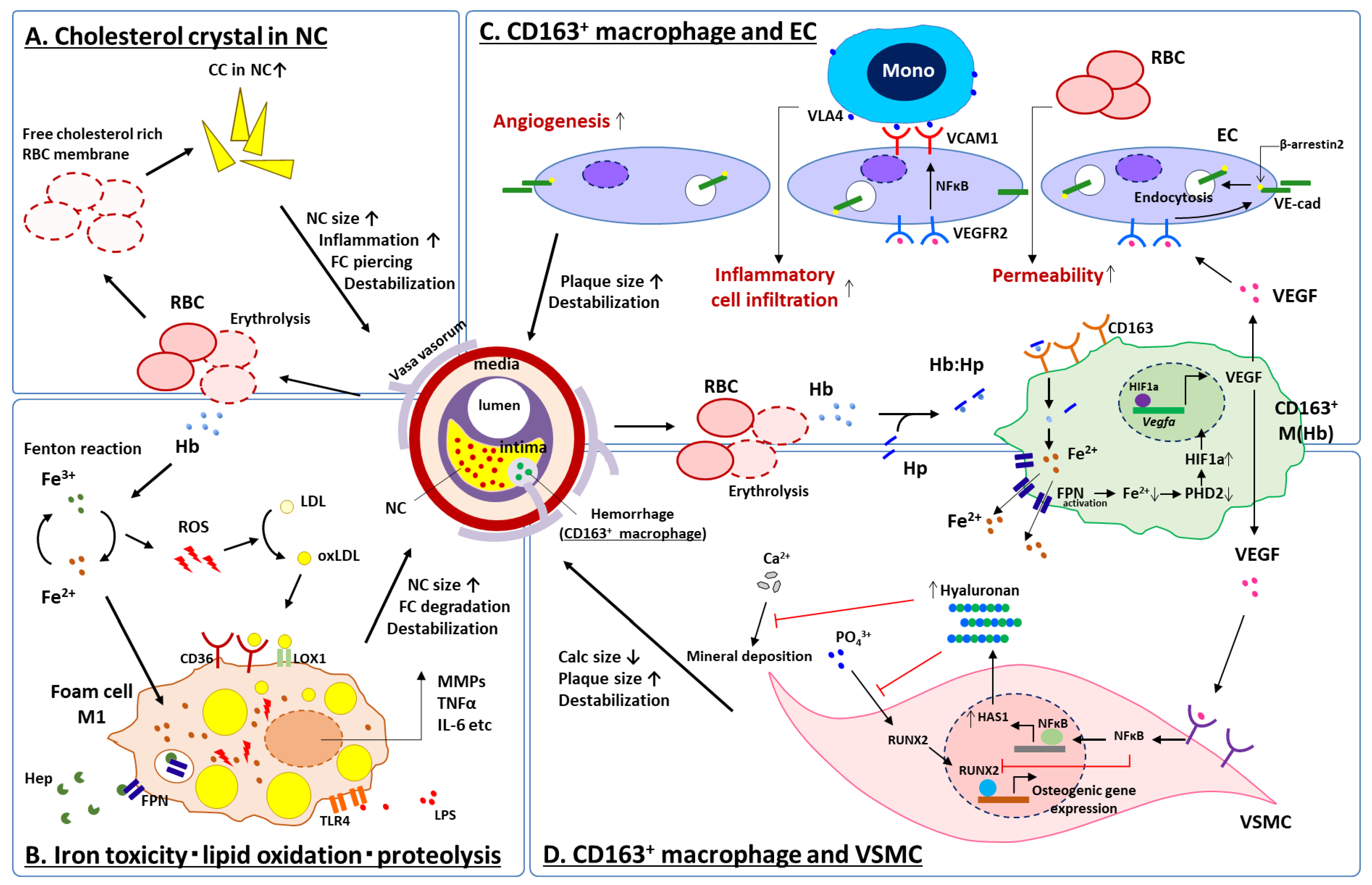
4. Intraplaque Hemorrhage, Cholesterol Crystal Accumulation and Iron-Derived Oxidative Stress
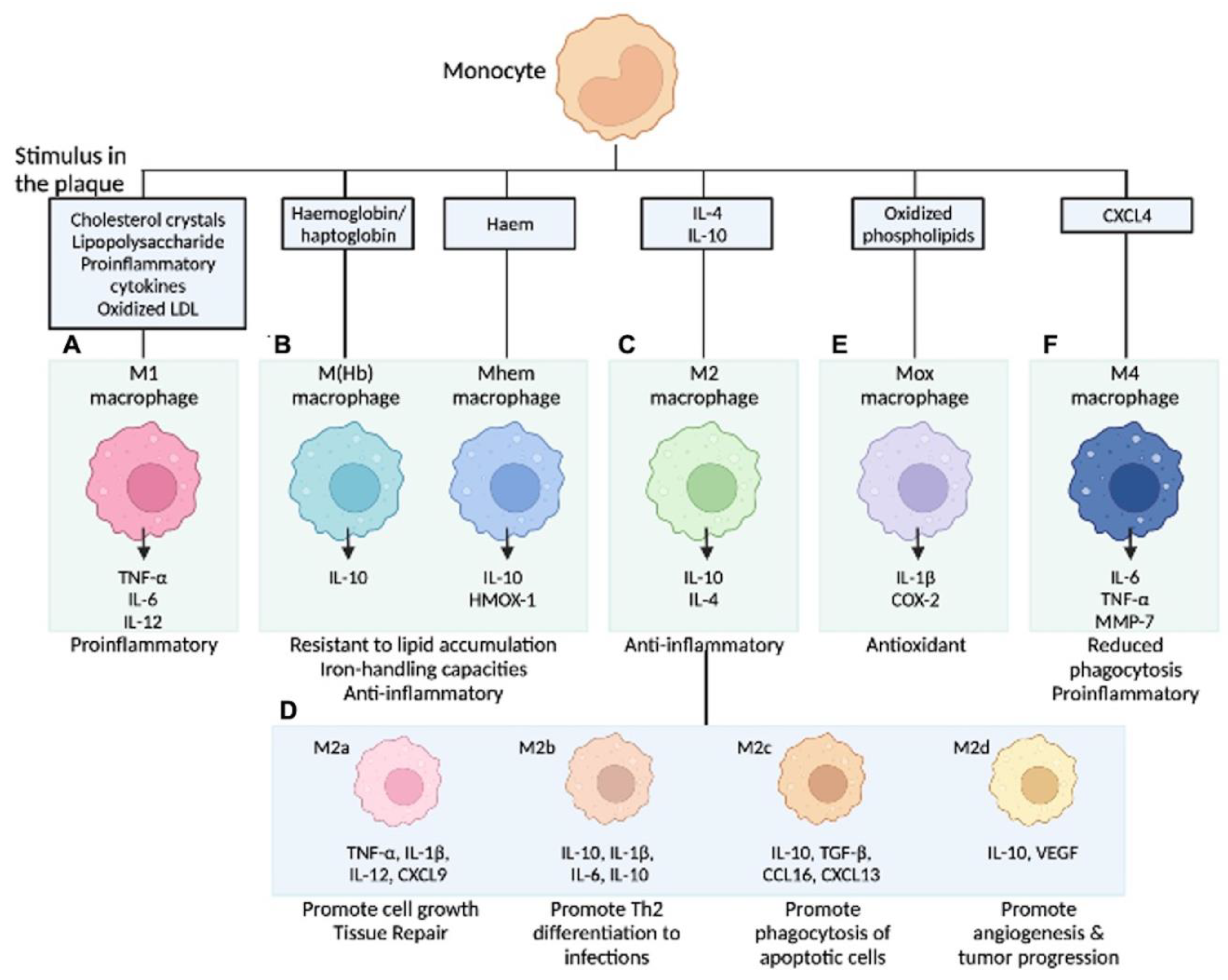
5. Diversity of Macrophage Phenotype at the Site of Atherosclerosis
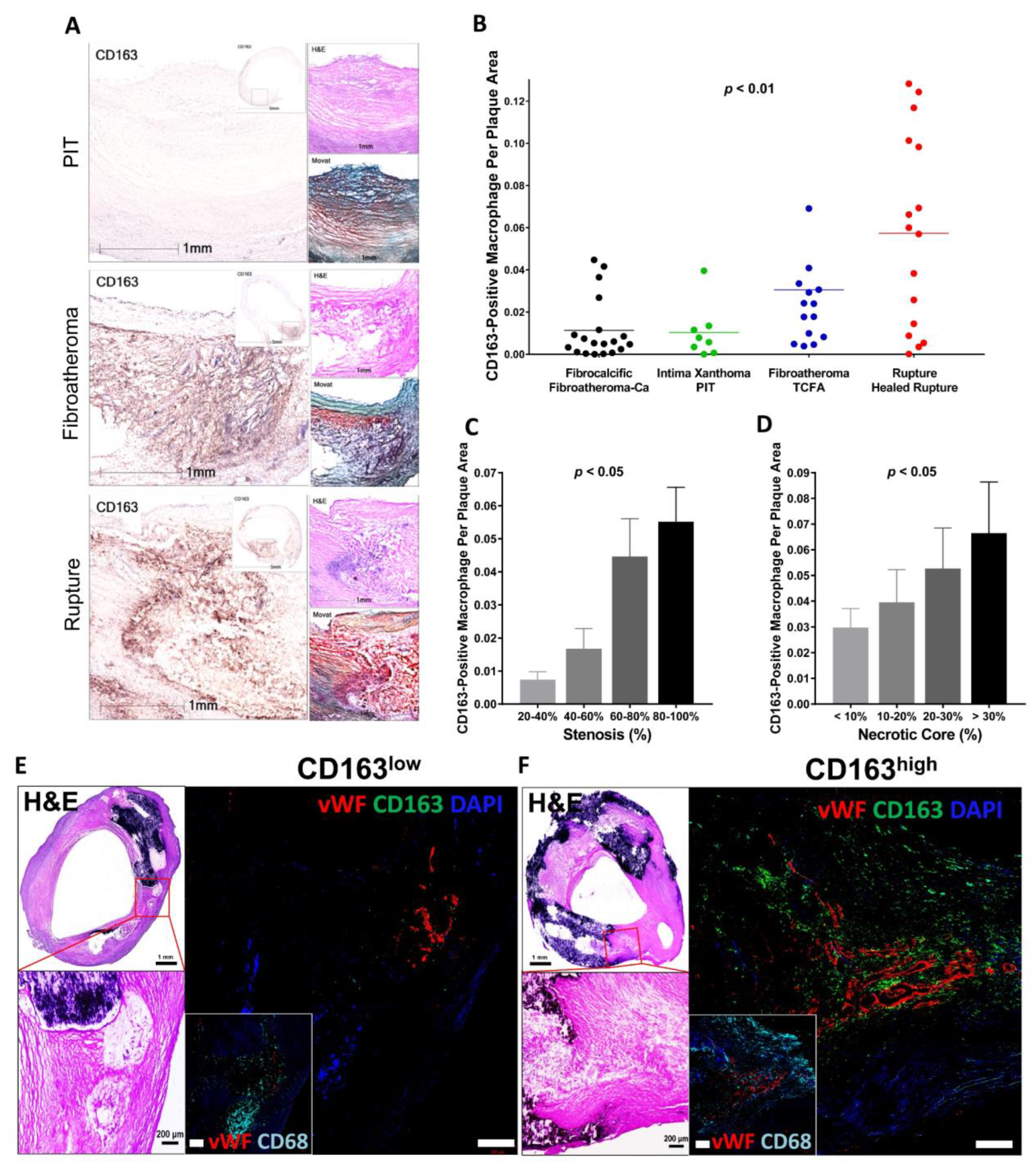
6. Intraplaque Hemorrhage and CD163+ Alternative Macrophages
6.1. CD163+ Macrophages, Microvascular Permeability and Inflammation
6.2. CD163+ Macrophages and Vascular Calcification
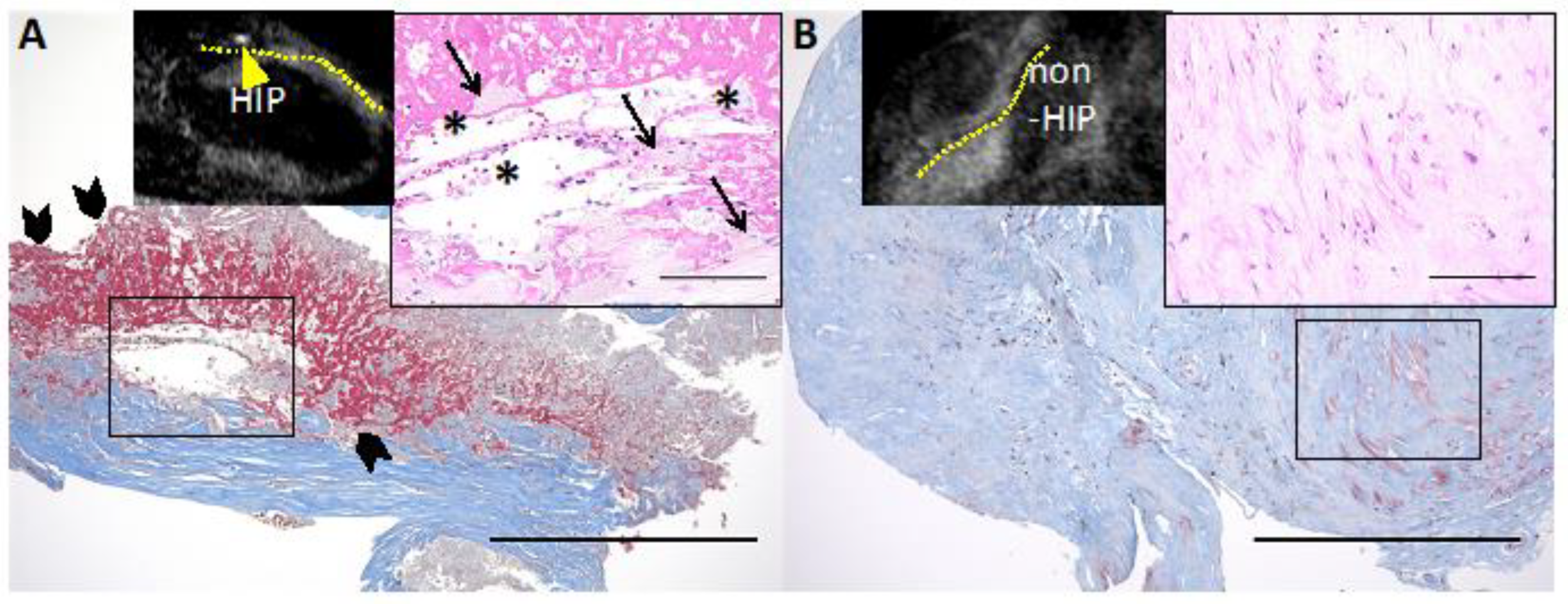
7. Intraplaque Hemorrhage and Clinical Prognosis Examined by Diagnostic Imaging Modalities
| VSL | Authors/Study | Population | Country | Modality | Characteristics of IPH | No. of Pt (lesion) | Mean F/O (months) | Endpoint | Results | Interpretation |
|---|---|---|---|---|---|---|---|---|---|---|
| CoA | Usui E. et al. 2021 [12] | Pts who underwent PCI due to ACS or SAP. NCLs of PCI-treated VSL. | JPN | OCT | LIA + CC | 566 (735) | 30 | MACE ** | CC + LIA were found in 114/735 NCLs (15.5%). Untreated NCLs with LIA + CC had an increased risk for NCL-MACE (adjusted HR 3.09 [95% CI 1.27–7.50]). | An OCT-detected LIA + CC in an NCL was associated with subsequent NCL-MACE. |
| CoA | Noguchi T. et al. [10] | Pts with SAP underwent cardiac CT and MRI | JPN | MRI | HIP * | 568 | 55 | MACE ** | HIP with PMRs ≥ 1.4 was the independent predictor of future coronary events (HR: 3.96 [95% CI 1.92–8.17]). | HIPs may represent a novel predictive factor. |
| CA | Rotterdam study [74] | Asy CA stenosis Pts detected by US w/o history of CAD and/or stroke | NLD | MRI | High intensity PL on 3D-T1W-GRE | 1349 | 61.2 | Stroke, cardiac death, CoA revascularization | MRI-derived IPH was associated with incident stroke and CHD (adjusted HR: 2.42 [95% CI: 1.30–4.50], and 1.95 [95% CI 1.20–3.14]). | IPH in the CA P is an independent RF for stroke and CHD. IPH can be a marker of plaque vulnerability. |
| CA | Saam T. et al. [9] | Meta analysis of 8 studies: Sym and/or Asy CA stenosis (30–99% stenosis) | USA, CAN, JPN, UK | MRI | (1) High intensity PL on 3D-T1W-GRE or (2) classification of IPH by levels of T1W, T2W/PDW and TOF intensities | 689 (712) | 19.6 | Stroke, TIA | MRI-derived IPH was associated with an ∼6-fold higher risk for events (HR: 5.69 [95% CI 2.98–10.87]). | Presence of IPH on MRI strongly predicts cerebrovascular events. |
| CA | Gupta A. et al. [76] | Meta analysis of 9 studies: Sym and/or Asy CA stenosis (<50–79% stenosis) | USA, CAN, JPN, NLD, CHE, UK | MRI | (1) High intensity PL on 3D-T1W-GRE or (2) classification of IPH by levels of T1W, T2W/PDW and TOF intensities | 678 (702) | 20.2 | Stroke, TIA | The HR for MRI-defined IPH as predictor of subsequent stroke/TIA were 4.59 [95% CI 2.91–7.24]. | MRI-defined IPH is associated with risk for stroke or TIA. Plaque composition by MRI gives stroke risk information beyond degree of stenosis. |
| CA | Singh N. et al. [82] | Asy CA atherosclerosis Pts detected by US (50–70% stenosis) | CAN | MRI | High intensity PL on 3D-T1W-GRE | 75 (98) | 24.9 | Stroke, TIA | MR-derived IPH was associated with an increased risk of cerebrovascular events (HR 3.59 [95% CI 2.48–4.71]). | MRI-defined IPH is associated with stroke/TIA in Asy CA stenosis. The absence of IPH at MRI may be a marker of plaque stability and of a lower risk of future event. |
| CA | Takaya N. et al. [83] | Asy CA atherosclerosis Pts detected by US (50–79% stenosis) | USA | MRI | Classification of IPH (fresh, recent, old) by levels of T1W, T2W/PDW and TOF intensities | 154 | 38.2 | Stroke, TIA | Presence of MRI-defined IPH (HR 5.2 [95% CI 1.6–17.3]) and larger mean IPH area (HR for 10 mm2 increase 2.6 [95% CI 1.4–4.6]) were associated with subsequent symptoms. | IPH by MRI is associated with future cerebrovascular events in patients with asymptomatic moderate carotid stenosis. |
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Cornelissen, A.; Sato, Y.; Mori, M.; Kawakami, R.; Kawai, K.; Ghosh, S.K.B.; Xu, W.; Abebe, B.G.; Dikongue, A.; et al. Vulnerable Plaque in Patients with Acute Coronary Syndrome: Identification, Importance, and Management. US Cardiol. Rev. 2022, 16, e01. [Google Scholar] [CrossRef]
- Kramer, M.C.; Rittersma, S.Z.; de Winter, R.J.; Ladich, E.R.; Fowler, D.R.; Liang, Y.H.; Kutys, R.; Carter-Monroe, N.; Kolodgie, F.D.; van der Wal, A.C.; et al. Relationship of thrombus healing to underlying plaque morphology in sudden coronary death. J. Am. Coll. Cardiol. 2010, 55, 122–132. [Google Scholar] [CrossRef]
- Jia, H.; Abtahian, F.; Aguirre, A.D.; Lee, S.; Chia, S.; Lowe, H.; Kato, K.; Yonetsu, T.; Vergallo, R.; Hu, S.; et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J. Am. Coll. Cardiol. 2013, 62, 1748–1758. [Google Scholar] [CrossRef]
- Michel, J.B.; Virmani, R.; Arbustini, E.; Pasterkamp, G. Intraplaque haemorrhages as the trigger of plaque vulnerability. Eur. Heart J. 2011, 32, 1977–1985. [Google Scholar] [CrossRef]
- Takaya, N.; Yuan, C.; Chu, B.; Saam, T.; Polissar, N.L.; Jarvik, G.P.; Isaac, C.; McDonough, J.; Natiello, C.; Small, R.; et al. Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: A high-resolution magnetic resonance imaging study. Circulation 2005, 111, 2768–2775. [Google Scholar] [CrossRef]
- Schindler, A.; Schinner, R.; Altaf, N.; Hosseini, A.A.; Simpson, R.J.; Esposito-Bauer, L.; Singh, N.; Kwee, R.M.; Kurosaki, Y.; Yamagata, S.; et al. Prediction of Stroke Risk by Detection of Hemorrhage in Carotid Plaques: Meta-Analysis of Individual Patient Data. JACC Cardiovasc. Imaging 2020, 13, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Saam, T.; Hetterich, H.; Hoffmann, V.; Yuan, C.; Dichgans, M.; Poppert, H.; Koeppel, T.; Hoffmann, U.; Reiser, M.F.; Bamberg, F. Meta-analysis and systematic review of the predictive value of carotid plaque hemorrhage on cerebrovascular events by magnetic resonance imaging. J. Am. Coll. Cardiol. 2013, 62, 1081–1091. [Google Scholar] [CrossRef]
- Noguchi, T.; Kawasaki, T.; Tanaka, A.; Yasuda, S.; Goto, Y.; Ishihara, M.; Nishimura, K.; Miyamoto, Y.; Node, K.; Koga, N. High-intensity signals in coronary plaques on noncontrast T1-weighted magnetic resonance imaging as a novel determinant of coronary events. J. Am. Coll. Cardiol. 2014, 63, 989–999. [Google Scholar] [CrossRef]
- Uzu, K.; Kawakami, R.; Sawada, T.; Takaya, T.; Taniguchi, Y.; Hirota, S.; Fujii, K.; Yasaka, Y.; Kawai, H. Histopathological Characterization of High-Intensity Signals in Coronary Plaques on Noncontrast T1-Weighted Magnetic Resonance Imaging. JACC Cardiovasc. Imaging 2021, 14, 518–519. [Google Scholar] [CrossRef]
- Usui, E.; Matsumura, M.; Mintz, G.S.; Zhou, Z.; Hada, M.; Yamaguchi, M.; Hoshino, M.; Kanaji, Y.; Sugiyama, T.; Murai, T.; et al. Clinical outcomes of low-intensity area without attenuation and cholesterol crystals in non-culprit lesions assessed by optical coherence tomography. Atherosclerosis 2021, 332, 41–47. [Google Scholar] [CrossRef]
- Paterson, J.C. Vascularization and hemorrhage of the intima of coronary atherosclerotic arteries. Arch. Pathol. 1936, 22, 312–324. [Google Scholar]
- Wartman, W.B. Occlusion of the coronary arteries by hemorrhage into their walls. Am. Heart J. 1938, 5, 459–470. [Google Scholar] [CrossRef]
- Glagov, S.; Weisenberg, E.; Zarins, C.K.; Stankunavicius, R.; Kolettis, G.J. Compensatory enlargement of human atherosclerotic coronary arteries. N. Engl. J. Med. 1987, 316, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.P.; Kolodgie, F.D.; Farb, A.; Weber, D.K.; Malcom, G.T.; Smialek, J.; Virmani, R. Healed plaque ruptures and sudden coronary death: Evidence that subclinical rupture has a role in plaque progression. Circulation 2001, 103, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.; Davies, M.J. Mechanisms of progression in native coronary artery disease: Role of healed plaque disruption. Heart 1999, 82, 265–268. [Google Scholar] [CrossRef]
- Kolodgie, F.D.; Gold, H.K.; Burke, A.P.; Fowler, D.R.; Kruth, H.S.; Weber, D.K.; Farb, A.; Guerrero, L.J.; Hayase, M.; Kutys, R.; et al. Intraplaque hemorrhage and progression of coronary atheroma. N. Engl. J. Med. 2003, 349, 2316–2325. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, H.; Baluk, P.; Morikawa, S.; McLean, J.W.; Thurston, G.; Roberge, S.; Jain, R.K.; McDonald, D.M. Openings between defective endothelial cells explain tumor vessel leakiness. Am. J. Pathol. 2000, 156, 1363–1380. [Google Scholar] [CrossRef]
- Dejana, E. Endothelial cell-cell junctions: Happy together. Nat. Rev. Mol. Cell Biol. 2004, 5, 261–270. [Google Scholar] [CrossRef]
- Sluimer, J.C.; Kolodgie, F.D.; Bijnens, A.P.; Maxfield, K.; Pacheco, E.; Kutys, B.; Duimel, H.; Frederik, P.M.; van Hinsbergh, V.W.; Virmani, R.; et al. Thin-walled microvessels in human coronary atherosclerotic plaques show incomplete endothelial junctions relevance of compromised structural integrity for intraplaque microvascular leakage. J. Am. Coll. Cardiol. 2009, 53, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Kaartinen, M.; Penttilä, A.; Kovanen, P.T. Mast cells accompany microvessels in human coronary atheromas: Implications for intimal neovascularization and hemorrhage. Atherosclerosis 1996, 123, 123–131. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, P.; Emini Veseli, B.; Van der Veken, B.; Roth, L.; Martinet, W.; De Meyer, G.R.Y. Pharmacological strategies to inhibit Intraplaque angiogenesis in atherosclerosis. Vasc. Pharmacol. 2019, 112, 72–78. [Google Scholar] [CrossRef] [PubMed]
- de Vries, M.R.; Niessen, H.W.; Löwik, C.W.; Hamming, J.F.; Jukema, J.W.; Quax, P.H. Plaque rupture complications in murine atherosclerotic vein grafts can be prevented by TIMP-1 overexpression. PLoS ONE 2012, 7, e47134. [Google Scholar] [CrossRef]
- Sedding, D.G.; Boyle, E.C.; Demandt, J.A.F.; Sluimer, J.C.; Dutzmann, J.; Haverich, A.; Bauersachs, J. Vasa Vasorum Angiogenesis: Key Player in the Initiation and Progression of Atherosclerosis and Potential Target for the Treatment of Cardiovascular Disease. Front. Immunol. 2018, 9, 706. [Google Scholar] [CrossRef]
- Moreno, P.R.; Purushothaman, K.R.; Zias, E.; Sanz, J.; Fuster, V. Neovascularization in human atherosclerosis. Curr. Mol. Med. 2006, 6, 457–477. [Google Scholar] [CrossRef]
- Galis, Z.S.; Sukhova, G.K.; Lark, M.W.; Libby, P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J. Clin. Investig. 1994, 94, 2493–2503. [Google Scholar] [CrossRef]
- Pedrigi, R.M.; de Silva, R.; Bovens, S.M.; Mehta, V.V.; Petretto, E.; Krams, R. Thin-cap fibroatheroma rupture is associated with a fine interplay of shear and wall stress. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2224–2231. [Google Scholar] [CrossRef]
- Cunningham, K.S.; Gotlieb, A.I. The role of shear stress in the pathogenesis of atherosclerosis. Lab. Investig. 2005, 85, 9–23. [Google Scholar] [CrossRef]
- Wentzel, J.J.; Chatzizisis, Y.S.; Gijsen, F.J.; Giannoglou, G.D.; Feldman, C.L.; Stone, P.H. Endothelial shear stress in the evolution of coronary atherosclerotic plaque and vascular remodelling: Current understanding and remaining questions. Cardiovasc. Res. 2012, 96, 234–243. [Google Scholar] [CrossRef]
- Malek, A.M.; Alper, S.L.; Izumo, S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 1999, 282, 2035–2042. [Google Scholar] [CrossRef]
- Slager, C.J.; Wentzel, J.J.; Gijsen, F.J.; Schuurbiers, J.C.; van der Wal, A.C.; van der Steen, A.F.; Serruys, P.W. The role of shear stress in the generation of rupture-prone vulnerable plaques. Nat. Clin. Pract. Cardiovasc. Med. 2005, 2, 401–407. [Google Scholar] [CrossRef]
- Lucerna, M.; Zernecke, A.; de Nooijer, R.; de Jager, S.C.; Bot, I.; van der Lans, C.; Kholova, I.; Liehn, E.A.; van Berkel, T.J.; Yla-Herttuala, S.; et al. Vascular endothelial growth factor-A induces plaque expansion in ApoE knock-out mice by promoting de novo leukocyte recruitment. Blood 2007, 109, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; van Haperen, R.; de Waard, M.; van Damme, L.C.; Tempel, D.; Hanemaaijer, L.; van Cappellen, G.W.; Bos, J.; Slager, C.J.; Duncker, D.J.; et al. Shear stress affects the intracellular distribution of eNOS: Direct demonstration by a novel in vivo technique. Blood 2005, 106, 3691–3698. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Ando, J. New molecular mechanisms for cardiovascular disease:blood flow sensing mechanism in vascular endothelial cells. J. Pharmacol. Sci. 2011, 116, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Metaxa, E.; Meng, H.; Kaluvala, S.R.; Szymanski, M.P.; Paluch, R.A.; Kolega, J. Nitric oxide-dependent stimulation of endothelial cell proliferation by sustained high flow. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H736–H742. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Sinha, S.; Majumder, S.; Muley, A.; Siamwala, J.H.; Gupta, R.; Chatterjee, S. Shear stress promotes nitric oxide production in endothelial cells by sub-cellular delocalization of eNOS: A basis for shear stress mediated angiogenesis. Nitric Oxide 2010, 22, 304–315. [Google Scholar] [CrossRef]
- Choi, Y.S.; Choi, H.J.; Min, J.K.; Pyun, B.J.; Maeng, Y.S.; Park, H.; Kim, J.; Kim, Y.M.; Kwon, Y.G. Interleukin-33 induces angiogenesis and vascular permeability through ST2/TRAF6-mediated endothelial nitric oxide production. Blood 2009, 114, 3117–3126. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qiu, J.; Luo, S.; Xie, X.; Zheng, Y.; Zhang, K.; Ye, Z.; Liu, W.; Gregersen, H.; Wang, G. High shear stress induces atherosclerotic vulnerable plaque formation through angiogenesis. Regen. Biomater. 2016, 3, 257–267. [Google Scholar] [CrossRef]
- Virmani, R.; Narula, J.; Farb, A. When neoangiogenesis ricochets. Am. Heart J. 1998, 136, 937–939. [Google Scholar] [CrossRef] [PubMed]
- Tangirala, R.K.; Jerome, W.G.; Jones, N.L.; Small, D.M.; Johnson, W.J.; Glick, J.M.; Mahlberg, F.H.; Rothblat, G.H. Formation of cholesterol monohydrate crystals in macrophage-derived foam cells. J. Lipid Res. 1994, 35, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Kolodgie, F.D.; Burke, A.P.; Nakazawa, G.; Cheng, Q.; Xu, X.; Virmani, R. Free cholesterol in atherosclerotic plaques: Where does it come from? Curr. Opin. Lipidol. 2007, 18, 500–507. [Google Scholar] [CrossRef]
- Warnatsch, A.; Ioannou, M.; Wang, Q.; Papayannopoulos, V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015, 349, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Vedre, A.; Pathak, D.R.; Crimp, M.; Lum, C.; Koochesfahani, M.; Abela, G.S. Physical factors that trigger cholesterol crystallization leading to plaque rupture. Atherosclerosis 2009, 203, 89–96. [Google Scholar] [CrossRef]
- Gravastrand, C.S.; Steinkjer, B.; Halvorsen, B.; Landsem, A.; Skjelland, M.; Jacobsen, E.A.; Woodruff, T.M.; Lambris, J.D.; Mollnes, T.E.; Brekke, O.L.; et al. Cholesterol Crystals Induce Coagulation Activation through Complement-Dependent Expression of Monocytic Tissue Factor. J. Immunol. 2019, 203, 853–863. [Google Scholar] [CrossRef]
- Crichton, R.R.; Wilmet, S.; Legssyer, R.; Ward, R.J. Molecular and cellular mechanisms of iron homeostasis and toxicity in mammalian cells. J. Inorg. Biochem. 2002, 91, 9–18. [Google Scholar] [CrossRef]
- Finn, A.V.; Nakano, M.; Polavarapu, R.; Karmali, V.; Saeed, O.; Zhao, X.; Yazdani, S.; Otsuka, F.; Davis, T.; Habib, A.; et al. Hemoglobin directs macrophage differentiation and prevents foam cell formation in human atherosclerotic plaques. J. Am. Coll. Cardiol. 2012, 59, 166–177. [Google Scholar] [CrossRef]
- Sindrilaru, A.; Peters, T.; Wieschalka, S.; Baican, C.; Baican, A.; Peter, H.; Hainzl, A.; Schatz, S.; Qi, Y.; Schlecht, A.; et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J. Clin. Investig. 2011, 121, 985–997. [Google Scholar] [CrossRef]
- Cominacini, L.; Pasini, A.F.; Garbin, U.; Davoli, A.; Tosetti, M.L.; Campagnola, M.; Rigoni, A.; Pastorino, A.M.; Lo Cascio, V.; Sawamura, T. Oxidized low density lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF-kappaB through an increased production of intracellular reactive oxygen species. J. Biol. Chem. 2000, 275, 12633–12638. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, H.; Chen, Y.; Liu, X.; Tian, J.; Shen, W. The role of macrophage iron overload and ferroptosis in atherosclerosis. Biomolecules 2022, 12, 1702. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.J.; Tabas, I. Macrophages in the pathogenesis of atherosclerosis. Cell 2011, 145, 341–355. [Google Scholar] [CrossRef]
- Otsuka, F.; Kramer, M.C.; Woudstra, P.; Yahagi, K.; Ladich, E.; Finn, A.V.; de Winter, R.J.; Kolodgie, F.D.; Wight, T.N.; Davis, H.R.; et al. Natural progression of atherosclerosis from pathologic intimal thickening to late fibroatheroma in human coronary arteries: A pathology study. Atherosclerosis 2015, 241, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Schrijvers, D.M.; De Meyer, G.R.; Kockx, M.M.; Herman, A.G.; Martinet, W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1256–1261. [Google Scholar] [CrossRef]
- Libby, P. Mechanisms of acute coronary syndromes and their implications for therapy. N. Engl. J. Med. 2013, 368, 2004–2013. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef]
- Libby, P.; Hansson, G.K. From Focal Lipid Storage to Systemic Inflammation: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 1594–1607. [Google Scholar] [CrossRef]
- Chinetti-Gbaguidi, G.; Colin, S.; Staels, B. Macrophage subsets in atherosclerosis. Nat. Rev. Cardiol. 2015, 12, 10–17. [Google Scholar] [CrossRef]
- Kawai, K.; Vozenilek, A.E.; Kawakami, R.; Sato, Y.; Ghosh, S.K.B.; Virmani, R.; Finn, A.V. Understanding the role of alternative macrophage phenotypes in human atherosclerosis. Expert Rev. Cardiovasc. Ther. 2022, 20, 689–705. [Google Scholar] [CrossRef]
- Fernandez, D.M.; Rahman, A.H.; Fernandez, N.F.; Chudnovskiy, A.; Amir, E.D.; Amadori, L.; Khan, N.S.; Wong, C.K.; Shamailova, R.; Hill, C.A.; et al. Single-cell immune landscape of human atherosclerotic plaques. Nat. Med. 2019, 25, 1576–1588. [Google Scholar]
- Boyle, J.J.; Johns, M.; Kampfer, T.; Nguyen, A.T.; Game, L.; Schaer, D.J.; Mason, J.C.; Haskard, D.O. Activating transcription factor 1 directs Mhem atheroprotective macrophages through coordinated iron handling and foam cell protection. Circ. Res. 2012, 110, 20–33. [Google Scholar] [PubMed]
- Nagy, E.; Eaton, J.W.; Jeney, V.; Soares, M.P.; Varga, Z.; Galajda, Z.; Szentmiklósi, J.; Méhes, G.; Csonka, T.; Smith, A.; et al. Red cells, hemoglobin, heme, iron, and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, M.; Graversen, J.H.; Jacobsen, C.; Sonne, O.; Hoffman, H.J.; Law, S.K.; Moestrup, S.K. Identification of the haemoglobin scavenger receptor. Nature 2001, 409, 198–201. [Google Scholar] [CrossRef]
- Nielsen, M.J.; Møller, H.J.; Moestrup, S.K. Hemoglobin and heme scavenger receptors. Antioxid. Redox Signal 2010, 12, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, E.; Hultman, K.; Edsfeldt, A.; Persson, A.; Nitulescu, M.; Nilsson, J.; Gonçalves, I.; Björkbacka, H. CD163+ macrophages are associated with a vulnerable plaque phenotype in human carotid plaques. Sci. Rep. 2020, 10, 14362. [Google Scholar] [CrossRef]
- Guo, L.; Akahori, H.; Harari, E.; Smith, S.L.; Polavarapu, R.; Karmali, V.; Otsuka, F.; Gannon, R.L.; Braumann, R.E.; Dickinson, M.H.; et al. CD163+ macrophages promote angiogenesis and vascular permeability accompanied by inflammation in atherosclerosis. J. Clin. Investig. 2018, 128, 1106–1124. [Google Scholar] [CrossRef]
- Sakamoto, A.; Kawakami, R.; Mori, M.; Guo, L.; Paek, K.H.; Mosquera, J.V.; Cornelissen, A.; Ghosh, S.K.B.; Kawai, K.; Konishi, T.; et al. CD163+ macrophages restrain vascular calcification, promoting the development of high-risk plaque. JCI Insight 2023, 8, e154922. [Google Scholar] [CrossRef]
- Mori, H.; Torii, S.; Kutyna, M.; Sakamoto, A.; Finn, A.V.; Virmani, R. Coronary Artery Calcification and its Progression: What Does it Really Mean? JACC Cardiovasc. Imaging 2018, 11, 127–142. [Google Scholar]
- Criqui, M.H.; Denenberg, J.O.; Ix, J.H.; McClelland, R.L.; Wassel, C.L.; Rifkin, D.E.; Carr, J.J.; Budoff, M.J.; Allison, M.A. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA 2014, 311, 271–278. [Google Scholar] [CrossRef]
- Vengrenyuk, Y.; Carlier, S.; Xanthos, S.; Cardoso, L.; Ganatos, P.; Virmani, R.; Einav, S.; Gilchrist, L.; Weinbaum, S. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proc. Natl. Acad. Sci. USA 2006, 103, 14678–14683. [Google Scholar] [PubMed]
- Qiao, Y.; Etesami, M.; Malhotra, S.; Astor, B.C.; Virmani, R.; Kolodgie, F.D.; Trout, H.H., 3rd; Wasserman, B.A. Identification of intraplaque hemorrhage on MR angiography images: A comparison of contrast-enhanced mask and time-of-flight techniques. AJNR Am. J. Neuroradiol. 2011, 32, 454–459. [Google Scholar] [PubMed]
- van den Bouwhuijsen, Q.J.; Vernooij, M.W.; Hofman, A.; Krestin, G.P.; van der Lugt, A.; Witteman, J.C. Determinants of magnetic resonance imaging detected carotid plaque components: The Rotterdam Study. Eur. Heart J. 2012, 33, 221–229. [Google Scholar] [PubMed]
- Bos, D.; Arshi, B.; van den Bouwhuijsen, Q.J.A.; Ikram, M.K.; Selwaness, M.; Vernooij, M.W.; Kavousi, M.; van der Lugt, A. Atherosclerotic Carotid Plaque Composition and Incident Stroke and Coronary Events. J. Am. Coll. Cardiol. 2021, 77, 1426–1435. [Google Scholar] [PubMed]
- Zhao, X.Q.; Sun, J.; Hippe, D.S.; Isquith, D.A.; Canton, G.; Yamada, K.; Balu, N.; Crouse, J.R., 3rd; Anderson, T.J.; Huston, J., 3rd; et al. Magnetic Resonance Imaging of Intraplaque Hemorrhage and Plaque Lipid Content With Continued Lipid-Lowering Therapy: Results of a Magnetic Resonance Imaging Substudy in AIM-HIGH. Circ. Cardiovasc. Imaging 2022, 15, e014229. [Google Scholar]
- Gupta, A.; Baradaran, H.; Schweitzer, A.D.; Kamel, H.; Pandya, A.; Delgado, D.; Dunning, A.; Mushlin, A.I.; Sanelli, P.C. Carotid plaque MRI and stroke risk: A systematic review and meta-analysis. Stroke 2013, 44, 3071–3077. [Google Scholar]
- Saba, L.; Francone, M.; Bassareo, P.P.; Lai, L.; Sanfilippo, R.; Montisci, R.; Suri, J.S.; De Cecco, C.N.; Faa, G. CT Attenuation Analysis of Carotid Intraplaque Hemorrhage. AJNR Am. J. Neuroradiol. 2018, 39, 131–137. [Google Scholar]
- U-King-Im, J.M.; Fox, A.J.; Aviv, R.I.; Howard, P.; Yeung, R.; Moody, A.R.; Symons, S.P. Characterization of carotid plaque hemorrhage: A CT angiography and MR intraplaque hemorrhage study. Stroke 2010, 41, 1623–1629. [Google Scholar]
- Fujimoto, S.; Kondo, T.; Kodama, T.; Takase, S.; Narula, J. Delayed plaque enhancement by CT angiography. JACC Cardiovasc. Imaging 2012, 5, 1181–1182. [Google Scholar]
- Liu, W.; Xie, Y.; Wang, C.; Du, Y.; Nguyen, C.; Wang, Z.; Fan, Z.; Dong, L.; Liu, Y.; Bi, X.; et al. Atherosclerosis T1-weighted characterization (CATCH): Evaluation of the accuracy for identifying intraplaque hemorrhage with histological validation in carotid and coronary artery specimens. J. Cardiovasc. Magn. Reson. 2018, 20, 27. [Google Scholar]
- Sato, S.; Matsumoto, H.; Li, D.; Ohya, H.; Mori, H.; Sakai, K.; Ogura, K.; Oishi, Y.; Masaki, R.; Tanaka, H.; et al. Coronary High-Intensity Plaques at T1-weighted MRI in Stable Coronary Artery Disease: Comparison with Near-Infrared Spectroscopy Intravascular US. Radiology 2022, 302, 557–565. [Google Scholar] [PubMed]
- Singh, N.; Moody, A.R.; Gladstone, D.J.; Leung, G.; Ravikumar, R.; Zhan, J.; Maggisano, R. Moderate carotid artery stenosis: MR imaging-depicted intraplaque hemorrhage predicts risk of cerebrovascular ischemic events in asymptomatic men. Radiology 2009, 252, 502–508. [Google Scholar] [PubMed]
- Takaya, N.; Yuan, C.; Chu, B.; Saam, T.; Underhill, H.; Cai, J.; Tran, N.; Polissar, N.L.; Isaac, C.; Ferguson, M.S.; et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: A prospective assessment with MRI—Initial results. Stroke 2006, 37, 818–823. [Google Scholar] [PubMed]
- Htun, N.M.; Chen, Y.C.; Lim, B.; Schiller, T.; Maghzal, G.J.; Huang, A.L.; Elgass, K.D.; Rivera, J.; Schneider, H.G.; Wood, B.R.; et al. Near-infrared autofluorescence induced by intraplaque hemorrhage and heme degradation as marker for high-risk atherosclerotic plaques. Nat. Commun. 2017, 8, 75. [Google Scholar] [PubMed]
- Ughi, G.J.; Wang, H.; Gerbaud, E.; Gardecki, J.A.; Fard, A.M.; Hamidi, E.; Vacas-Jacques, P.; Rosenberg, M.; Jaffer, F.A.; Tearney, G.J. Clinical Characterization of Coronary Atherosclerosis With Dual-Modality OCT and Near-Infrared Autofluorescence Imaging. JACC Cardiovasc. Imaging 2016, 9, 1304–1314. [Google Scholar] [PubMed]
- Wang, H.; Gardecki, J.A.; Ughi, G.J.; Jacques, P.V.; Hamidi, E.; Tearney, G.J. Ex vivo catheter-based imaging of coronary atherosclerosis using multimodality OCT and NIRAF excited at 633 nm. Biomed. Opt. Express 2015, 6, 1363–1375. [Google Scholar]
- Kunio, M.; Gardecki, J.A.; Watanabe, K.; Nishimiya, K.; Verma, S.; Jaffer, F.A.; Tearney, G.J. Histopathological correlation of near infrared autofluorescence in human cadaver coronary arteries. Atherosclerosis 2022, 344, 31–39. [Google Scholar]
- Matsuura, Y.; Kanter, J.E.; Bornfeldt, K.E. Highlighting Residual Atherosclerotic Cardiovascular Disease Risk. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e1–e9. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakamoto, A.; Suwa, K.; Kawakami, R.; Finn, A.V.; Maekawa, Y.; Virmani, R.; Finn, A.V. Significance of Intra-plaque Hemorrhage for the Development of High-Risk Vulnerable Plaque: Current Understanding from Basic to Clinical Points of View. Int. J. Mol. Sci. 2023, 24, 13298. https://doi.org/10.3390/ijms241713298
Sakamoto A, Suwa K, Kawakami R, Finn AV, Maekawa Y, Virmani R, Finn AV. Significance of Intra-plaque Hemorrhage for the Development of High-Risk Vulnerable Plaque: Current Understanding from Basic to Clinical Points of View. International Journal of Molecular Sciences. 2023; 24(17):13298. https://doi.org/10.3390/ijms241713298
Chicago/Turabian StyleSakamoto, Atsushi, Kenichiro Suwa, Rika Kawakami, Alexandra V. Finn, Yuichiro Maekawa, Renu Virmani, and Aloke V. Finn. 2023. "Significance of Intra-plaque Hemorrhage for the Development of High-Risk Vulnerable Plaque: Current Understanding from Basic to Clinical Points of View" International Journal of Molecular Sciences 24, no. 17: 13298. https://doi.org/10.3390/ijms241713298
APA StyleSakamoto, A., Suwa, K., Kawakami, R., Finn, A. V., Maekawa, Y., Virmani, R., & Finn, A. V. (2023). Significance of Intra-plaque Hemorrhage for the Development of High-Risk Vulnerable Plaque: Current Understanding from Basic to Clinical Points of View. International Journal of Molecular Sciences, 24(17), 13298. https://doi.org/10.3390/ijms241713298





