New Ruthenium Nitrosyl Complexes Combining Potentially Photoactive Nitrosyl Group with the Magnetic Nitroxide Radicals as Ligands
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure and Synthesis
2.2. Hirshfeld Surfaces Analysis
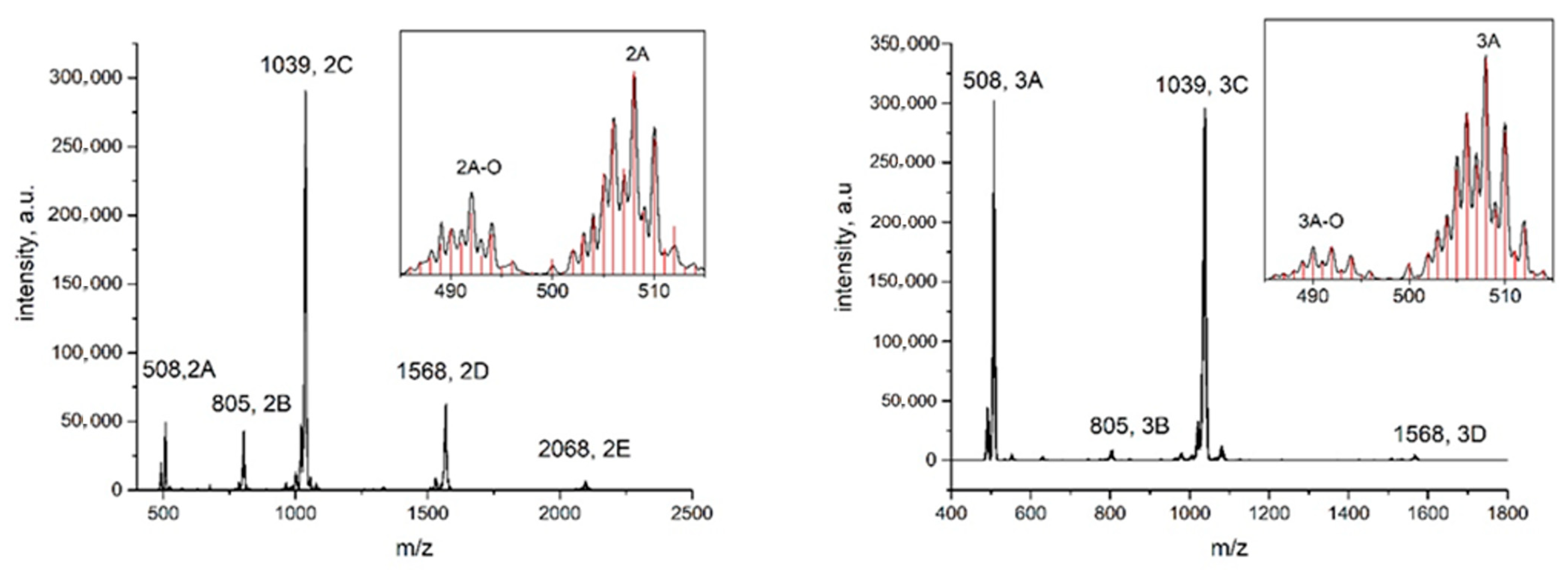
2.3. Mass-Spectrometry
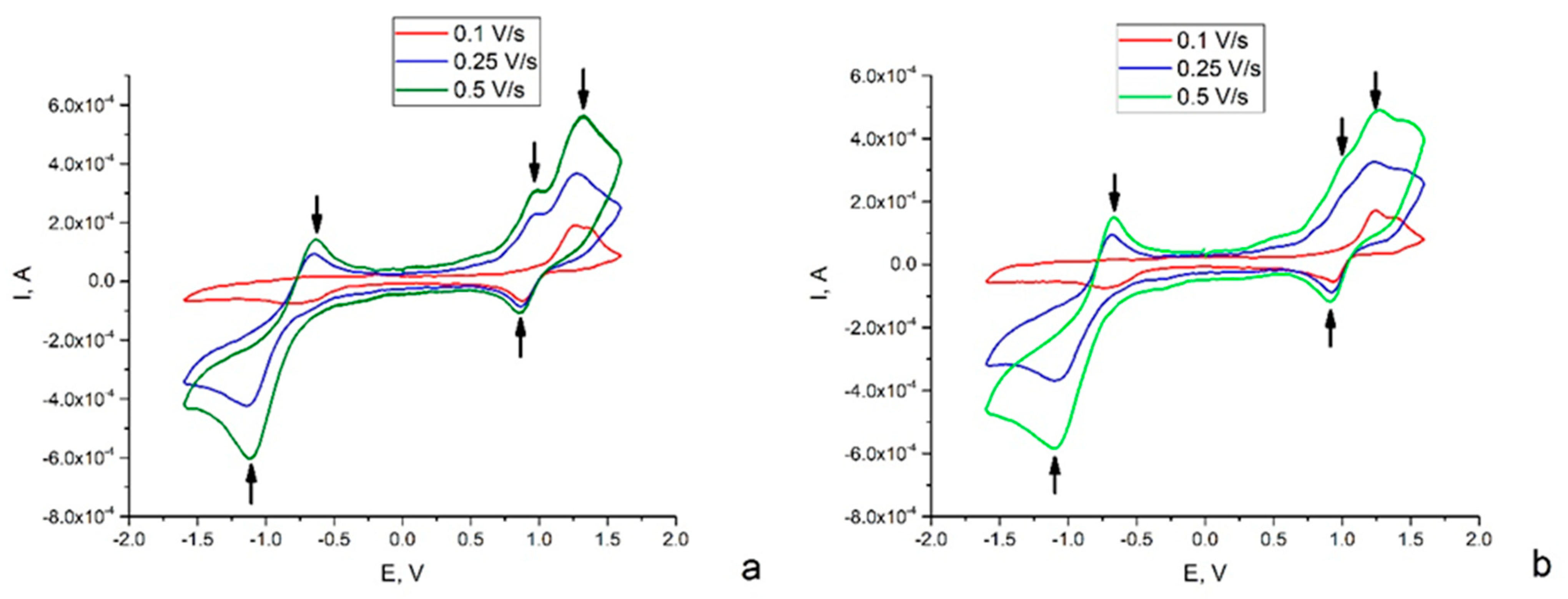
2.4. Electrochemistry
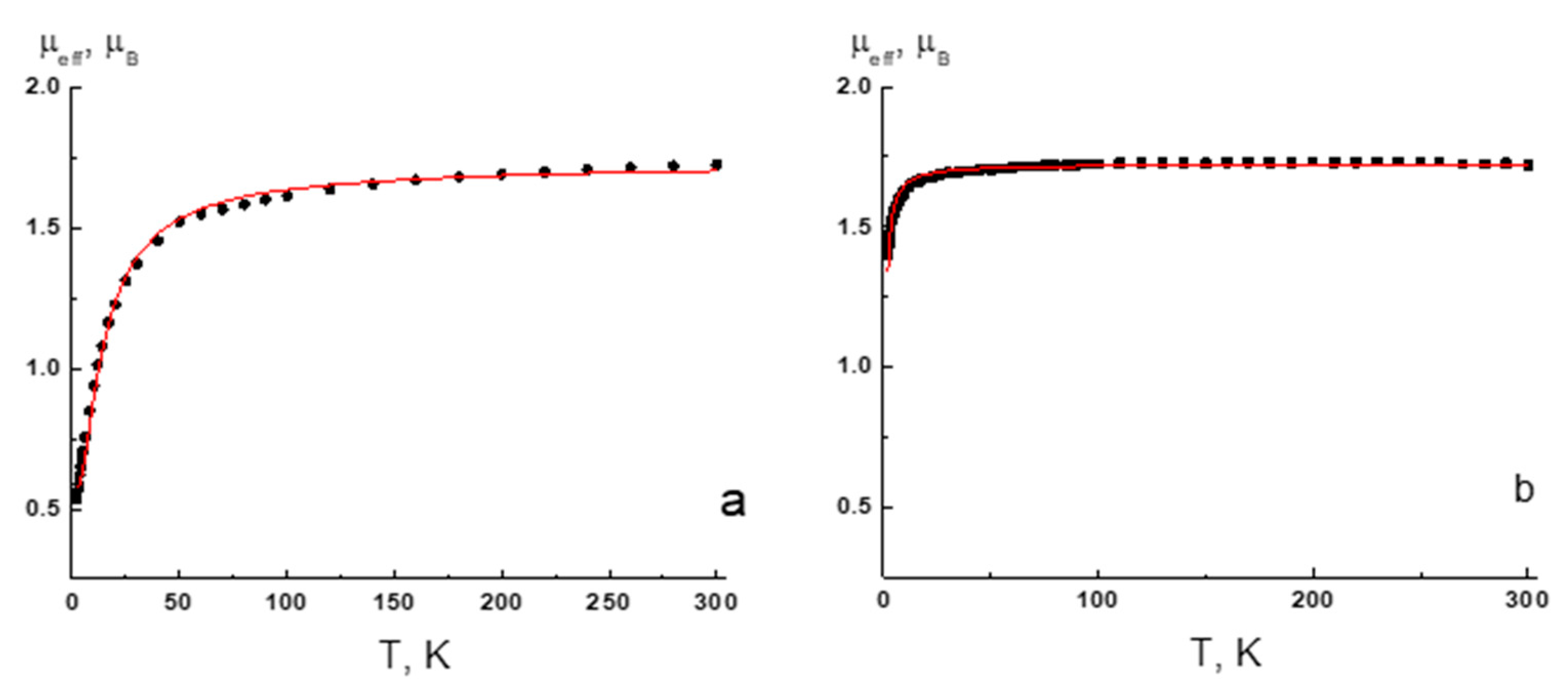
2.5. Magnetic Measurements
3. Materials and Methods
3.1. General Procedures and Materials
3.2. Synthesis of Na[RuNOCl4L1]
3.3. Synthesis of Na[RuNOCl4L2]·H2O
3.4. Physical methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luneau, D.; Rey, P. Magnetism of Metal-Nitroxide Compounds Involving Bis-Chelating Imidazole and Benzimidazole Substituted Nitronyl Nitroxide Free Radicals. Coord. Chem. Rev. 2005, 249, 2591–2611. [Google Scholar] [CrossRef]
- Gass, I.A.; Tewary, S.; Rajaraman, G.; Asadi, M.; Lupton, D.W.; Moubaraki, B.; Chastanet, G.; Létard, J.-F.; Murray, K.S. Solvate-Dependent Spin Crossover and Exchange in Cobalt(II) Oxazolidine Nitroxide Chelates. Inorg. Chem. 2014, 53, 5055–5066. [Google Scholar] [CrossRef] [PubMed]
- Ovcharenko, V.; Bagryanskaya, E. Breathing Crystals from Copper Nitroxyl Complexes. In Spin-Crossover Materials; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 239–280. [Google Scholar] [CrossRef]
- Romanenko, G.V.; Maryunina, K.Y.; Bogomyakov, A.S.; Sagdeev, R.Z.; Ovcharenko, V.I. Relationship between the Thermally Induced Reorientations of Aromatic Solvate Molecules in Cu(Hfac)2–Nitroxide Breathing Crystals and the Character of the Magnetic Anomaly. Inorg. Chem. 2011, 50, 6597–6609. [Google Scholar] [CrossRef]
- Ershova, I.V.; Piskunov, A.V.; Cherkasov, V.K. Complexes of Diamagnetic Cations with Radical Anion Ligands. Russ. Chem. Rev. 2020, 89, 1157. [Google Scholar] [CrossRef]
- Oshio, H.; Watanabe, T.; Ohto, A.; Ito, T.; Ikoma, T.; Tero-Kubota, S. Ferromagnetic Interactions between Imino Nitroxides through Diamagnetic Metal Ions: Crystal Structures, Magnetism, and Electronic Properties of [M(I)(Imino Nitroxide)2](PF6) (M = Cu(I) and Ag(I)). Inorg. Chem. 1997, 36, 3014–3021. [Google Scholar] [CrossRef]
- Sato, O.; Iyoda, T.; Fujishima, A.; Hashimoto, K. Photoinduced Magnetization of a Cobalt-Iron Cyanide. Science 1996, 272, 704–705. [Google Scholar] [CrossRef]
- Stefańczyk, O.; Ohkoshi, S. Photoswitchable High-Dimensional CoII–[WV(CN)8] Networks: Past, Present, and Future. J. Appl. Phys. 2021, 129, 110901. [Google Scholar] [CrossRef]
- Arczyński, M.; Stanek, J.; Sieklucka, B.; Dunbar, K.R.; Pinkowicz, D. Site-Selective Photoswitching of Two Distinct Magnetic Chromophores in a Propeller-Like Molecule To Achieve Four Different Magnetic States. J. Am. Chem. Soc. 2019, 141, 19067–19077. [Google Scholar] [CrossRef]
- Gütlich, P.; Hauser, A.; Spiering, H. Thermal and Optical Switching of Iron(II) Complexes. Angew. Chem. Int. Ed. Engl. 1994, 33, 2024–2054. [Google Scholar] [CrossRef]
- Matsuda, K.; Irie, M. Photoswitching of Magnetic Properties by Using Diarylethene Photochromic Spin Coupler. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 2000, 345, 155–160. [Google Scholar] [CrossRef]
- Rogacz, K.; Brzozowska, M.; Baś, S.; Kurpiewska, K.; Pinkowicz, D. Low-Coordinate Erbium(III) Single-Molecule Magnets with Photochromic Behavior. Inorg. Chem. 2022, 61, 16295–16306. [Google Scholar] [CrossRef]
- Dommaschk, M.; Schütt, C.; Venkataramani, S.; Jana, U.; Näther, C.; Sönnichsen, F.D.; Herges, R. Rational Design of a Room Temperature Molecular Spin Switch. The Light-Driven Coordination Induced Spin State Switch (LD-CISSS) Approach. Dalt. Trans. 2014, 43, 17395–17405. [Google Scholar] [CrossRef] [PubMed]
- Kaszub, W.; Marino, A.; Lorenc, M.; Collet, E.; Bagryanskaya, E.G.; Tretyakov, E.V.; Ovcharenko, V.I.; Fedin, M.V. Ultrafast Photoswitching in a Copper-Nitroxide-Based Molecular Magnet. Angew. Chem. 2014, 53, 10636–10640. [Google Scholar] [CrossRef] [PubMed]
- Canton, S.E.; Biednov, M.; Pápai, M.; Lima, F.A.; Choi, T.-K.; Otte, F.; Jiang, Y.; Frankenberger, P.; Knoll, M.; Zalden, P.; et al. Ultrafast Jahn-Teller Photoswitching in Cobalt Single-Ion Magnets. Adv. Sci. 2023, 10, 2206880. [Google Scholar] [CrossRef]
- Schaniel, D.; Imlau, M.; Weisemoeller, T.; Woike, T.; Krämer, K.W.; Güdel, H.-U. Photoinduced Nitrosyl Linkage Isomers Uncover a Variety of Unconventional Photorefractive Media. Adv. Mater. 2007, 19, 723–726. [Google Scholar] [CrossRef]
- Mikhailov, A.A.; Stolyarova, E.D.; Kostin, G.A. PHOTOCHEMISTRY OF RUTHENIUM NITROSYL COMPLEXES IN SOLIDS AND SOLUTIONS AND ITS POTENTIAL APPLICATIONS. J. Struct. Chem. 2021, 62, 497–516. [Google Scholar] [CrossRef]
- Mikhailov, A.A.; Wenger, E.; Kostin, G.A.; Schaniel, D. Room-Temperature Photogeneration of Nitrosyl Linkage Isomers in Ruthenium Nitrosyl Complexes. Chem.-A Eur. J. 2019, 25, 7569–7574. [Google Scholar] [CrossRef]
- Kostin, G.A.; Tolstikov, S.E.; Kuratieva, N.V.; Nadolinny, V.A.; Ovcharenko, V.I. FIRST EXAMPLE OF RUTHENIUM NITROSO COMPLEXES WITH A NITROXYL RADICAL AS A LIGAND. J. Struct. Chem. 2023, 64, 169–178. [Google Scholar] [CrossRef]
- Rintoul, L.; Micallef, A.S.; Bottle, S.E. The Vibrational Group Frequency of the N–O Stretching Band of Nitroxide Stable Free Radicals. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 70, 713–717. [Google Scholar] [CrossRef]
- Rathgeb, A.; Böhm, A.; Novak, M.S.; Gavriluta, A.; Dömötör, O.; Tommasino, J.B.; Enyedy, É.A.; Shova, S.; Meier, S.; Jakupec, M.A.; et al. Ruthenium-Nitrosyl Complexes with Glycine, l-Alanine, l-Valine, l-Proline, d-Proline, l-Serine, l-Threonine, and l-Tyrosine: Synthesis, X-Ray Diffraction Structures, Spectroscopic and Electrochemical Properties, and Antiproliferative Activity. Inorg. Chem. 2014, 53, 2718–2729. [Google Scholar] [CrossRef]
- Büchel, G.E.; Gavriluta, A.; Novak, M.; Meier, S.M.; Jakupec, M.A.; Cuzan, O.; Turta, C.; Tommasino, J.-B.; Jeanneau, E.; Novitchi, G.; et al. Striking Difference in Antiproliferative Activity of Ruthenium- and Osmium-Nitrosyl Complexes with Azole Heterocycles. Inorg. Chem. 2013, 52, 6273–6285. [Google Scholar] [CrossRef]
- Rechitskaya, E.D.; Kuratieva, N.V.; Lider, E.V.; Eremina, J.A.; Klyushova, L.S.; Eltsov, I.V.; Kostin, G.A. Tuning of Cytotoxic Activity by Bio-Mimetic Ligands in Ruthenium Nitrosyl Complexes. J. Mol. Struct. 2020, 1219, 128565. [Google Scholar] [CrossRef]
- Coe, B.J.; Glenwright, S.J. Trans-Effects in Octahedral Transition Metal Complexes. Coord. Chem. Rev. 2000, 203, 5–80. [Google Scholar] [CrossRef]
- Stolyarova, E.D.; Mikhailov, A.A.; Ulantikov, A.A.; Eremina, J.A.; Klyushova, L.S.; Kuratieva, N.V.; Nadolinny, V.A.; Kostin, G.A. Blue-to-Red Light Triggered Nitric Oxide Release in Cytotoxic/Cytostatic Ruthenium Nitrosyl Complexes Bearing Biomimetic Ligands. J. Photochem. Photobiol. A Chem. 2021, 421, 113520. [Google Scholar] [CrossRef]
- Rechitskaya, E.D.; Vorobiev, V.A.; Kuratieva, N.V.; Kostin, G.A. MIXED-LIGAND NITROSYL AND 3-CYANOPYRIDINE COMPLEX OF RUTHENIUM(II): SYNTHESIS, CRYSTAL STRUCTURE, AND BOND ISOMERISM. J. Struct. Chem. 2021, 62, 256–264. [Google Scholar] [CrossRef]
- Piggot, P.M.T.; Hall, L.A.; White, A.J.P.; Williams, D.J. Synthesis of Ruthenium(II) Monosubstituted Squarates: 1. Procedural Considerations. Inorganica Chim. Acta 2004, 357, 250–258. [Google Scholar] [CrossRef]
- Chyba, J.; Novák, M.; Munzarová, P.; Novotný, J.; Marek, R. Through-Space Paramagnetic NMR Effects in Host–Guest Complexes: Potential Ruthenium(III) Metallodrugs with Macrocyclic Carriers. Inorg. Chem. 2018, 57, 8735–8747. [Google Scholar] [CrossRef]
- Hołyńska, M. Iridium(III) Products Isolated in a Reaction of IrCl3 with Phenyl 2-Pyridyl Ketoxime. Z. Krist.-Cryst. Mater. 2013, 228, 72–76. [Google Scholar] [CrossRef]
- Müller, U.; Noll, A. (Na-15-Krone-5)2ReCl6·4 CH2Cl2, Eine Struktur Mit CH2Cl2-Molekülen in Pseudohexagonalen Kanälen. Z. Krist.-Cryst. Mater. 2003, 218, 699–702. [Google Scholar] [CrossRef]
- Liddle, S.T.; Clegg, W. A Homologous Series of Crown-Ether-Complexed Alkali Metal Amides as Discrete Ion-Pair Species: Synthesis and Structures of [M(12-Crown-4)2][PyNPh·PyN(H)Ph] (M=Li, Na and K). Polyhedron 2003, 22, 3507–3513. [Google Scholar] [CrossRef]
- Liebing, P.; Schmeide, M.; Kühling, M.; Witzorke, J. The Alkali Metal Salts of Methyl Xanthic Acid. Eur. J. Inorg. Chem. 2020, 2020, 2428–2434. [Google Scholar] [CrossRef]
- Vostrikova, K.E.; Peresypkina, E.V.; Drebushchak, V.A. Cucurbituril-Assisted Transformation of Nitronyl Nitroxide into Imino Nitroxide in the Solid State. CrystEngComm 2011, 13, 3241–3245. [Google Scholar] [CrossRef]
- Luneau, D. Coordination Chemistry of Nitronyl Nitroxide Radicals Has Memory. Eur. J. Inorg. Chem. 2020, 2020, 597–604. [Google Scholar] [CrossRef]
- Goldstein, S.; Russo, A.; Samuni, A. Reactions of PTIO and Carboxy-PTIO with ·NO, ·NO2, and O2¯*. J. Biol. Chem. 2003, 278, 50949–50955. [Google Scholar] [CrossRef] [PubMed]
- Budnikova, Y.G.; Gryaznova, T.V.; Kadirov, M.K.; Tret’yakov, E.V.; Kholin, K.V.; Ovcharenko, V.I.; Sagdeev, R.Z.; Sinyashin, O.G. Electrochemistry of Nitronyl and Imino Nitroxides. Russ. J. Phys. Chem. A 2009, 83, 1976–1980. [Google Scholar] [CrossRef]
- Lee, J.; Lee, E.; Kim, S.; Bang, G.S.; Shultz, D.A.; Schmidt, R.D.; Forbes, M.D.E.; Lee, H. Nitronyl Nitroxide Radicals as Organic Memory Elements with Both N- and p-Type Properties. Angew. Chem. 2011, 123, 4506–4510. [Google Scholar] [CrossRef]
- Fedyushin, P.A.; Zayakin, I.A.; Tolstikov, S.E.; Lalov, A.V.; Akyeva, A.Y.; Syroeshkin, M.A.; Romanenko, G.V.; Tretyakov, E.V.; Egorov, M.P.; Ovcharenko, V.I. Synthesis and Redox Properties of Imidazol-2-Yl-Substituted Nitronyl Nitroxides. Russ. Chem. Bull. 2022, 71, 722–734. [Google Scholar] [CrossRef]
- Zayakin, I.A.; Akyeva, A.Y.; Syroeshkin, M.A.; Bagryanskaya, I.Y.; Tretyakov, E.V.; Egorov, M.P. Synthesis, Structure, and Electrochemistry of Nitronyl Nitroxide-Substituted 1,4-Naphthoquinone. Russ. Chem. Bull. 2023, 72, 213–222. [Google Scholar] [CrossRef]
- Souza, D.A.; Moreno, Y.; Ponzio, E.A.; Resende, J.A.L.C.; Jordão, A.K.; Cunha, A.C.; Ferreira, V.F.; Novak, M.A.; Vaz, M.G.F. Synthesis, Crystal Structure, Magnetism and Electrochemical Properties of Two Copper(II) Furoyltrifluoroacetonate Complexes with Nitroxide Radical. Inorganica Chim. Acta 2011, 370, 469–473. [Google Scholar] [CrossRef]
- Swain, P.; Mallika, C.; Jagadeeswara Rao, C.; Kamachi Mudali, U.; Natarajan, R. Electrochemical Studies on the Reduction Behaviour of Ruthenium Nitrosyl Ions in Nitric Acid Medium. J. Appl. Electrochem. 2015, 45, 209–216. [Google Scholar] [CrossRef]
- Minakshi, M.; Mitchell, D.R.G.; Munnangi, A.R.; Barlow, A.J.; Fichtner, M. New Insights into the Electrochemistry of Magnesium Molybdate Hierarchical Architectures for High Performance Sodium Devices. Nanoscale 2018, 10, 13277–13288. [Google Scholar] [CrossRef] [PubMed]
- Barmi, M.J.; Minakshi, M. Tuning the Redox Properties of the Nanostructured CoMoO4 Electrode: Effects of Surfactant Content and Synthesis Temperature. ChemPlusChem 2016, 81, 964–977. [Google Scholar] [CrossRef]
- Emel’yanov, V.A.; Gromilov, S.A.; Baidina, I.A.; Virovets, A.V.; Belyaev, A.V.; Logvinenko, V.A. Synthesis and Crystal Structure of Na2[RuNOCl5]·6H2O. J. Struct. Chem. 1999, 40, 883–891. [Google Scholar] [CrossRef]
- Tolstikov, S.E.; Artiukhova, N.A.; Romanenko, G.V.; Bogomyakov, A.S.; Zueva, E.M.; Barskaya, I.Y.; Fedin, M.V.; Maryunina, K.Y.; Tretyakov, E.V.; Sagdeev, R.Z.; et al. Heterospin Complex Showing Spin Transition at Room Temperature. Polyhedron 2015, 100, 132–138. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, M.; Cui, G.; Peng, S. A Class of Novel Nitronyl Nitroxide Labeling Basic and Acidic Amino Acids: Synthesis, Application for Preparing ESR Optionally Labeling Peptides, and Bioactivity Investigations. Bioorganic Med. Chem. 2008, 16, 4019–4028. [Google Scholar] [CrossRef]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A Powerful New Program for the Analysis of Anisotropic Monomeric and Exchange-Coupled Polynuclear d- and f-Block Complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld Surface Analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
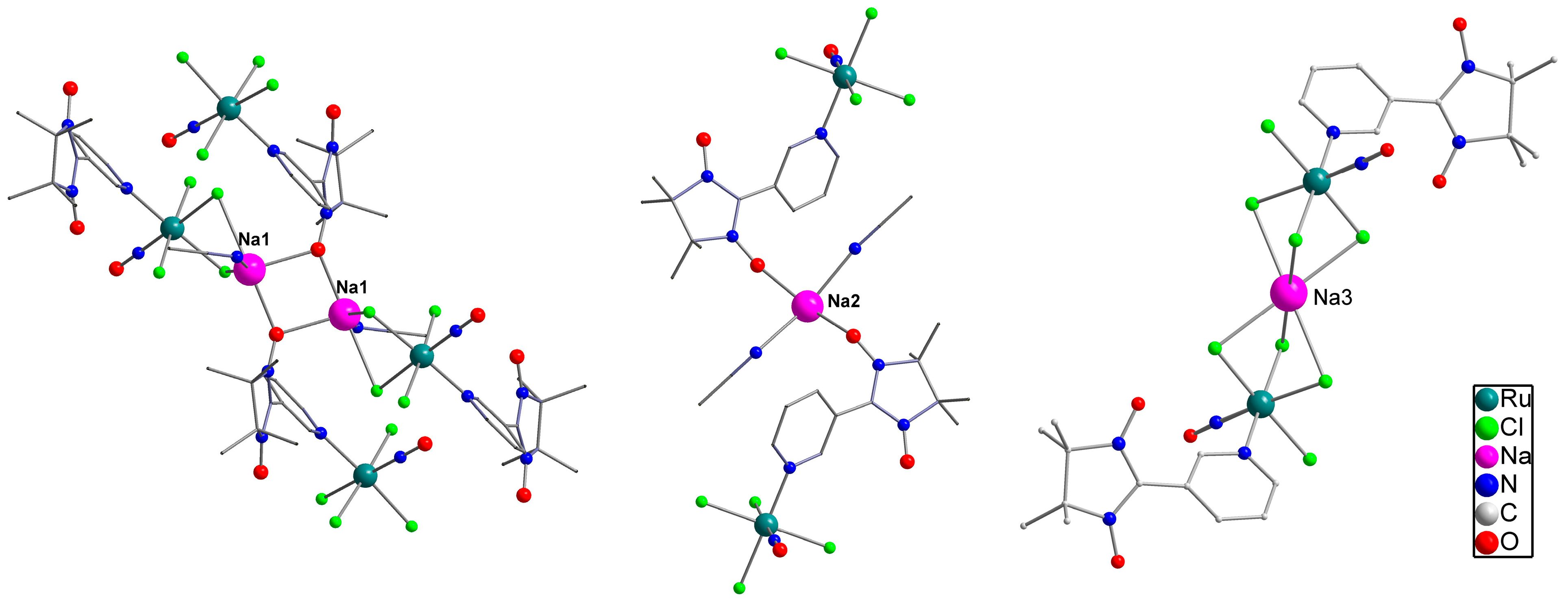

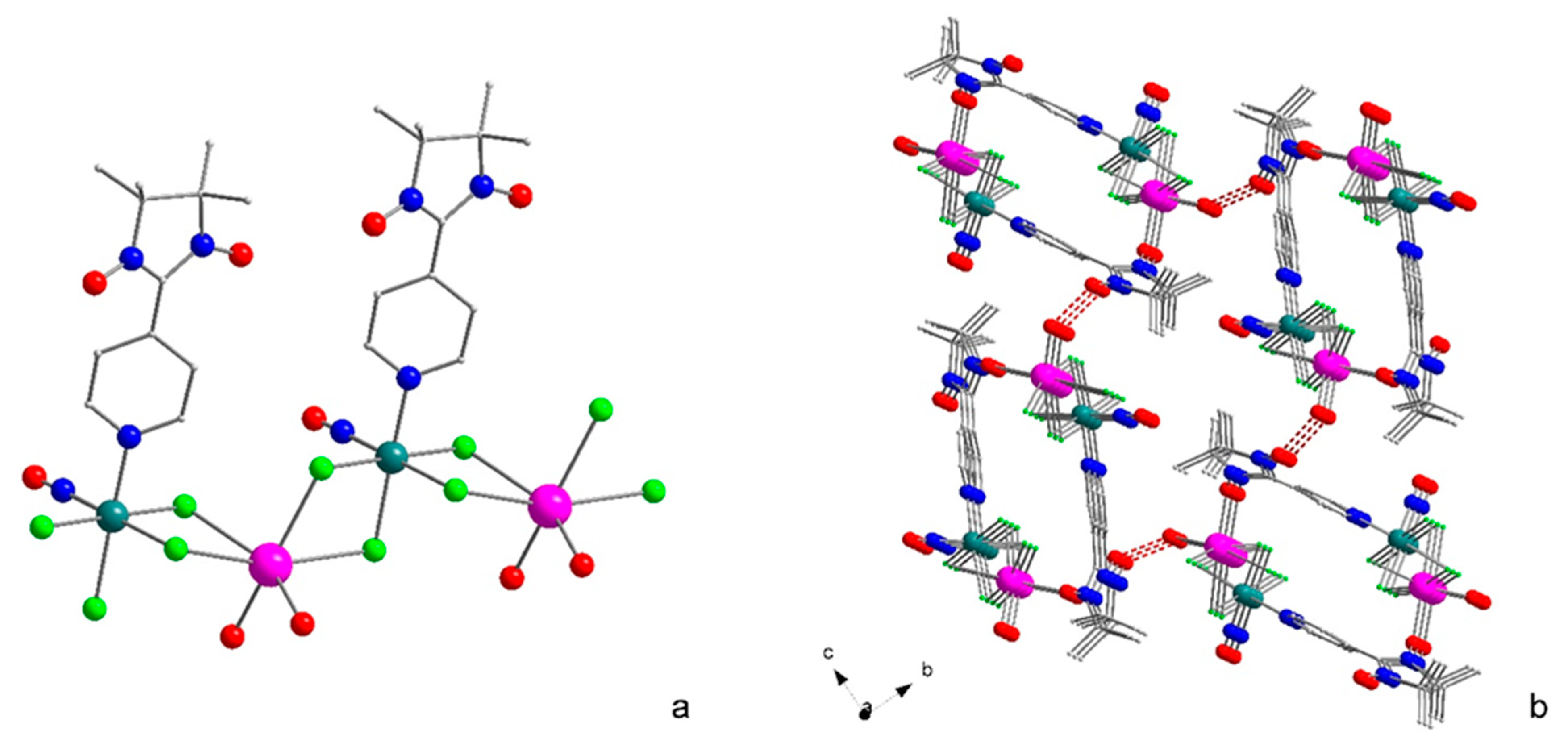
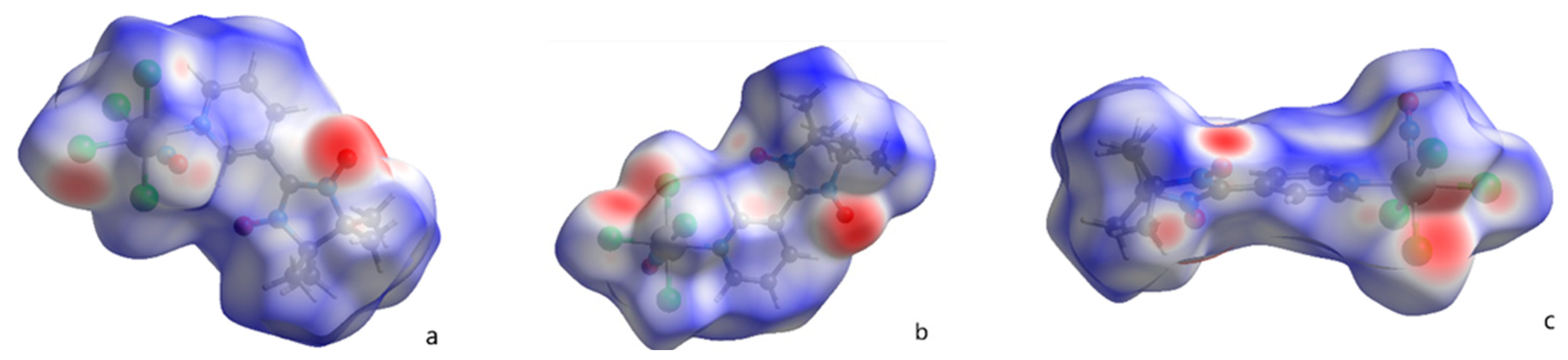

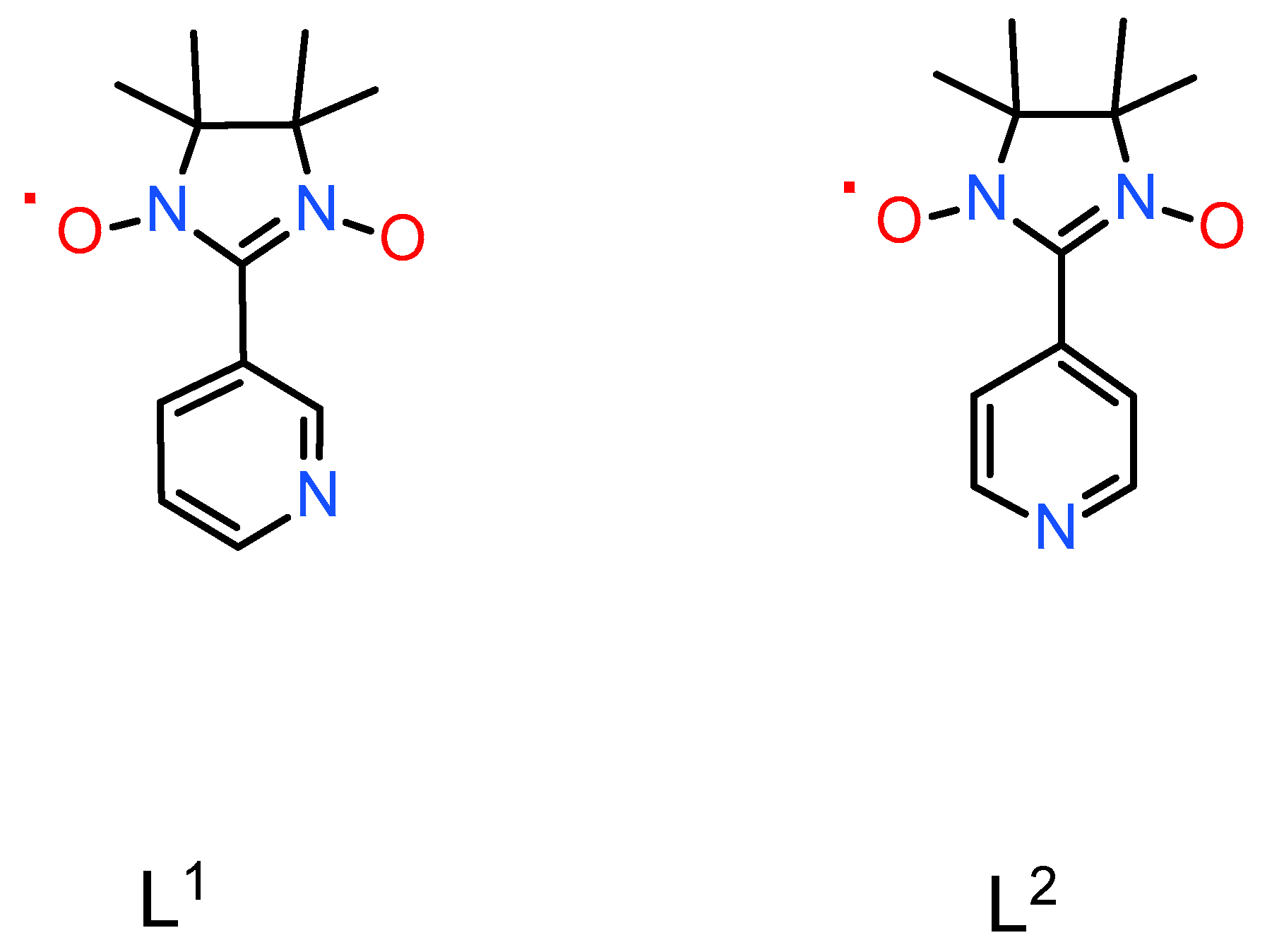
| Parameter | NaRuNOCl4L 1 (1) [19] | NaRuNOCl4L 1 (2) | NaRuNOCl4L 2 (3) |
|---|---|---|---|
| d (Ru-Cl), Å | 2.349(1)–2.398(1) | 2.345(1)–2.382(1) | 2.347(1)–2.400(1) |
| d (Ru-NL), Å | 2.102(3) | 2.111(2)–2.118(2) | 2.110(4) |
| d (Ru-NO), Å | 1.726(3) | 1.736(3)–1.752(3) | 1.799(4) |
| d (RuN-O), Å | 1.133(4) | 1.110(3)–1.124(3) | 1.011(5) |
| ∠Ru-N-O, ° | 178.1(3) | 173.6(3)–179.0(3) | 177.8(4) |
| d (N-O(L)), Å | 1.275(3)–1.303(3) | 1.273(3)–1.296(3) | 1.260(5)–1.272(5) |
| ∠NN-Py *, ° | 49.5(4) | 41.0(4), 48.2(4) | 33.5(2) |
| Complex Forms | (2) | (3) |
|---|---|---|
| m/z | ||
| RuNOCl4 | 274 | 274 |
| RuNOCl4L− (A) | 508 | 508 |
| (A)-O | 492 | 492 |
| NaRuNOCl4(RuNOCl4L) (B) | 805 | 805 |
| Na(RuNOCl4L)2 (C) | 1039 | 1039 |
| (C)-O | 1023 | 1023 |
| (C) + CH3CN | - | 1080 |
| Na2(RuNOCl4L)3 (D) | 1568 | 1568 |
| Na3(RuNOCl4L)4 (E) | 2068 | - |
| Scan Rate, V/s | Epc, V | Epa, V | |||
|---|---|---|---|---|---|
| N-O·→ N-O− | N+ = O → N-O· | N-O− → N-O· | N-O → N+ = O· | (Ru2+-NO+) →(Ru3+-NO+) | |
| Complex (2) | |||||
| 0.1 | −0.75 | 0.88 | ND | 1.26 | 1.36 |
| 0.25 | −1.14 | 0.86 | −0.65 | 0.97 | 1.28 |
| 0.5 | −1.13 | 0.86 | −0.64 | 0.98 | 1.31 |
| Complex (3) | |||||
| 0.1 | −0.73 | 0.93 | ND | 1.24 | 1.39 |
| 0.25 | −1.09 | 0.92 | −0.68 | 1.01 | 1.23 |
| 0.5 | −1.10 | 0.90 | −0.67 | 1.00 | 1.26 |
| (2) | (3) | |
|---|---|---|
| Empirical formula | C28H38Cl8N10Na2O6Ru2 | C12H18Cl4N4NaO4Ru |
| Formula weight | 1142.40 | 548.16 |
| Crystal system | triclinic | orthorhombic |
| Space group | P-1 | P212121 |
| a/Å | 10.4158(2) | 6.9913(2) |
| b/Å | 10.8548(2) | 15.1057(3) |
| c/Å | 20.9285(4) | 18.7215(5) |
| α/° | 99.831(1) | 90 |
| β/° | 94.477(1) | 90 |
| γ/° | 109.036(1) | 90 |
| Volume/Å3 | 2181.12(7) | 1977.15(9) |
| Z | 2 | 4 |
| ρcalcg/cm3 | 1.739 | 1.842 |
| μ/mm−1 | 1.254 | 1.381 |
| F(000) | 1140.0 | 1092.0 |
| 2Θ range for data collection/° | 4.714 to 66.502 | 3.464 to 66.392 |
| Index ranges | −16 ≤ h ≤ 16, −16 ≤ k ≤ 16, −32 ≤ l ≤ 29 | −10 ≤ h ≤ 10, −23 ≤ k ≤ 22, −28 ≤ l ≤ 28 |
| Reflections collected | 43,674 | 26,074 |
| Independent reflections | 16,301 [Rint = 0.0452, Rsigma = 0.0718] | 7563 [Rint = 0.0388, Rsigma = 0.0426] |
| Data/restraints/parameters | 16,301/0/515 | 7563/4/245 |
| Goodness-of-fit on F2 | 0.861 | 1.058 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0460, wR2 = 0.1217 | R1 = 0.0400, wR2 = 0.0838 |
| Final R indexes [all data] | R1 = 0.0835, wR2 = 0.1466 | R1 = 0.0486, wR2 = 0.0875 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostin, G.A.; Kozlov, R.; Bogomyakov, A.; Tolstikov, S.; Sheven, D.; Korenev, S. New Ruthenium Nitrosyl Complexes Combining Potentially Photoactive Nitrosyl Group with the Magnetic Nitroxide Radicals as Ligands. Int. J. Mol. Sci. 2023, 24, 13371. https://doi.org/10.3390/ijms241713371
Kostin GA, Kozlov R, Bogomyakov A, Tolstikov S, Sheven D, Korenev S. New Ruthenium Nitrosyl Complexes Combining Potentially Photoactive Nitrosyl Group with the Magnetic Nitroxide Radicals as Ligands. International Journal of Molecular Sciences. 2023; 24(17):13371. https://doi.org/10.3390/ijms241713371
Chicago/Turabian StyleKostin, Gennadiy A., Ruslan Kozlov, Artem Bogomyakov, Svyatoslav Tolstikov, Dmitriy Sheven, and Sergey Korenev. 2023. "New Ruthenium Nitrosyl Complexes Combining Potentially Photoactive Nitrosyl Group with the Magnetic Nitroxide Radicals as Ligands" International Journal of Molecular Sciences 24, no. 17: 13371. https://doi.org/10.3390/ijms241713371
APA StyleKostin, G. A., Kozlov, R., Bogomyakov, A., Tolstikov, S., Sheven, D., & Korenev, S. (2023). New Ruthenium Nitrosyl Complexes Combining Potentially Photoactive Nitrosyl Group with the Magnetic Nitroxide Radicals as Ligands. International Journal of Molecular Sciences, 24(17), 13371. https://doi.org/10.3390/ijms241713371







