The Case for the Target of Rapamycin Pathway as a Candidate Circadian Oscillator
Abstract
:1. Introduction
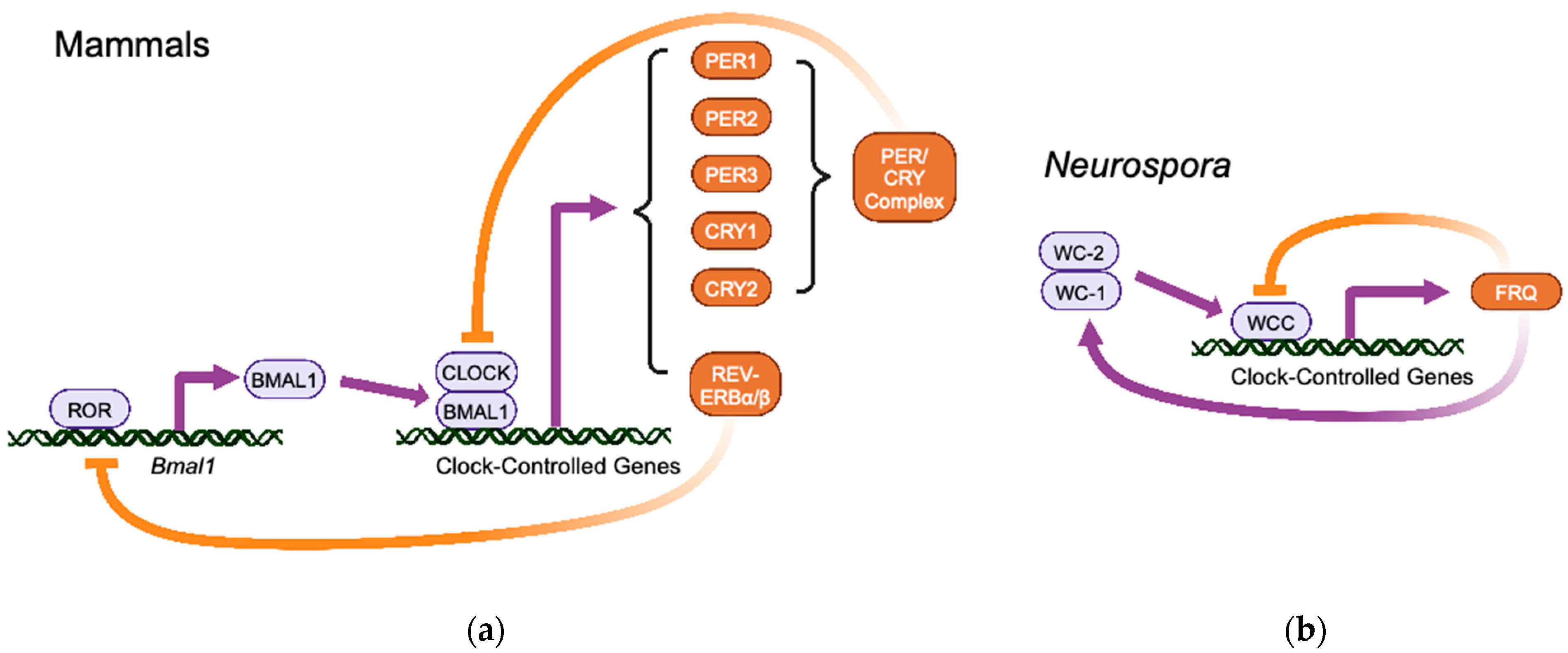
2. The TTFL Model of Circadian Oscillators in Eukaryotes
3. Rhythmicity in the Absence of the Canonical TTFL
3.1. Are TTFL Models Enough?
3.2. Non-TTFL Rhythms
4. The TOR (Target of Rapamycin) Pathway
4.1. TOR Complexes
4.2. Targets of TORC1 Output
4.3. Regulators of TORC1
4.4. TORC2
4.5. The TOR Pathway in Plants
5. TOR and the Circadian System
5.1. Criteria for Identifying an Oscillator
5.2. Is TOR Activity Rhythmic?
5.3. Does the TTFL Influence TOR?
5.4. Does TOR Influence Circadian Timing in the Presence of the TTFL?
5.5. Does TOR Participate in Circadian Timing in the Absence of the TTFL?
6. The TOR Pathway as a Self-Sustained Oscillator
6.1. The Relationship between TOR Rhythmicity and “Metabolic Oscillators”
6.2. Advantages of TOR as an Oscillator Candidate
7. Feedback Regulation of TOR Activity
7.1. Feedback Loops as the Basis for Oscillators
7.2. Protein Synthesis Feedback on TOR
7.3. Feedback from Autophagy to TOR
8. Ultradian Metabolic Rhythms in Yeast
9. Problems and Unanswered Questions
9.1. Is TOR Activity Rhythmic in the Absence of a TTFL?
9.2. Can the Kinetics of TOR Feedback Loops Account for 24 h Rhythmicity?
9.3. What about Cells without TOR?
9.4. Are TOR Effects on Rhythmicity “Merely Pleiotropic”?
9.5. What Is the TTFL for?
10. Conclusions
Funding
Conflicts of Interest
References
- Pittendrigh, C.S. Circadian rhythms and the circadian organization of living systems. Cold Spring Harb. Symp. Quant. Biol. 1960, 25, 159–184. [Google Scholar] [CrossRef] [PubMed]
- Hardin, P.E.; Hall, J.C.; Rosbash, M. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature 1990, 343, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Patke, A.; Young, M.W.; Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020, 21, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.L.; Loros, J.J.; Dunlap, J.C. The circadian clock of Neurospora crassa. FEMS Microbiol. Rev. 2012, 36, 95–110. [Google Scholar] [CrossRef]
- Bell-Pedersen, D. Understanding circadian rhythmicity in Neurospora crassa: From behavior to genes and back again. Fungal Genet. Biol. 2000, 29, 1–18. [Google Scholar] [CrossRef]
- Lakin-Thomas, P.; Bell-Pedersen, D.; Brody, S. The genetics of circadian rhythms in Neurospora. In The Genetics of Circadian Rhythms; Brody, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 55–104. [Google Scholar] [CrossRef]
- Greenham, K.; McClung, C.R. Integrating circadian dynamics with physiological processes in plants. Nat. Rev. Genet. 2015, 16, 598–610. [Google Scholar] [CrossRef]
- Petersen, J.; Rredhi, A.; Szyttenholm, J.; Mittag, M. Evolution of circadian clocks along the green lineage. Plant Physiol. 2022, 190, 924–937. [Google Scholar] [CrossRef]
- Roenneberg, T.; Merrow, M. Circadian systems and metabolism. J. Biol. Rhythm. 1999, 14, 449–459. [Google Scholar] [CrossRef]
- Hastings, M.H.; Maywood, E.S.; O’Neill, J.S. Cellular circadian pacemaking and the role of cytosolic rhythms. Curr. Biol. 2008, 18, R805–R815. [Google Scholar] [CrossRef]
- Qin, X.; Byrne, M.; Xu, Y.; Mori, T.; Johnson, C.H. Coupling of a core post-translational pacemaker to a slave transcription/translation feedback loop in a circadian system. PLoS Biol. 2010, 8, e1000394. [Google Scholar] [CrossRef]
- van Ooijen, G.; Millar, A.J. Non-transcriptional oscillators in circadian timekeeping. Trends Biochem. Sci. 2012, 37, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Milev, N.B.; Rhee, S.G.; Reddy, A.B. Cellular timekeeping: It’s redox o’clock. Cold Spring Harb. Perspect. Biol. 2018, 10, a027698. [Google Scholar] [CrossRef] [PubMed]
- Milev, N.B.; Reddy, A.B. Circadian redox oscillations and metabolism. Trends Endocrinol. Metab. 2015, 26, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Sugiyama, S.; Byrne, M.; Johnson, C.H.; Uchihashi, T.; Ando, T. Revealing circadian mechanisms of integration and resilience by visualizing clock proteins working in real time. Nat. Commun. 2018, 9, 3245. [Google Scholar] [CrossRef]
- Lakin-Thomas, P.L. Transcriptional feedback oscillators: Maybe, maybe not…. J. Biol. Rhythm. 2006, 21, 83–92. [Google Scholar] [CrossRef]
- Lakin-Thomas, P. Circadian rhythms, metabolic oscillators, and the target of rapamycin (TOR) pathway: The Neurospora connection. Curr. Genet. 2019, 65, 339–349. [Google Scholar] [CrossRef]
- Putker, M.; O’Neill, J.S. Reciprocal control of the circadian clock and cellular redox state—A critical appraisal. Mol. Cells 2016, 39, 6–19. [Google Scholar] [CrossRef]
- Roy, S.; Beauchemin, M.; Dagenais-Bellefeuille, S.; Letourneau, L.; Cappadocia, M.; Morse, D. The Lingulodinium circadian system lacks rhythmic changes in transcript abundance. BMC Biol. 2014, 12, 107. [Google Scholar] [CrossRef]
- Ray, S.; Valekunja, U.K.; Stangherlin, A.; Howell, S.A.; Snijders, A.P.; Damodaran, G.; Reddy, A.B. Circadian rhythms in the absence of the clock gene Bmal1. Science 2020, 367, 800. [Google Scholar] [CrossRef]
- Putker, M.; Wong, D.C.S.; Seinkmane, E.; Rzechorzek, N.M.; Zeng, A.; Hoyle, N.P.; Chesham, J.E.; Edwards, M.D.; Feeney, K.A.; Fischer, R.; et al. CRYPTOCHROMES confer robustness, not rhythmicity, to circadian timekeeping. EMBO J. 2021, 40, e106745. [Google Scholar] [CrossRef]
- Krahmer, J.; Hindle, M.; Perby, L.K.; Mogensen, H.K.; Nielsen, T.H.; Halliday, K.J.; van Ooijen, G.; Le Bihan, T.; Millar, A.J. The circadian clock gene circuit controls protein and phosphoprotein rhythms in Arabidopsis thaliana. Mol. Cell. Proteom. 2022, 21, 100172. [Google Scholar] [CrossRef]
- Edgar, R.S.; Green, E.W.; Zhao, Y.; Van Ooijen, G.; Olmedo, M.; Qin, X.; Xu, Y.; Pan, M.; Valekunja, U.K.; Feeney, K.A.; et al. Peroxiredoxins are conserved markers of circadian rhythms. Nature 2012, 485, 459–464. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J.S.; van Ooijen, G.; Dixon, L.E.; Troein, C.; Corellou, F.; Bouget, F.-Y.; Reddy, A.B.; Millar, A.J. Circadian rhythms persist without transcription in a eukaryote. Nature 2011, 469, 554–558. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J.S.; Reddy, A.B. Circadian clocks in human red blood cells. Nature 2011, 469, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Causton, H.C.; Feeney, K.A.; Ziegler, C.A.; O’Neill, J.S. Metabolic cycles in yeast share features conserved among circadian rhythms. Curr. Biol. 2015, 25, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, L.; Adhvaryu, K.K.; Kafes, E.; Motavaze, K.; Lakin-Thomas, P. A component of the TOR (Target Of Rapamycin) nutrient-sensing pathway plays a role in circadian rhythmicity in Neurospora crassa. PLoS Genet. 2018, 14, e1007457. [Google Scholar] [CrossRef]
- Li, S.; Motavaze, K.; Kafes, E.; Suntharalingam, S.; Lakin-Thomas, P. A new mutation affecting FRQ-less rhythms in the circadian system of Neurospora crassa. PLoS Genet. 2011, 7, e1002151. [Google Scholar] [CrossRef]
- Eskandari, R.; Ratnayake, L.; Lakin-Thomas, P.L. Shared components of the FRQ-less oscillator and TOR pathway maintain rhythmicity in Neurospora. J. Biol. Rhythm. 2021, 36, 329–345. [Google Scholar] [CrossRef]
- Loewith, R.; Hall, M.N. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics 2011, 189, 1177–1201. [Google Scholar] [CrossRef]
- Albert, V.; Hall, M.N. mTOR signaling in cellular and organismal energetics. Curr. Opin. Cell Biol. 2015, 33, 55–66. [Google Scholar] [CrossRef]
- Eltschinger, S.; Loewith, R. TOR complexes and the maintenance of cellular homeostasis. Trends Cell Biol. 2016, 26, 148–159. [Google Scholar] [CrossRef]
- González, A.; Hall, M.N. Nutrient sensing and TOR signaling in yeast and mammals. EMBO J. 2017, 36, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR signaling in growth, metabolism, and disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Thorner, J. TOR complex 2 is a master regulator of plasma membrane homeostasis. Biochem. J. 2022, 479, 1917–1940. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Zhang, N.; Li, J.; Shen, X.; Sheen, J.; Xiong, Y. TOR kinase, a GPS in the complex nutrient and hormonal signaling networks to guide plant growth and development. J. Exp. Bot. 2022, 73, 7041–7054. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Merrow, M. Molecular circadian oscillators: An alternative hypothesis. J. Biol. Rhythm. 1998, 13, 167–179. [Google Scholar] [CrossRef]
- Cornu, M.; Oppliger, W.; Albert, V.; Robitaille, A.M.; Trapani, F.; Quagliata, L.; Fuhrer, T.; Sauer, U.; Terracciano, L.; Hall, M.N. Hepatic mTORC1 controls locomotor activity, body temperature, and lipid metabolism through FGF21. Proc. Natl. Acad. Sci. USA 2014, 111, 11592–11599. [Google Scholar] [CrossRef]
- Khapre, R.V.; Kondratova, A.A.; Patel, S.; Dubrovsky, Y.; Wrobel, M.; Antoch, M.P.; Kondratov, R.V. BMAL1-dependent regulation of the mTOR signaling pathway delays aging. Aging 2014, 6, 48–57. [Google Scholar] [CrossRef]
- Khapre, R.V.; Patel, S.A.; Kondratova, A.A.; Chaudhary, A.; Velingkaar, N.; Antoch, M.P.; Kondratov, R.V. Metabolic clock generates nutrient anticipation rhythms in mTOR signaling. Aging 2014, 6, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, H.; Matsunaga, N.; Fujioka, T.; Okazaki, F.; Akagawa, Y.; Tsurudome, Y.; Ono, M.; Kuwano, M.; Koyanagi, S.; Ohdo, S. Circadian regulation of mTOR by the ubiquitin pathway in renal cell carcinoma. Cancer Res. 2014, 74, 543–551. [Google Scholar] [CrossRef]
- Drägert, K.; Bhattacharya, I.; Hall, M.N.; Humar, R.; Battegay, E.; Haas, E. Basal mTORC2 activity and expression of its components display diurnal variation in mouse perivascular adipose tissue. Biochem. Biophys. Res. Commun. 2016, 473, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Dang, F.; Li, P.; Wang, P.; Xu, Q.; Liu, Z.; Li, Y.; Wu, Y.; Chen, Y.; Liu, Y. The circadian protein Period2 suppresses mTORC1 activity via recruiting Tsc1 to mTORC1 complex. Cell Metab. 2019, 29, 653–667.e6. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Anderson, F.E.; Jung, Y.J.; Dziema, H.; Obrietan, K. Circadian regulation of mammalian Target of Rapamycin signaling in the mouse suprachiasmatic nucleus. Neuroscience 2011, 181, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Jouffe, C.; Cretenet, G.; Symul, L.; Martin, E.; Atger, F.; Naef, F.; Gachon, F. The circadian clock coordinates ribosome biogenesis. PLoS Biol. 2013, 11, e1001455. [Google Scholar] [CrossRef]
- Robles, M.S.; Humphrey, S.J.; Mann, M. Phosphorylation is a central mechanism for circadian control of metabolism and physiology. Cell Metab. 2017, 25, 118–127. [Google Scholar] [CrossRef]
- Choudhary, M.K.; Nomura, Y.; Wang, L.; Nakagami, H.; Somers, D.E. Quantitative circadian phosphoproteomic analysis of Arabidopsis reveals extensive clock control of key components in physiological, metabolic, and signaling pathways. Mol. Cell. Proteom. 2015, 14, 2243–2260. [Google Scholar] [CrossRef]
- Enganti, R.; Cho, S.K.; Toperzer, J.D.; Urquidi-Camacho, R.A.; Cakir, O.S.; Ray, A.P.; Abraham, P.E.; Hettich, R.L.; von Arnim, A.G. Phosphorylation of ribosomal protein RPS6 integrates light signals and circadian clock signals. Front. Plant Sci. 2018, 8, 2210. [Google Scholar] [CrossRef]
- Urrea-Castellanos, R.; Caldana, C.; Henriques, R. Growing at the right time: Interconnecting the TOR pathway with photoperiod and circadian regulation. J. Exp. Bot. 2022, 73, 7006–7015. [Google Scholar] [CrossRef]
- Greenwood, M.; Locke, J.C. The circadian clock coordinates plant development through specificity at the tissue and cellular level. Curr. Opin. Plant Biol. 2020, 53, 65–72. [Google Scholar] [CrossRef]
- Zhang, Y.; Giacchetti, S.; Parouchev, A.; Hadadi, E.; Li, X.; Dallmann, R.; Xandri-Monje, H.; Portier, L.; Adam, R.; Lévi, F.; et al. Dosing time dependent in vitro pharmacodynamics of Everolimus despite a defective circadian clock. Cell Cycle 2018, 17, 33–42. [Google Scholar] [CrossRef]
- Huang, C.C.Y.; Ko, M.L.; Ko, G.Y.P. A new functional role for mechanistic/mammalian target of rapamycin complex 1 (mTORC1) in the circadian regulation of L-type voltage-gated calcium channels in avian cone photoreceptors. PLoS ONE 2013, 8, e73315. [Google Scholar] [CrossRef] [PubMed]
- Akhtari, G. The Relationship between TOR Activity and the Circadian Clock of Neurospora crassa. Master’s Thesis, York University, Toronto, ON, Canada, 2023. Available online: https://hdl.handle.net/10315/41332 (accessed on 24 August 2023).
- Karki, S.; Castillo, K.; Ding, Z.; Kerr, O.; Lamb, T.M.; Wu, C.; Sachs, M.S.; Bell-Pedersen, D. Circadian clock control of eIF2α phosphorylation is necessary for rhythmic translation initiation. Proc. Natl. Acad. Sci. USA 2020, 117, 10935–10945. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Lamb, T.M.; Boukhris, A.; Porter, R.; Bell-Pedersen, D. Circadian clock control of translation initiation factor eIF2α activity requires eIF2γ-dependent recruitment of rhythmic PPP-1 phosphatase in Neurospora crassa. mBio 2021, 12, 10–1128. [Google Scholar] [CrossRef]
- Diernfellner, A.C.R.; Querfurth, C.; Salazar, C.; Höfer, T.; Brunner, M. Phosphorylation modulates rapid nucleocytoplasmic shuttling and cytoplasmic accumulation of Neurospora clock protein FRQ on a circadian time scale. Genes Dev. 2009, 23, 2192–2200. [Google Scholar] [CrossRef] [PubMed]
- Cao, R. mTOR signaling, translational control, and the circadian clock. Front. Genet. 2018, 9, 367. [Google Scholar] [CrossRef]
- Cao, R.; Robinson, B.; Xu, H.; Gkogkas, C.; Khoutorsky, A.; Alain, T.; Yanagiya, A.; Nevarko, T.; Liu, A.; Amir, S.; et al. Translational control of entrainment and synchrony of the suprachiasmatic circadian clock by mTOR/4E-BP1 signaling. Neuron 2013, 79, 712–724. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Stowie, A.; de Zavalia, N.; Leise, T.; Pathak, S.S.; Drewes, L.R.; Davidson, A.J.; Amir, S.; Sonenberg, N.; Cao, R. mTOR signaling in VIP neurons regulates circadian clock synchrony and olfaction. Proc. Natl. Acad. Sci. USA 2018, 115, E3296–E3304. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, C.; Kathale, N.D.; Liu, D.; Lee, C.; Freeman, D.A.; Hogenesch, J.B.; Cao, R.; Liu, A.C. mTOR signaling regulates central and peripheral circadian clock function. PLoS Genet. 2018, 14, e1007369. [Google Scholar] [CrossRef]
- Feeney, K.A.; Hansen, L.L.; Putker, M.; Olivares-Yañez, C.; Day, J.; Eades, L.J.; Larrondo, L.F.; Hoyle, N.P.; O’Neill, J.S.; Van Ooijen, G. Daily magnesium fluxes regulate cellular timekeeping and energy balance. Nature 2016, 532, 375–379. [Google Scholar] [CrossRef]
- Lipton, J.O.; Boyle, L.M.; Yuan, E.D.; Hochstrasser, K.J.; Chifamba, F.F.; Nathan, A.; Tsai, P.T.; Davis, F.; Sahin, M. Aberrant proteostasis of BMAL1 underlies circadian abnormalities in a paradigmatic mTOR-opathy. Cell Rep. 2017, 20, 868–880. [Google Scholar] [CrossRef]
- Zheng, X.; Sehgal, A. AKT and TOR signaling set the pace of the circadian pacemaker. Curr. Biol. 2010, 20, 1203–1208. [Google Scholar] [CrossRef]
- Zhang, N.; Meng, Y.; Li, X.; Zhou, Y.; Ma, L.; Fu, L.; Schwarzländer, M.; Liu, H.; Xiong, Y. Metabolite-mediated TOR signaling regulates the circadian clock in Arabidopsis. Proc. Natl. Acad. Sci. USA 2019, 116, 25395–25397. [Google Scholar] [CrossRef]
- Diernfellner, A.C.R.; Lauinger, L.; Shostak, A.; Brunner, M. A pathway linking translation stress to checkpoint kinase 2 signaling in Neurospora crassa. Proc. Natl. Acad. Sci. USA 2019, 116, 17271–17279. [Google Scholar] [CrossRef] [PubMed]
- Stangherlin, A.; Seinkmane, E.; O’Neill, J.S. Understanding circadian regulation of mammalian cell function, protein homeostasis, and metabolism. Curr. Opin. Syst. Biol. 2021, 28, 100391. [Google Scholar] [CrossRef] [PubMed]
- Roustan, V.; Jain, A.; Teige, M.; Ebersberger, I.; Weckwerth, W. An evolutionary perspective of AMPK-TOR signaling in the three domains of life. J. Exp. Bot. 2016, 67, 3897–3907. [Google Scholar] [CrossRef]
- Gressner, A.M.; Wool, I.G. The stimulation of the phosphorylation of ribosomal protein S6 by cycloheximide and puromycin. Biochem. Biophys. Res. Commun. 1974, 60, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.J.; Schreiber, S.L. A signaling pathway to translational control. Cell 1996, 86, 517–520. [Google Scholar] [CrossRef]
- Urban, J.; Soulard, A.; Huber, A.; Lippman, S.; Mukhopadhyay, D.; Deloche, O.; Wanke, V.; Anrather, D.; Ammerer, G.; Riezman, H.; et al. Sch9 Is a major target of TORC1 in Saccharomyces cerevisiae. Mol. Cell 2007, 26, 663–674. [Google Scholar] [CrossRef]
- Beugnet, A.; Tee, A.R.; Taylor, P.M.; Proud, C.G. Regulation of targets of mTOR (mammalian target of rapamycin) signalling by intracellular amino acid availability. Biochem. J. 2003, 372, 555–566. [Google Scholar] [CrossRef]
- Lempiäinen, H.; Uotila, A.; Urban, J.; Dohnal, I.; Ammerer, G.; Loewith, R.; Shore, D. Sfp1 interaction with TORC1 and Mrs6 reveals feedback regulation on TOR signaling. Mol. Cell 2009, 33, 704–716. [Google Scholar] [CrossRef]
- Kimball, S.R.; Do, A.N.D.; Kutzler, L.; Cavener, D.R.; Jefferson, L.S. Rapid turnover of the mTOR complex 1 (mTORC1) repressor REDD1 and activation of mTORC1 signaling following inhibition of protein synthesis. J. Biol. Chem. 2008, 283, 3465–3475. [Google Scholar] [CrossRef] [PubMed]
- Koromilas, A.E. M(en)TORship lessons on life and death by the integrated stress response. Biochim. Et Biophys. Acta Gen. Subj. 2019, 1863, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; McPhee, C.K.; Zheng, L.; Mardones, G.A.; Rong, Y.; Peng, J.; Mi, N.; Zhao, Y.; Liu, Z.; Wan, F.; et al. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature 2010, 465, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.S.; Huh, W.K. Bidirectional regulation between TORC1 and autophagy in Saccharomyces cerevisiae. Autophagy 2011, 7, 854–862. [Google Scholar] [CrossRef]
- Papagiannakis, A.; Niebel, B.; Wit, E.C.; Heinemann, M. Autonomous metabolic oscillations robustly gate the early and late cell cycle. Mol. Cell 2017, 65, 285–295. [Google Scholar] [CrossRef]
- Baumgartner, B.L.; O’Laughlin, R.; Jin, M.; Tsimring, L.S.; Hao, N.; Hasty, J. Flavin-based metabolic cycles are integral features of growth and division in single yeast cells. Sci. Rep. 2018, 8, 18045. [Google Scholar] [CrossRef]
- Amponsah, P.S.; Yahya, G.; Zimmermann, J.; Mai, M.; Mergel, S.; Mühlhaus, T.; Storchova, Z.; Morgan, B. Peroxiredoxins couple metabolism and cell division in an ultradian cycle. Nat. Chem. Biol. 2021, 17, 477–484. [Google Scholar] [CrossRef]
- O’Neill, J.S. Redox-coupled rhythm and brews. Nat. Chem. Biol. 2021, 17, 373–374. [Google Scholar] [CrossRef]
- Guerra, P.; Vuillemenot, L.A.P.E.; van Oppen, Y.B.; Been, M.; Milias-Argeitis, A. TORC1 and PKA activity towards ribosome biogenesis oscillates in synchrony with the budding yeast cell cycle. J. Cell Sci. 2022, 135, jcs260378. [Google Scholar] [CrossRef]
- Gardner, G.F.; Feldman, J.F. Temperature compensation of circadian period length in clock mutants of Neurospora crassa. Plant Physiol. 1981, 68, 1244–1248. [Google Scholar] [CrossRef]
- Kelliher, C.M.; Stevenson, E.L.; Loros, J.J.; Dunlap, J.C. Nutritional compensation of the circadian clock is a conserved process influenced by gene expression regulation and mRNA stability. PLoS Biol. 2023, 21, e3001961. [Google Scholar] [CrossRef] [PubMed]
- Loros, J.J.; Feldman, J.F. Loss of temperature compensation of circadian period length in the frq-9 mutant of Neurospora crassa. J. Biol. Rhythm. 1986, 1, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Aronson, B.D.; Johnson, K.A.; Dunlap, J.C. Circadian clock locus Frequency: Protein encoded by a single open reading frame defines period length and temperature compensation. Proc. Natl. Acad. Sci. USA 1994, 91, 7683–7687. [Google Scholar] [CrossRef]
- Lakin-Thomas, P.L.; Brody, S. Circadian rhythms in Neurospora crassa: Lipid deficiencies restore robust rhythmicity to null frequency and white-collar mutants. Proc. Natl. Acad. Sci. USA 2000, 97, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Granshaw, T.; Tsukamoto, M.; Brody, S. Circadian rhythms in Neurospora crassa: Farnesol or geraniol allow expression of rhythmicity in the otherwise arrhythmic strains frq10, wc-1, and wc-2. J. Biol. Rhythm. 2003, 18, 287–296. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakin-Thomas, P. The Case for the Target of Rapamycin Pathway as a Candidate Circadian Oscillator. Int. J. Mol. Sci. 2023, 24, 13307. https://doi.org/10.3390/ijms241713307
Lakin-Thomas P. The Case for the Target of Rapamycin Pathway as a Candidate Circadian Oscillator. International Journal of Molecular Sciences. 2023; 24(17):13307. https://doi.org/10.3390/ijms241713307
Chicago/Turabian StyleLakin-Thomas, Patricia. 2023. "The Case for the Target of Rapamycin Pathway as a Candidate Circadian Oscillator" International Journal of Molecular Sciences 24, no. 17: 13307. https://doi.org/10.3390/ijms241713307
APA StyleLakin-Thomas, P. (2023). The Case for the Target of Rapamycin Pathway as a Candidate Circadian Oscillator. International Journal of Molecular Sciences, 24(17), 13307. https://doi.org/10.3390/ijms241713307





