Regulation of Glycoprotein VI-Dependent Platelet Activation and Thrombus Formation by Heparan Sulfate Proteoglycan Perlecan
Abstract
:1. Introduction
2. Results
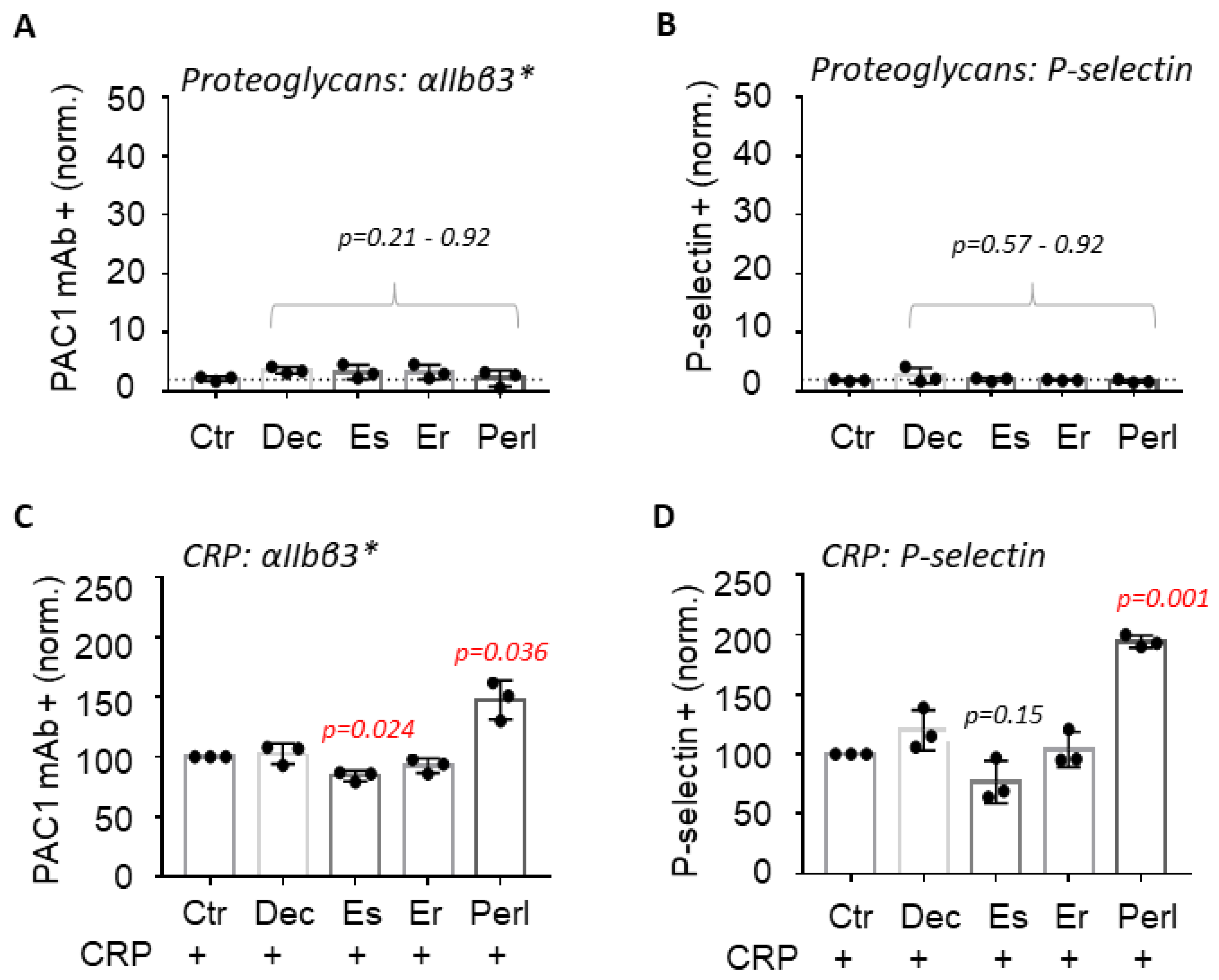
2.1. Perlecan Enhances GPVI-Induced Platelet Activation
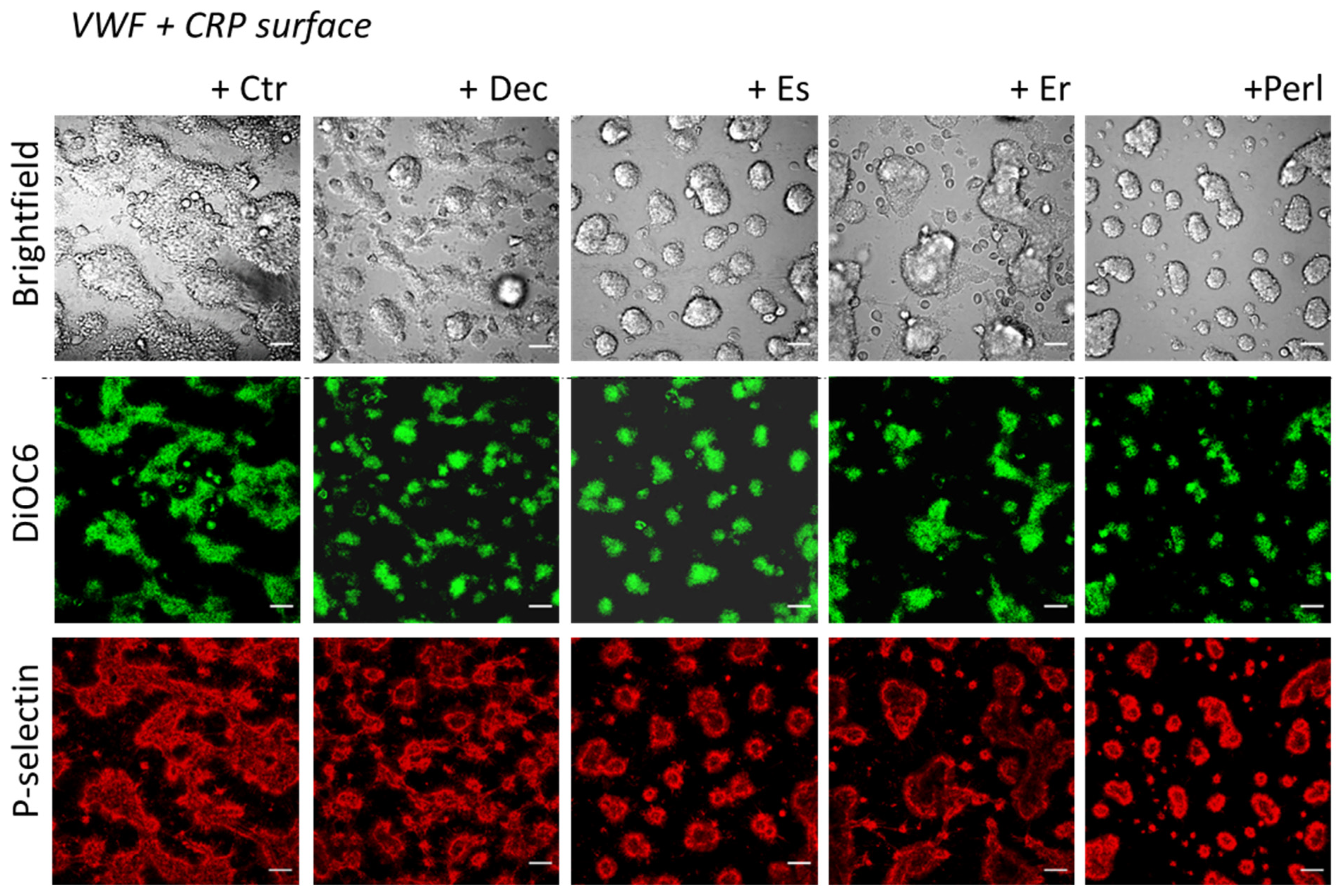
2.2. Perlecan Induces Platelet Spreading
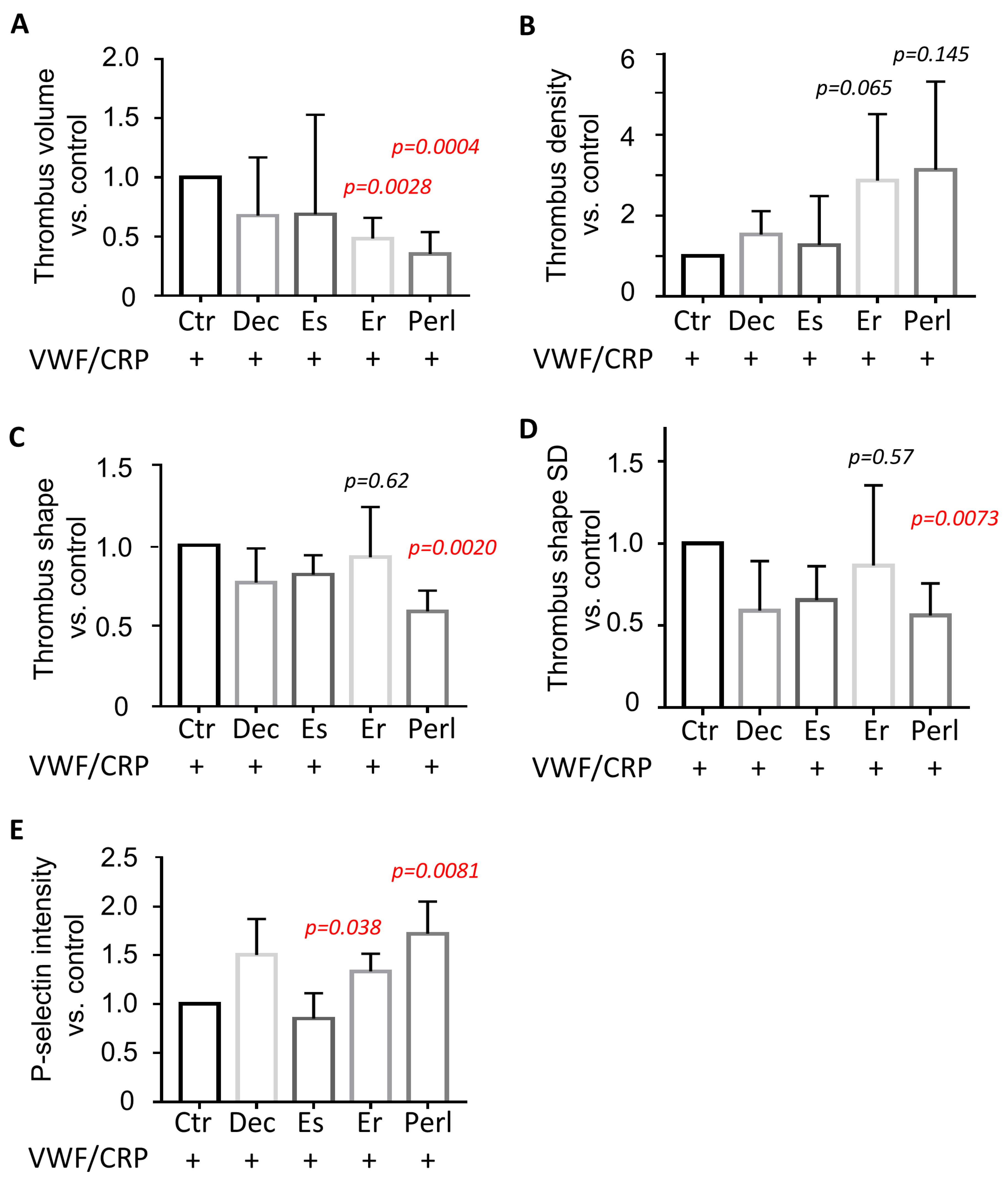
2.3. Dual Modulating Effect of Perlecan in GPVI-Dependent Thrombus Formation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Blood Donors and Platelet Isolation
4.3. Flow Cytometry
4.4. Platelet Spreading
4.5. Microfluidic Thrombus Formation
4.6. Assessment of Thrombus Parameters
4.7. Culturing of Endothelial Cells
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thorne, J.T.; Segal, T.R.; Chang, S.; Jorge, S.; Segars, J.H.; Leppert, P.C. Dynamic reciprocity between cells and their microenvironment in reproduction. Biol. Reprod. 2015, 92, 25. [Google Scholar] [CrossRef] [PubMed]
- Viola, M.; Karousou, E.; D’Angelo, M.L.; Moretto, P.; Caon, I.; Luca, G.; Passi, A.; Vigetti, D. Extracellular matrix in atherosclerosis: Hyaluronan and proteoglycans insights. Curr. Med. Chem. 2016, 23, 2958–2971. [Google Scholar] [CrossRef]
- Crea, F.; Libby, P. Acute coronary syndromes: The way forward from mechanisms to precision treatment. Circulation 2017, 136, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Pasterkamp, G.; Crea, F.; Jang, I.K. Reassessing the mechanisms of acute coronary syndromes. Circ. Res. 2019, 124, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc. Res. 2021, 117, 2525–2536. [Google Scholar] [CrossRef]
- Kolodgie, F.D.; Burke, A.P.; Wight, T.N.; Virmani, R. The accumulation of specific types of proteoglycans in eroded plaques: A role in coronary thrombosis in the absence of rupture. Curr. Opin. Lipidol. 2004, 15, 575–582. [Google Scholar] [CrossRef]
- Talusan, P.; Bedri, S.; Yang, S.; Kattapuram, T.; Silva, N.; Roughley, P.J.; Stone, J.R. Analysis of intimal proteoglycans in atherosclerosis-prone and atherosclerosis-resistant human arteries by mass spectrometry. Mol. Cell. Proteom. 2005, 4, 1350–1357. [Google Scholar] [CrossRef]
- Vaisar, T.; Hu, J.H.; Airhart, N.; Fox, K.; Heinecke, J.; Nicosia, R.F.; Kohler, T.; Potter, Z.E.; Simon, G.M.; Dix, M.M.; et al. Parallel murine and human plaque proteomics reveals pathways of plaque rupture. Circ. Res. 2020, 127, 997–1022. [Google Scholar] [CrossRef]
- Guidetti, G.; Bertoni, A.; Viola, M.; Tira, E.; Balduini, C.; Torti, M. The small proteoglycan decorin supports adhesion and activation of human platelets. Blood 2002, 100, 1707–1714. [Google Scholar] [CrossRef]
- Hoermann, H.; Krueger, I.; Maurus, N.; Reusswig, F.; Sun, Y.; Kohlmorgen, C.; Grandoch, M.; Fischer, J.W.; Elvers, M. The proteoglycan biglycan modulates platelet adhesion and thrombus formation in a GPVI-dependent manner. Int. J. Mol. Sci. 2021, 22, 12168. [Google Scholar] [CrossRef]
- Lord, M.S.; Yu, W.; Cheng, B.; Simmons, A.; Poole-Warren, L.; Whitelock, J.M. The modulation of platelet and endothelial cell adhesion to vascular graft materials by perlecan. Biomaterials 2009, 30, 4898–4906. [Google Scholar] [CrossRef]
- Vögtle, T.; Sharma, S.; Mori, J.; Nagy, Z.; Semeniak, D.; Scandola, C.; Geer, M.J.; Smith, C.W.; Lane, J.; Pollack, S.; et al. Heparan sulfates are critical regulators of the inhibitory megakaryocyte-platelet receptor G6b-B. eLife 2019, 8, e46840. [Google Scholar] [CrossRef]
- Bix, G.; Iozzo, R.A.; Woodall, B.; Burrows, M.; McQuillan, A.; Campbell, S.; Fields, G.B.; Iozzo, R.V. Endorepellin, the C-terminal angiostatic module of perlecan, enhances collagen-platelet responses via the α2β1-integrin receptor. Blood 2007, 109, 3745–3748. [Google Scholar] [CrossRef] [PubMed]
- Halfter, W.; Dong, S.; Schurer, B.; Cole, G.J. Collagen XVIII is a basement membrane heparan sulfate proteoglycan. J. Biol. Chem. 1998, 273, 25404–25412. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Moses, M.A.; Wiederschain, D.; Arbiser, J.L.; Folkman, J. The generation of endostatin is mediated by elastase. Cancer Res. 1999, 59, 6052–6056. [Google Scholar]
- Italiano, J.E.; Richardson, J.L.; Patel-Hett, S.; Battinelli, E.; Zaslavsky, A.; Short, S.; Ryeom, S.; Folkman, J.; Klement, G.L. Angiogenesis is regulated by a novel mechanism: Pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood 2008, 111, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Nieswandt, B.; Watson, S.P. Platelet-collagen interaction: Is GPVI the central receptor? Blood 2003, 102, 449–461. [Google Scholar] [CrossRef]
- De Witt, S.M.; Swieringa, F.; Cavill, R.; Lamers, M.M.; van Kruchten, R.; Mastenbroek, T.; Baaten, C.; Coort, S.; Pugh, N.; Schulz, A.; et al. Identification of platelet function defects by multi-parameter assessment of thrombus formation. Nat. Commun. 2014, 5, 4257. [Google Scholar] [CrossRef]
- Veninga, A.; Baaten, C.C.; De Simone, I.; Tullemans, B.M.; Kuijpers, M.J.; Heemskerk, J.W.; van der Meijden, P.E. Effects of platelet agonists and priming on the formation of platelet populations. Thromb. Haemost. 2022, 122, 726–738. [Google Scholar] [CrossRef]
- De Simone, I.; Baaten, C.C.; Gibbins, J.M.; Cate, H.T.; Heemskerk, J.W.; Jones, C.I.; van der Meijden, P.E. Repeated platelet activation and the potential of previously activated platelets to contribute to thrombus formation. J. Thromb. Haemost. 2023, 21, 1289–1306. [Google Scholar] [CrossRef]
- Petersen, R.; Lambourne, J.J.; Javierre, B.M.; Grassi, L.; Kreuzhuber, R.; Ruklisa, D.; Rosa, I.M.; Tome, R.A.; Elding, H.; van Geffen, J.P.; et al. Platelet function is modified by common sequence variation in megakaryocyte super enhancer. Nat. Commun. 2017, 8, 16058. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, Z.M.; Mendolicchio, G.L. Adhesion mechanisms in platelet function. Circ. Res. 2007, 100, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Nagy, M.; Perrella, G.; Dalby, A.; Becerra, M.F.; Garcia Quintanilla, L.; Pike, J.A.; Morgan, N.V.; Gardiner, E.E.; Heemskerk, J.W.; Azócar, L.; et al. Flow studies on human GPVI-deficient blood under coagulating and noncoagulating conditions. Blood Adv. 2020, 4, 2953–2961. [Google Scholar] [CrossRef]
- Pugh, N.; Simpson, A.M.; Smethurst, P.A.; de Groot, P.G.; Raynal, N.; Farndale, R.W. Synergism between platelet collagen receptors defined using receptor-specific collagen-mimetic peptide substrata in flowing blood. Blood 2010, 115, 5069–5079. [Google Scholar] [CrossRef]
- Perrella, G.; Huang, J.; Provenzale, I.; Swieringa, F.; Heubel-Moenen, F.C.; Farndale, R.W.; Roest, M.; van der Meijden, P.E.; Thomas, M.; Ariëns, R.A.; et al. Non-redundant roles of platelet glycoprotein VI and integrin αIIbβ3 in fibrin-mediated microthrombus formation. Arterioscler. Thromb. Vasc. Biol. 2021, 41, e97–e111. [Google Scholar] [CrossRef]
- Coxon, C.H.; Geer, M.J.; Senis, Y.A. ITIM receptors: More than just inhibitors of platelet activation. Blood 2017, 129, 3407–3418. [Google Scholar] [CrossRef] [PubMed]
- Mazharian, A.; Mori, J.; Wang, Y.J.; Heising, S.; Neel, B.G.; Watson, S.P.; Senis, Y.A. Megakaryocyte-specific deletion of the protein-tyrosine phosphatases Shp1 and Shp2 causes abnormal megakaryocyte development, platelet production, and function. Blood 2013, 121, 4205–4220. [Google Scholar] [CrossRef]
- Wee, J.L.; Jackson, D.E. The Ig-ITIM superfamily member Pecam-1 regulates the outside-in signaling properties of integrin αIIbβ3 in platelets. Blood 2005, 106, 3816–3823. [Google Scholar] [CrossRef]
- Alshahrani, M.M.; Kyriacou, R.P.; O’Malley, C.J.; Heinrich, G.; Najjar, S.M.; Jackson, D.E. Ceacam-2 positively regulates integrin αIIbβ3-mediated platelet functions. Platelets 2016, 27, 743–750. [Google Scholar] [CrossRef]
- Kuriri, F.A.; Burchall, G.; Alanazi, F.; Antonipillai, J.; Dobie, G.; Beachemin, N.; Jackson, D.E. Mice lacking Pecam-1 and Ceacam-1 have enhanced platelet secretion and thrombus growth: Novel link with PAR4. Thromb. Haemost. 2022, 122, 961–973. [Google Scholar]
- Gilio, K.; Harper, M.T.; Cosemans, J.M.; Konopatskaya, O.; Munnix, I.C.A.; Prinzen, L.; Leitges, M.; Liu, Q.; Molkentin, J.D.; Heemskerk, J.W.; et al. Functional divergence of platelet protein kinase C (PKC) isoforms in thrombus formation on collagen. J. Biol. Chem. 2010, 285, 23410–23419. [Google Scholar] [CrossRef] [PubMed]
- De Simone, I.; Baaten, C.C.; Jandrot-Perrus, M.; Gibbins, J.M.; ten Cate, H.; Heemskerk, J.W.; Jones, C.I.; van der Meijden, P.E. Coagulation factor XIIIa and activated protein C activate platelets via GPVI and PAR1. Int. J. Mol. Sci. 2022, 23, 10203. [Google Scholar] [CrossRef] [PubMed]
- Bolte, S.; Cordelières, F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006, 224, 213–232. [Google Scholar] [CrossRef] [PubMed]
- Castelló-Cros, R.; Cukierman, E. Stromagenesis during tumorigenesis: Characterization of tumor-associated fibroblasts and stroma-derived 3D matrices. Methods Mol. Biol. 2009, 522, 275–305. [Google Scholar]
- Gong, T.; Heng, B.C.; Xu, J.; Zhu, S.; Yuan, C.; Lo, E.C.; Zhang, C. Decellularized extracellular matrix of human umbilical vein endothelial cells promotes endothelial differentiation of stem cells from exfoliated deciduous teeth. J. Biomed. Mater. Res. 2017, 105, 1083–1093. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, J.; Zhu, Y.; Sun, X.; Guo, W.; Liu, X.; Jing, X.; Guo, G.; Guo, Q.; Peng, J.; et al. Extracellular matrix derived by human umbilical cord-deposited mesenchymal stem cells accelerates chondrocyte proliferation and differentiation potential in vitro. Cell Tissue Bank. 2019, 20, 351–365. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Provenzale, I.; De Simone, I.; Gibbins, J.M.; Heemskerk, J.W.M.; van der Meijden, P.E.J.; Jones, C.I. Regulation of Glycoprotein VI-Dependent Platelet Activation and Thrombus Formation by Heparan Sulfate Proteoglycan Perlecan. Int. J. Mol. Sci. 2023, 24, 13352. https://doi.org/10.3390/ijms241713352
Provenzale I, De Simone I, Gibbins JM, Heemskerk JWM, van der Meijden PEJ, Jones CI. Regulation of Glycoprotein VI-Dependent Platelet Activation and Thrombus Formation by Heparan Sulfate Proteoglycan Perlecan. International Journal of Molecular Sciences. 2023; 24(17):13352. https://doi.org/10.3390/ijms241713352
Chicago/Turabian StyleProvenzale, Isabella, Ilaria De Simone, Jonathan M. Gibbins, Johan W. M. Heemskerk, Paola E. J. van der Meijden, and Chris I. Jones. 2023. "Regulation of Glycoprotein VI-Dependent Platelet Activation and Thrombus Formation by Heparan Sulfate Proteoglycan Perlecan" International Journal of Molecular Sciences 24, no. 17: 13352. https://doi.org/10.3390/ijms241713352
APA StyleProvenzale, I., De Simone, I., Gibbins, J. M., Heemskerk, J. W. M., van der Meijden, P. E. J., & Jones, C. I. (2023). Regulation of Glycoprotein VI-Dependent Platelet Activation and Thrombus Formation by Heparan Sulfate Proteoglycan Perlecan. International Journal of Molecular Sciences, 24(17), 13352. https://doi.org/10.3390/ijms241713352





