Is a 2D Nanostructured Surface Capable of Changing the Corrosion and Magnetic Properties of an Amorphous Alloy?
Abstract
1. Introduction
2. Results and Discussion
2.1. Anodic Modification of the Alloy Surface in BmimNTf2
2.2. Electrochemical Corrosion Testing
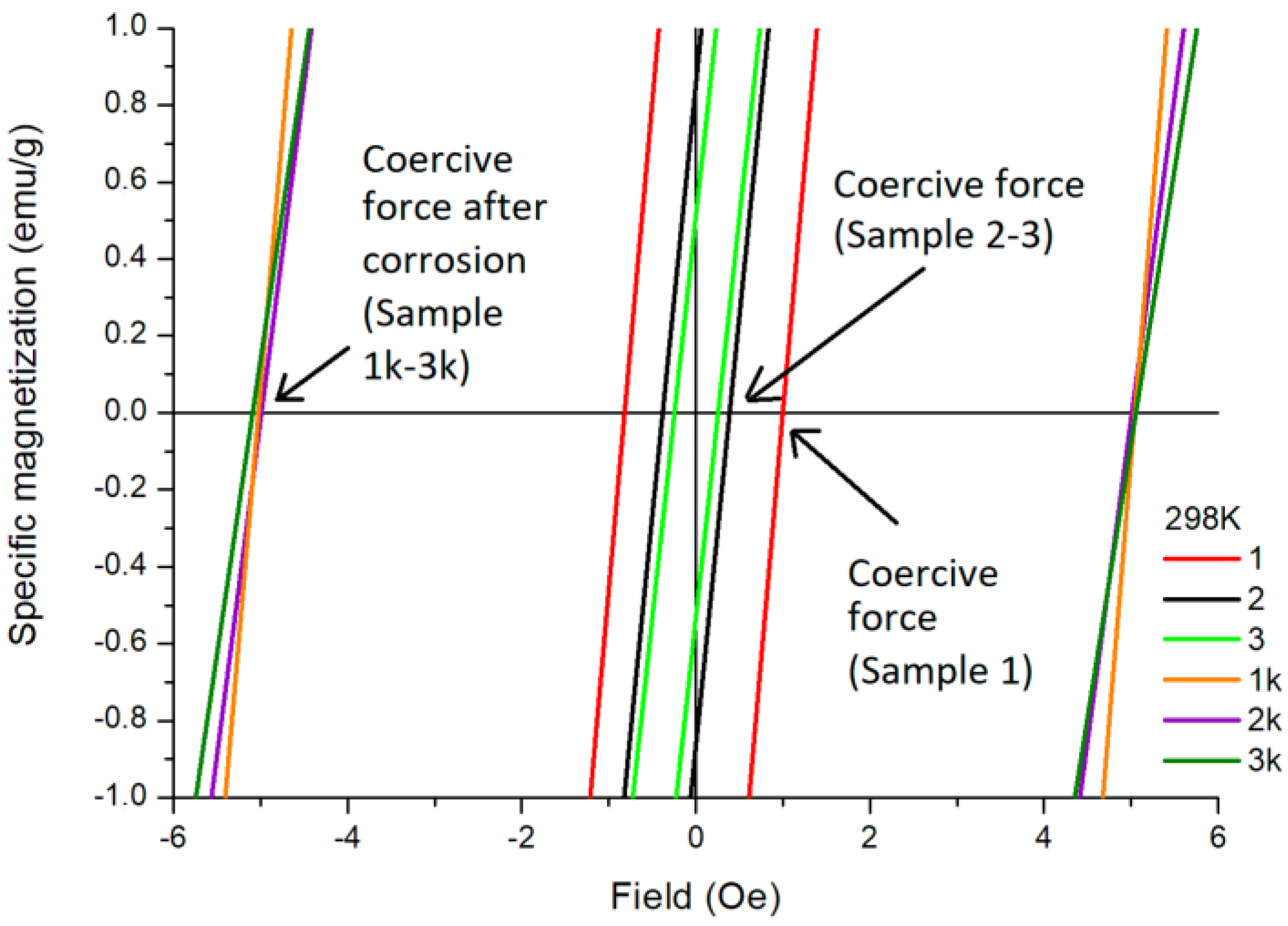
2.3. Magnetic Properties of Studied Samples
3. Materials and Methods
3.1. Materials
3.2. Material Characterization
3.3. Anodizing
3.4. Corrosion Testing
3.5. Impedance Response Testing
3.6. Magnetic Properties Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A. Abbreviation of the Designations of the Samples of the Co75Si15Fe5Cr4.5Al0.5 Amorphous Alloy (AA) Used and Crystalline Cobalt
| Sample Abbreviation | Description of the Sample | What Methods Were Used for |
| (1) | The sample AAs with “clean” (bare) surface | The elemental composition. Polarization curves, impedance spectra in Ringer’s solution, and 1 M Na2SO4 |
| (2) | The sample AAs contain natural oxide on the surface | |
| (3) | The sample AAs contain nanocells on the surface | |
| (4) | The sample of crystalline cobalt with “clean” (bare) surface | impedance spectra in Ringer’s solution. |
| (1k) | The sample AAs with “clean” (bare) surface after corrosion tests | The elemental composition after corrosion tests in Ringer’s solution and magnetic properties after corrosion tests in Ringer’s solution. |
| (2k) | The sample containing natural oxide on the surface after corrosion tests | |
| (3k) | The sample containing nanocells on the surface after corrosion tests |
References
- Xu, D.D.; Zhou, B.L.; Wang, Q.Q.; Zhou, J.; Yang, W.M.; Yuan, C.C.; Xue, L.; Fan, X.D.; Ma, L.Q.; Shen, B.L. Effects of Cr Addition on Thermal Stability, Soft Magnetic Properties and Corrosion Resistance of FeSiB Amorphous Alloys. Corros. Sci. 2018, 138, 20–27. [Google Scholar] [CrossRef]
- Gutfleisch, O.; Willard, M.A.; Brück, E.; Chen, C.H.; Sankar, S.G.; Liu, J.P. Magnetic Materials and Devices for the 21st Century: Stronger, Lighter, and More Energy Efficient. Adv. Mater. 2011, 23, 821–842. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, T.; Shen, B.; Li, B.; Zhang, M.; Zuo, S.; Liu, J.; Jiang, S.; Hu, F.; Sun, J. Microstructures and Magnetic Properties of Co-Substituted Ce–Fe–B Amorphous Alloys. J. Alloys Compd. 2020, 820, 153098. [Google Scholar] [CrossRef]
- Chiriac, H.; Herea, D.-D.; Corodeanu, S. Microwire Array for Giant Magneto-Impedance Detection of Magnetic Particles for Biosensor Prototype. J. Magn. Magn. Mater. 2007, 311, 425–428. [Google Scholar] [CrossRef]
- Wu, Q.; Li, J.; Zhang, P.; Pan, M.; Ge, H. Correlation between Magnetic Properties and Corrosion Resistance of SmCo5/α-Fe Nanocomposite Magnet. Int. J. Electrochem. Sci. 2019, 14, 4318–4326. [Google Scholar] [CrossRef]
- Ou, S.; Ma, D.; Li, Y.; Yubuta, K.; Tan, Z.; Wang, Y.; Zhang, W. Fabrication and Electrocatalytic Properties of Ferromagnetic Nanoporous PtFe by Dealloying an Amorphous Fe 60 Pt 10 B 30 Alloy. J. Alloys Compd. 2017, 706, 215–219. [Google Scholar] [CrossRef]
- Ackland, K.; Masood, A.; Kulkarni, S.; Stamenov, P. Ultra-Soft Magnetic Co-Fe-B-Si-Nb Amorphous Alloys for High Frequency Power Applications. AIP Adv. 2018, 8, 056129. [Google Scholar] [CrossRef]
- Xu, J.; Yang, Y.; Li, W.; Xie, Z.; Chen, X. Effect of Si Addition on Crystallization Behavior, Thermal Ability and Magnetic Properties in High Fe Content Fe-Si-B-P-Cu-C Alloy. Mater. Res. Bull. 2018, 97, 452–456. [Google Scholar] [CrossRef]
- Kurniawan, M.; Keylin, V.; McHenry, M.E. Alloy Substituents for Cost Reduction in Soft Magnetic Materials. J. Mater. Res. 2015, 30, 1072–1077. [Google Scholar] [CrossRef]
- Permyakova, I.E.; Glezer, A.M.; Savchenko, E.S.; Shchetinin, I.V. Effect of External Actions on the Magnetic Properties and Corrosion Resistance of Co70.5Fe0.5Cr4Si7B18 Amorphous Alloy. Bull. Russ. Acad. Sci. Phys. 2017, 81, 1310–1316. [Google Scholar] [CrossRef]
- Han, J.; Hong, J.; Kwon, S.; Choi-Yim, H. Effect of Cr Addition on Magnetic Properties and Corrosion Resistance of Optimized Co and Fe-Based Amorphous Alloys. Metals 2021, 11, 304. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, X.; Liu, H.; Fu, B.; Dong, Z.; Wang, Y. Microstructure, Corrosion Resistance, and Magnetic Properties of LaFe12.35-XCo0.65SixC0.15(x = 1.3, 1.4, 1.5) Compounds after Corrosion in Distilled Water. J. Supercond. Nov. Magn. 2022, 35, 1569–1574. [Google Scholar] [CrossRef]
- Nyby, C.; Guo, X.; Saal, J.E.; Chien, S.-C.; Gerard, A.Y.; Ke, H.; Li, T.; Lu, P.; Oberdorfer, C.; Sahu, S.; et al. Electrochemical Metrics for Corrosion Resistant Alloys. Sci. Data 2021, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Dong, C.; Li, X.; Xiao, K. Effects of Applied Magnetic Field on Corrosion of Beryllium Copper in NaCl Solution. J. Mater. Sci. Technol. 2010, 26, 355–361. [Google Scholar] [CrossRef]
- Chernavsky, P.A.; Kim, N.V.; Andrianov, V.A.; Perfiliev, Y.D.; Novakova, A.A.; Perov, N.S. The Influence of an External Magnetic Field on the Dynamics of Magnetite Reduction with Hydrogen. RSC Adv. 2021, 11, 15422–15427. [Google Scholar] [CrossRef]
- Herzer, G. Modern Soft Magnets: Amorphous and Nanocrystalline Materials. Acta Mater. 2013, 61, 718–734. [Google Scholar] [CrossRef]
- Veligatla, M.; Katakam, S.; Das, S.; Dahotre, N.; Gopalan, R.; Prabhu, D.; Arvindha Babu, D.; Choi-Yim, H.; Mukherjee, S. Effect of Iron on the Enhancement of Magnetic Properties for Cobalt-Based Soft Magnetic Metallic Glasses. Metall. Mater. Trans. A 2015, 46, 1019–1023. [Google Scholar] [CrossRef]
- Panda, A.K.; Mohanta, O.; Ghosh, M.; Mitra, A. Development of Nanostructured CoFe-Based Alloys for High Temperature Magnetic Applications. J. Nanosci. Nanotechnol. 2009, 9, 5600–5603. [Google Scholar] [CrossRef]
- Lebedeva, O.; Kultin, D.; Kustov, L. Electrochemical Synthesis of Unique Nanomaterials in Ionic Liquids. Nanomaterials 2021, 11, 3270. [Google Scholar] [CrossRef]
- Lebedeva, O.; Snytko, V.; Kuznetsova, I.; Kalmykov, K.; Kultin, D.; Root, N.; Philippova, S.; Dunaev, S.; Zakharov, A.; Kustov, L. Impact of Pretreatment of Metal Glass Fe70Cr15B15 on Anodization in 1-Butyl-3-Methylimidazolium Tetrafluoroborate Ionic Liquid. Metals 2020, 10, 583. [Google Scholar] [CrossRef]
- Lebedeva, O.; Kultin, D.; Kalmykov, K.; Snytko, V.; Kuznetsova, I.; Orekhov, A.; Zakharov, A.; Kustov, L. Nanorolls Decorated with Nanotubes as a Novel Type of Nanostructures: Fast Anodic Oxidation of Amorphous Fe–Cr–B Alloy in Hydrophobic Ionic Liquid. ACS Appl. Mater. Interfaces 2021, 13, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, O.; Kultin, D.; Zakharov, A.; Kustov, L. Advances in Application of Ionic Liquids: Fabrication of Surface Nanoscale Oxide Structures by Anodization of Metals and Alloys. Surf. Interfaces 2022, 34, 102345. [Google Scholar] [CrossRef]
- Gaikar, P.S.; Angre, A.P.; Wadhawa, G.; Ledade, P.V.; Mahmood, S.H.; Lambat, T.L. Green Synthesis of Cobalt Oxide Thin Films as an Electrode Material for Electrochemical Capacitor Application. Curr. Res. Green Sustain. Chem. 2022, 5, 100265. [Google Scholar] [CrossRef]
- Saverina, E.A.; Zinchenko, D.Y.; Farafonova, S.D.; Galushko, A.S.; Novikov, A.A.; Gorbachevskii, M.V.; Ananikov, V.P.; Egorov, M.P.; Jouikov, V.V.; Syroeshkin, M.A. Porous Silicon Preparation by Electrochemical Etching in Ionic Liquids. ACS Sustain. Chem. Eng. 2020, 8, 10259–10264. [Google Scholar] [CrossRef]
- Tateishi, K.; Ogino, H.; Waki, A.; Ohishi, T.; Murakami, M.; Asoh, H.; Ono, S. Anodization Behavior of Aluminum in Ionic Liquids with a Small Amount of Water. Electrochemistry 2013, 81, 440–447. [Google Scholar] [CrossRef][Green Version]
- Chaudhari, A.; Awale, A.S.; Chakrabarti, A.K. Surface Integrity Characterization of Austenitic, Martensitic and Ferritic Stainless Steel under Different Grinding Process. Mater. Res. Express 2019, 6, 1165c9. [Google Scholar] [CrossRef]
- Rellinghaus, B.; Fernandez DeAvila, S.; Armelles, G.; Beyers, R.; Kellock, A.; Weller, D. Natural Oxide Formation on Cobalt Investigated with the Magneto-Optical Kerr Effect. IEEE Trans. Magn. 1997, 33, 3238–3240. [Google Scholar] [CrossRef]
- Pontinha, M.; Faty, S.; Walls, M.G.; Ferreira, M.G.S.; Cunha Belo, M.D. Electronic Structure of Anodic Oxide Films Formed on Cobalt by Cyclic Voltammetry. Corros. Sci. 2006, 48, 2971–2986. [Google Scholar] [CrossRef]
- Kuznetsova, I.; Lebedeva, O.; Kultin, D.; Kalmykov, K.; Philippova, S.; Leonov, A.; Kustov, L. (Digital Presentation) Influence of Preliminary Anodization of Amorphous Alloy Co75Si15Fe5Cr4.5Al0.5 in Ionic Liquid on Corrosion Resistance. ECS Trans. 2022, 109, 87–94. [Google Scholar] [CrossRef]
- Schubert, N.; Schneider, M.; Michealis, A. The Mechanism of Anodic Dissolution of Cobalt in Neutral and Alkaline Electrolyte at High Current Density. Electrochim. Acta 2013, 113, 748–754. [Google Scholar] [CrossRef]
- Li, S.; Fang, M.; Xiao, Z.; Meng, X.; Lei, Q.; Jia, Y. Effect of Cr Addition on Corrosion Behavior of Cupronickel Alloy in 3.5 Wt% NaCl Solution. J. Mater. Res. Technol. 2023, 22, 2222–2238. [Google Scholar] [CrossRef]
- Lone, S.A.; Mardare, C.C.; Mardare, A.I.; Hassel, A.W. A Real Time Electrochemical Analysis of Co-Sputtered (Co-Cr) and (Co-Cr-Mo) Alloy Thin Film Libraries in Ringer’s Solution at 37 °C. Meet. Abstr. 2021, MA2021-011, 797. [Google Scholar] [CrossRef]
- Mezour, M.A.; Oweis, Y.; El-Hadad, A.A.; Algizani, S.; Tamimi, F.; Cerruti, M. Surface Modification of CoCr Alloys by Electrochemical Reduction of Diazonium Salts. RSC Adv. 2018, 8, 23191–23198. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, X.; Khan, F.; Ye, X.; Wang, S.; Chen, J. Field Deployable Impedance-Based Corrosion Sensor. Sci. Rep. 2022, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Menchaca, J.; Cabral-Miramontes, J.A.; Garay-Tapi, A.M.; Almeraya-Calderón, F. Influence of Microstructure on Corrosion Behavior of Zn-Al-Sr Alloys in Sodium Chloride Solution. Int. J. Electrochem. Sci. 2022, 17, 221038. [Google Scholar] [CrossRef]
- Gallant, D.; Simard, S. A Study on the Localized Corrosion of Cobalt in Bicarbonate Solutions Containing Halide Ions. Corros. Sci. 2005, 47, 1810–1838. [Google Scholar] [CrossRef]
- Abel, F.M.; Tzitzios, V.; Hadjipanayis, G.C. New Approach for Direct Chemical Synthesis of Hexagonal Co Nanoparticles. J. Magn. Magn. Mater. 2016, 400, 286–289. [Google Scholar] [CrossRef]
- Zhang, T.; Inoue, A. Bulk Glassy Alloys in (Fe, Co, Ni)-Si-B System. Mater. Trans. 2001, 42, 1015–1018. [Google Scholar] [CrossRef][Green Version]
- Vakhitov, R.M.; Shapayeva, T.B.; Solonetskiy, R.V.; Yumaguzin, A.R. Structure of Micromagnetic Formations Arising on Defects in Garnet-Ferrite Films. Phys. Met. Metallogr. 2017, 118, 541–545. [Google Scholar] [CrossRef]
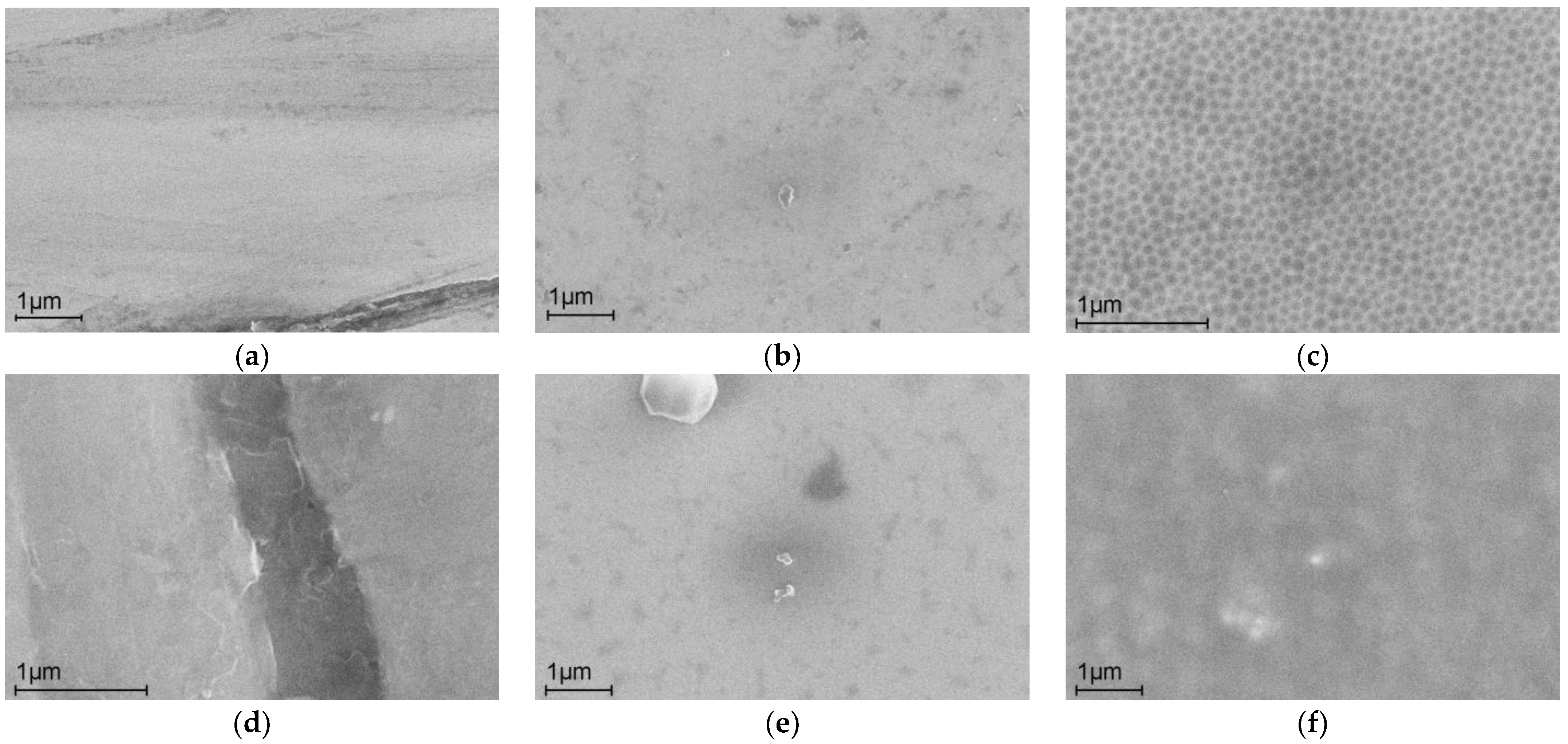
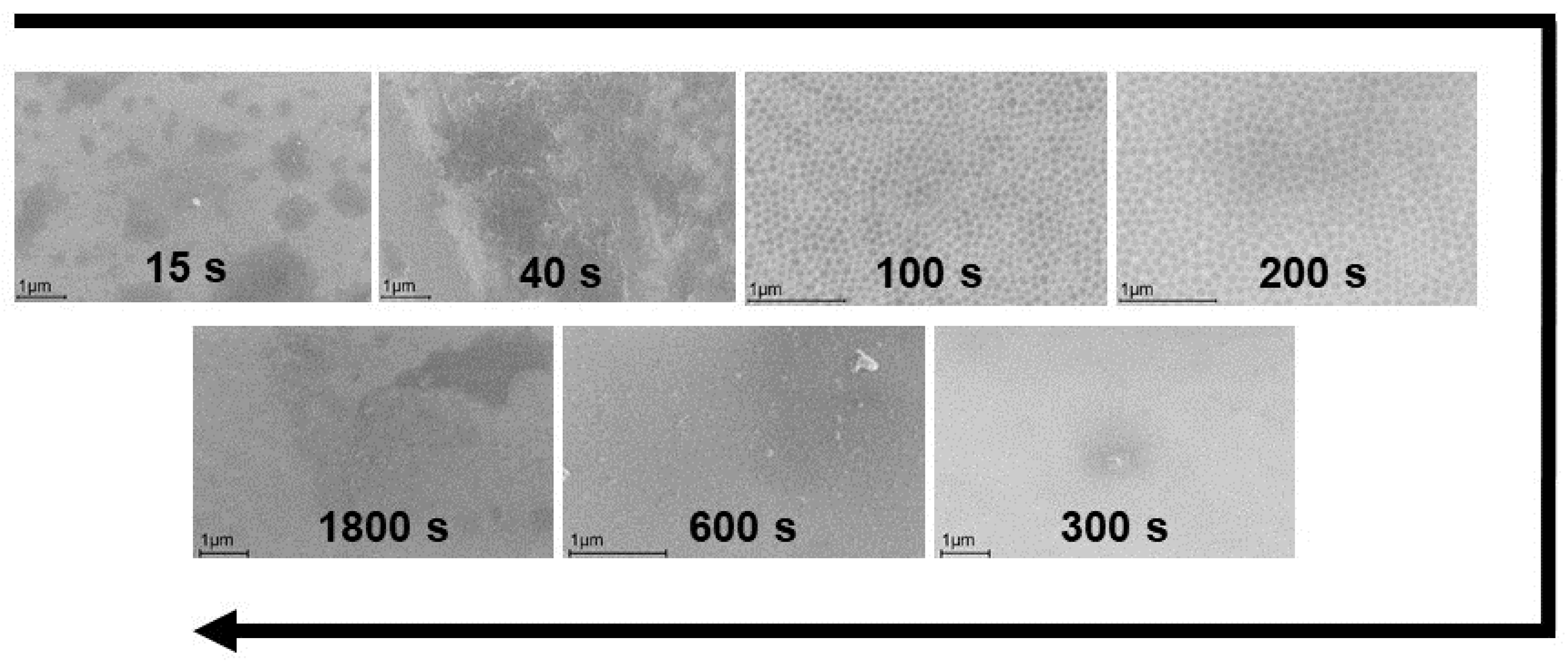
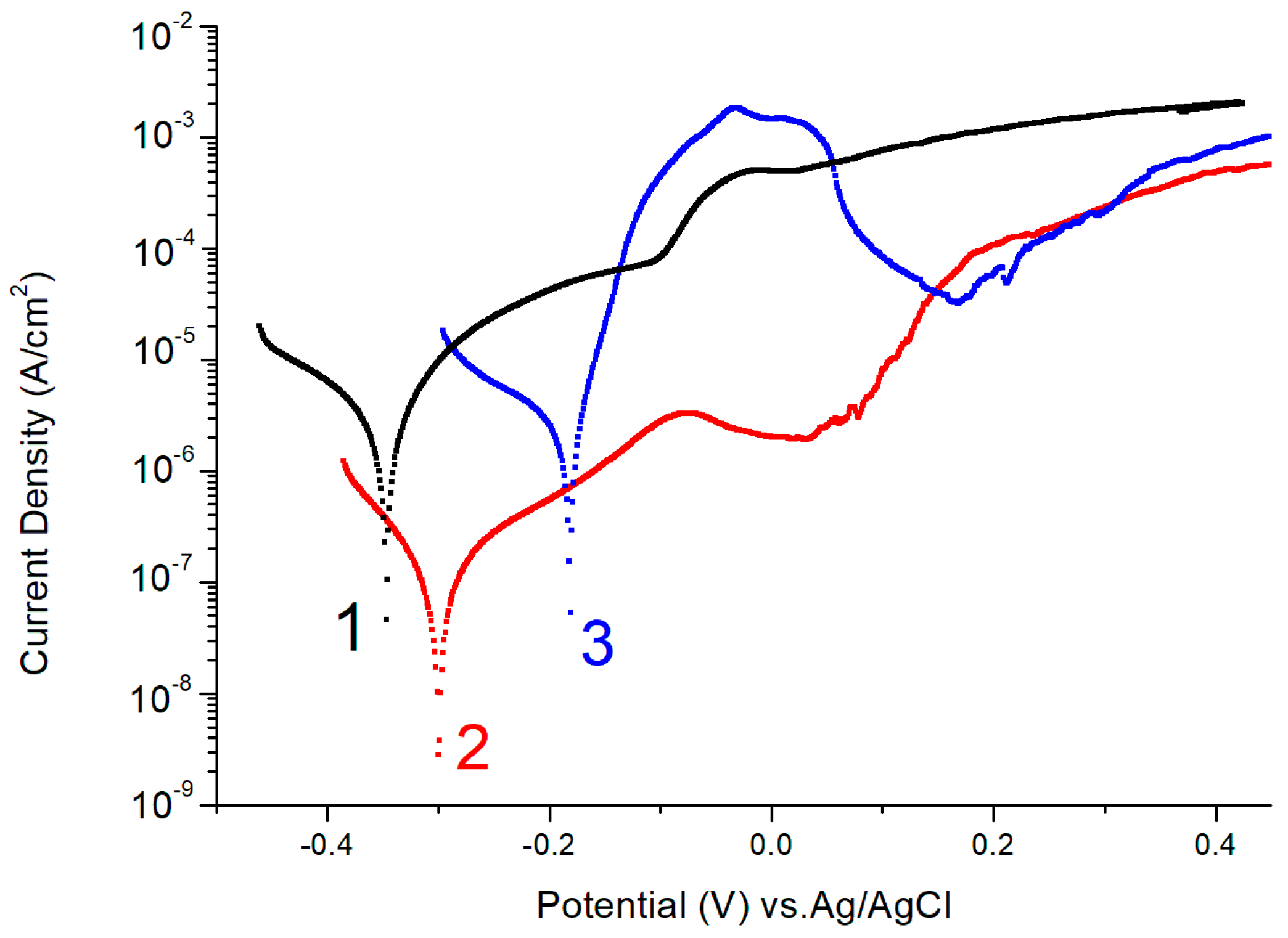

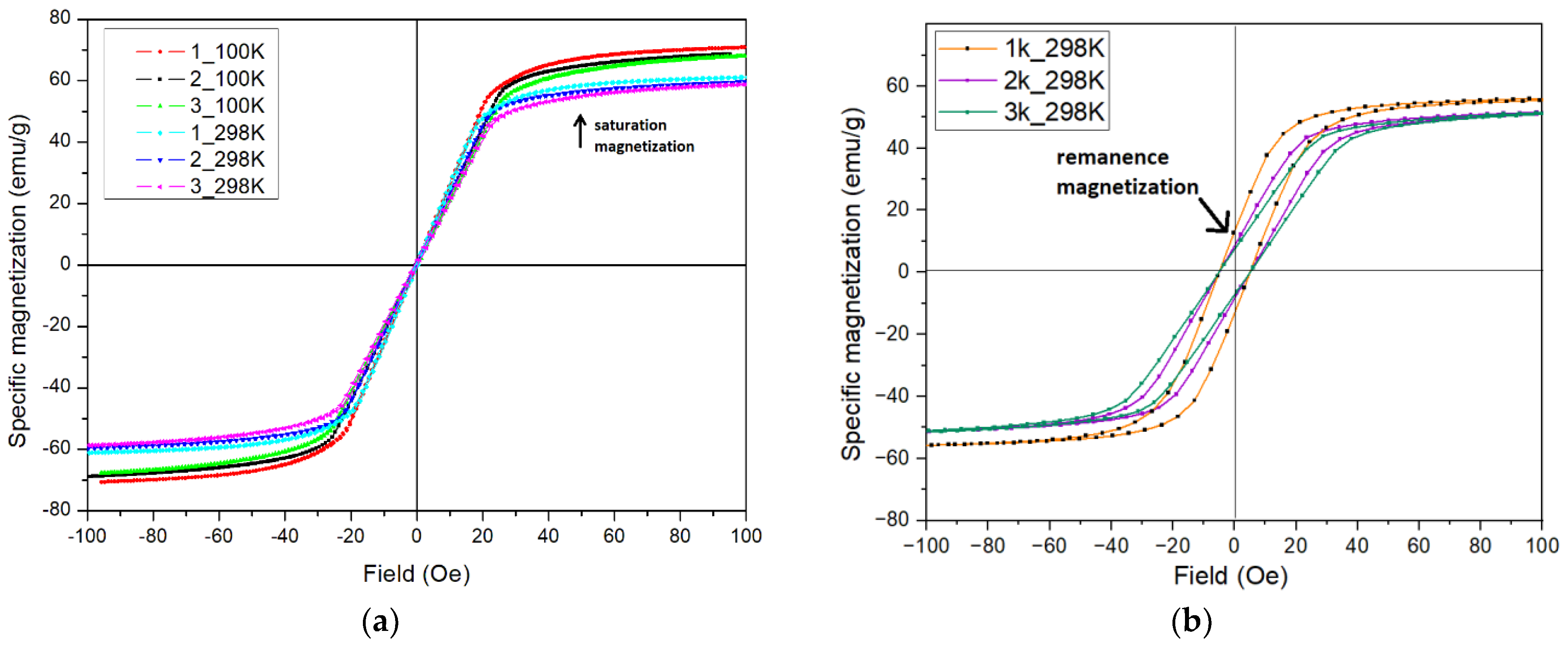

| Sample 1 | The Content of Elements before Corrosion Tests (at. %) | |||||
|---|---|---|---|---|---|---|
| Co | Si | Fe | Cr | Al | O | |
| (1) | 72.55 ± 2.40 | 14.06 ± 0.90 | 4.73 ± 0.37 | 3.94 ± 0.17 | 0.30 ± 0.22 | 4.42 ± 3.44 |
| (2) | 75.10 ± 0.49 | 14.27 ± 0.39 | 4.63 ± 0.37 | 4.19 ± 0.17 | 0.17 ± 0.16 | 1.65 ± 0.70 |
| (3) | 75.18 ± 0.49 | 14.54 ± 0.40 | 4.98 ± 0.25 | 4.21 ± 0.29 | 0.29 ± 0.15 | 0.81 ± 0.12 |
| The Content of Elements after Corrosion Tests (at. %) | ||||||
| (1k) | 70.65 ± 0.91 | 14.64 ± 0.61 | 4.76 ± 0.34 | 3.78 ± 0.24 | 0.34 ± 0.30 | 5.82 ± 1.07 |
| (2k) | 75.30 ± 1.12 | 14.37 ± 0.57 | 4.75 ± 0.24 | 4.16 ± 0.25 | 0.30 ± 0.27 | 1.13 ± 0.73 |
| (3k) | 71.39 ± 1.54 | 14.12 ± 0.28 | 4.85 ± 0.45 | 4.03 ± 0.26 | 0.23 ± 0.18 | 5.37 ± 1.47 |
| Sample | Ecorr exp (mV) | Ecorr calc (mV) | PR × 105 (Ohm) |
|---|---|---|---|
| (1) | −348 | −372 | 0.06 |
| (2) | −319 | −322 | 1.76 |
| (3) | −189 | −188 | 0.04 |
| Sample 1 | Environment | Rs/Ohm cm2 | Rp/Ohm cm2 | CPE/Ohm−1 cm−2 cN | N |
|---|---|---|---|---|---|
| (1) | Ringer’s solution | 20.4 ± 2% | 15455 ± 2% | 1.88 × 10–5 ± 3.4% | 0.88 ± 0.7% |
| (2) | 1 M Na2SO4 | 8.9 ± 3% | 173120 ± 3% | 6.34 × 10–6 ± 2.1% | 0.87 ± 0.4% |
| (3) | 1 M Na2SO4 | 10.3 ± 7% | 33546 ± 10% | 5.09 × 10–5 ± 5.2% | 0.84 ± 1.5% |
| (3) | Ringer’s solution | 26.3 ± 4% | 19669 ± 5% | 3.34 × 10–5 ± 5.8% | 0.83 ± 1.3% |
| (4) | Ringer’s solution | 34.0 ± 2% | 17117 ± 2% | 1.01 × 10–5 ± 2.5% | 0.79 ± 0.4% |
| Conditions | Sample 1 | Coercivity at 298 K (Oe) | Coercivity at 100 K (Oe) | Saturation Magnetization (Ms) at 298 K (emu/g) | Remanence Magnetization (Mr) at 298 K (emu/g) |
|---|---|---|---|---|---|
| Before corrosion testing | (1) | 0.35 | 0.16 | 61 | 0.9 |
| (2) | 0.38 | 0.08 | 60 | 0.4 | |
| (3) | 0.45 | 0.01 | 59 | 1.3 | |
| After corrosion testing | (1k) | 5.00 | 4.70 | 51 | 11.5 |
| (2k) | 4.95 | 4.50 | 48 | 9.0 | |
| (3k) | 5.10 | 4.80 | 47 | 8.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsova, I.; Lebedeva, O.; Kultin, D.; Perova, N.; Kalmykov, K.; Chernavskii, P.; Perov, N.; Kustov, L. Is a 2D Nanostructured Surface Capable of Changing the Corrosion and Magnetic Properties of an Amorphous Alloy? Int. J. Mol. Sci. 2023, 24, 13373. https://doi.org/10.3390/ijms241713373
Kuznetsova I, Lebedeva O, Kultin D, Perova N, Kalmykov K, Chernavskii P, Perov N, Kustov L. Is a 2D Nanostructured Surface Capable of Changing the Corrosion and Magnetic Properties of an Amorphous Alloy? International Journal of Molecular Sciences. 2023; 24(17):13373. https://doi.org/10.3390/ijms241713373
Chicago/Turabian StyleKuznetsova, Irina, Olga Lebedeva, Dmitry Kultin, Natalia Perova, Konstantin Kalmykov, Petr Chernavskii, Nikolai Perov, and Leonid Kustov. 2023. "Is a 2D Nanostructured Surface Capable of Changing the Corrosion and Magnetic Properties of an Amorphous Alloy?" International Journal of Molecular Sciences 24, no. 17: 13373. https://doi.org/10.3390/ijms241713373
APA StyleKuznetsova, I., Lebedeva, O., Kultin, D., Perova, N., Kalmykov, K., Chernavskii, P., Perov, N., & Kustov, L. (2023). Is a 2D Nanostructured Surface Capable of Changing the Corrosion and Magnetic Properties of an Amorphous Alloy? International Journal of Molecular Sciences, 24(17), 13373. https://doi.org/10.3390/ijms241713373








