Age-Dependent Growth-Related QTL Variations in Pacific Abalone, Haliotis discus hannai
Abstract
:1. Introduction
2. Results
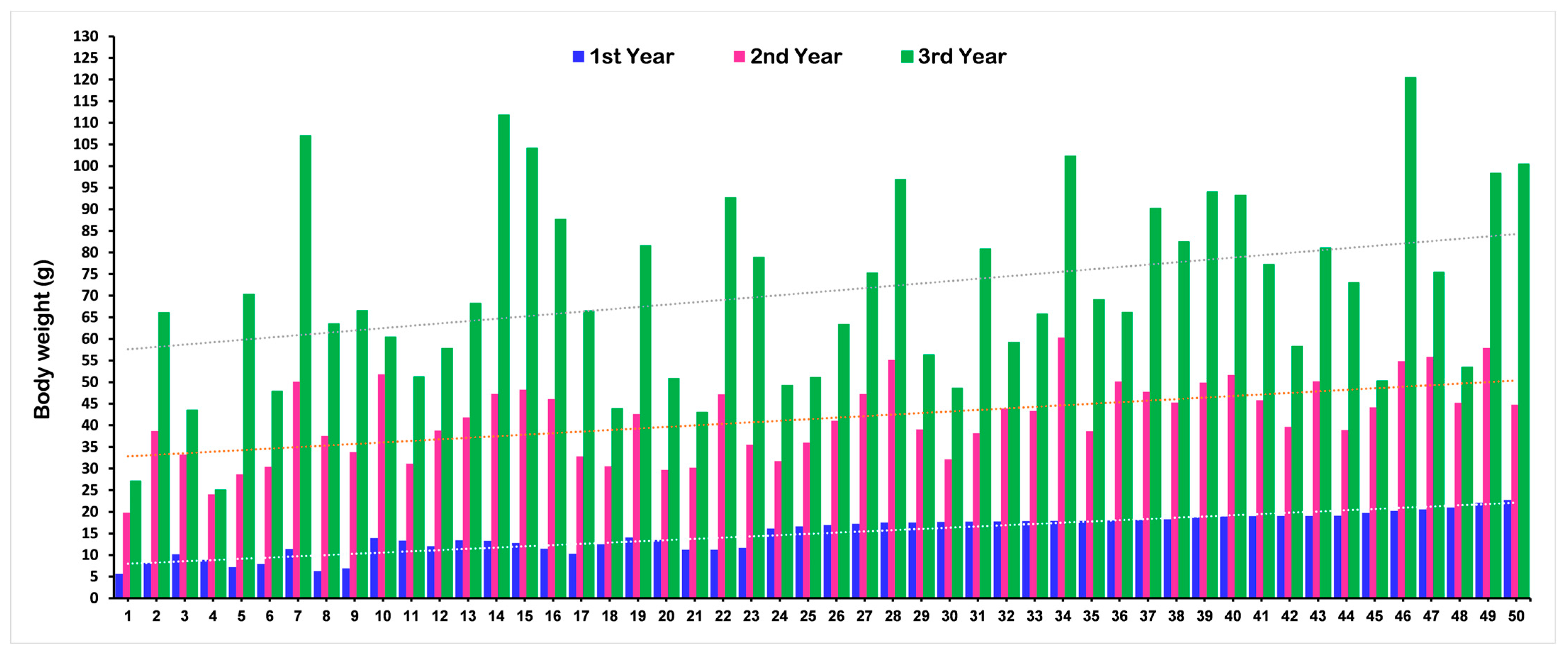
2.1. Variations in Phenotypic Traits
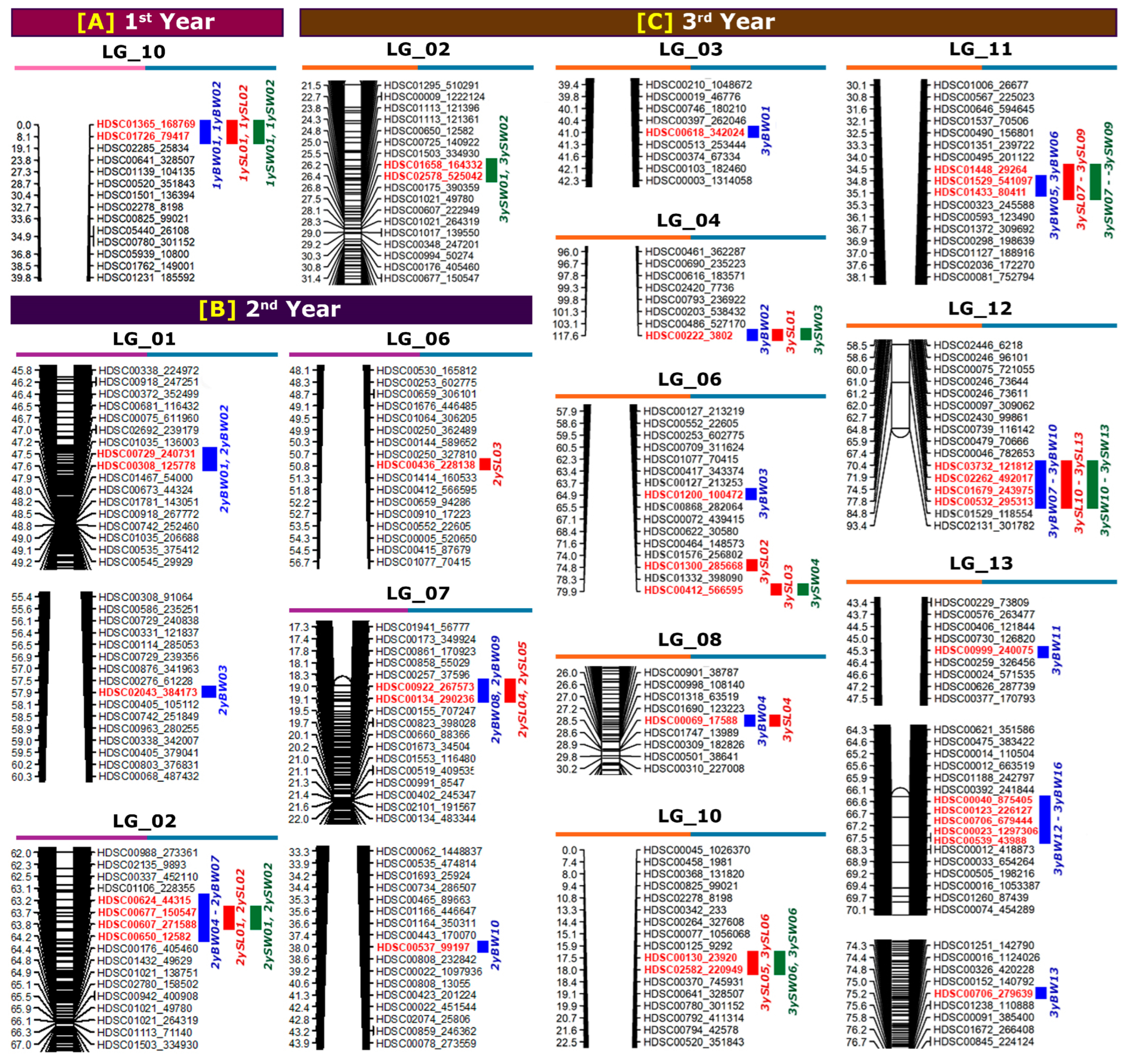
2.2. Genetic Linkage Map
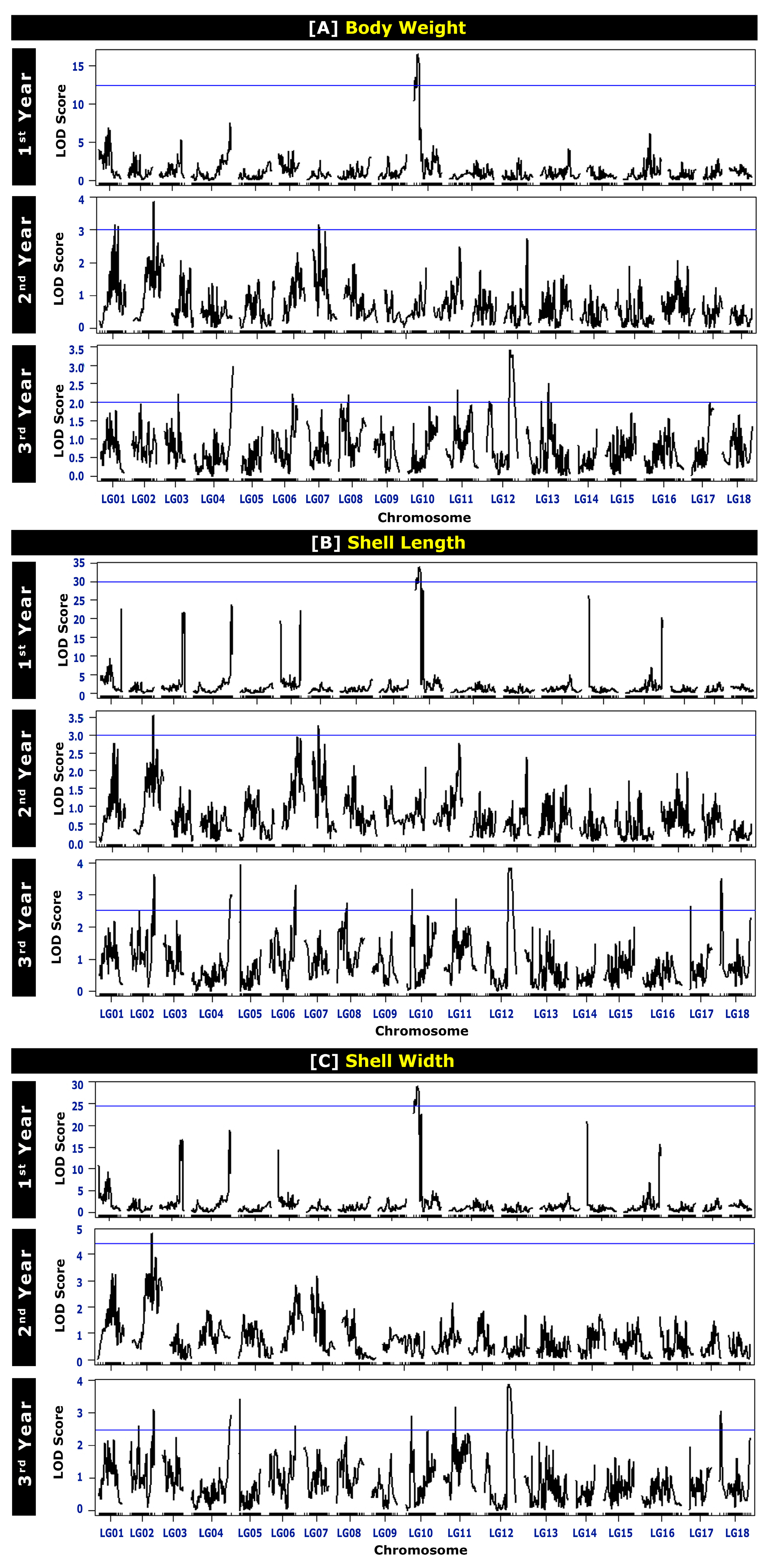
2.3. QTL Analysis
2.3.1. QTLs Associated with Body Weight (BW) at Different Ages
2.3.2. QTLs Associated with Shell Length (SL) at Different Ages
2.3.3. QTLs Associated with Shell Width (SW) at Different Ages
3. Discussion
4. Materials and Methods
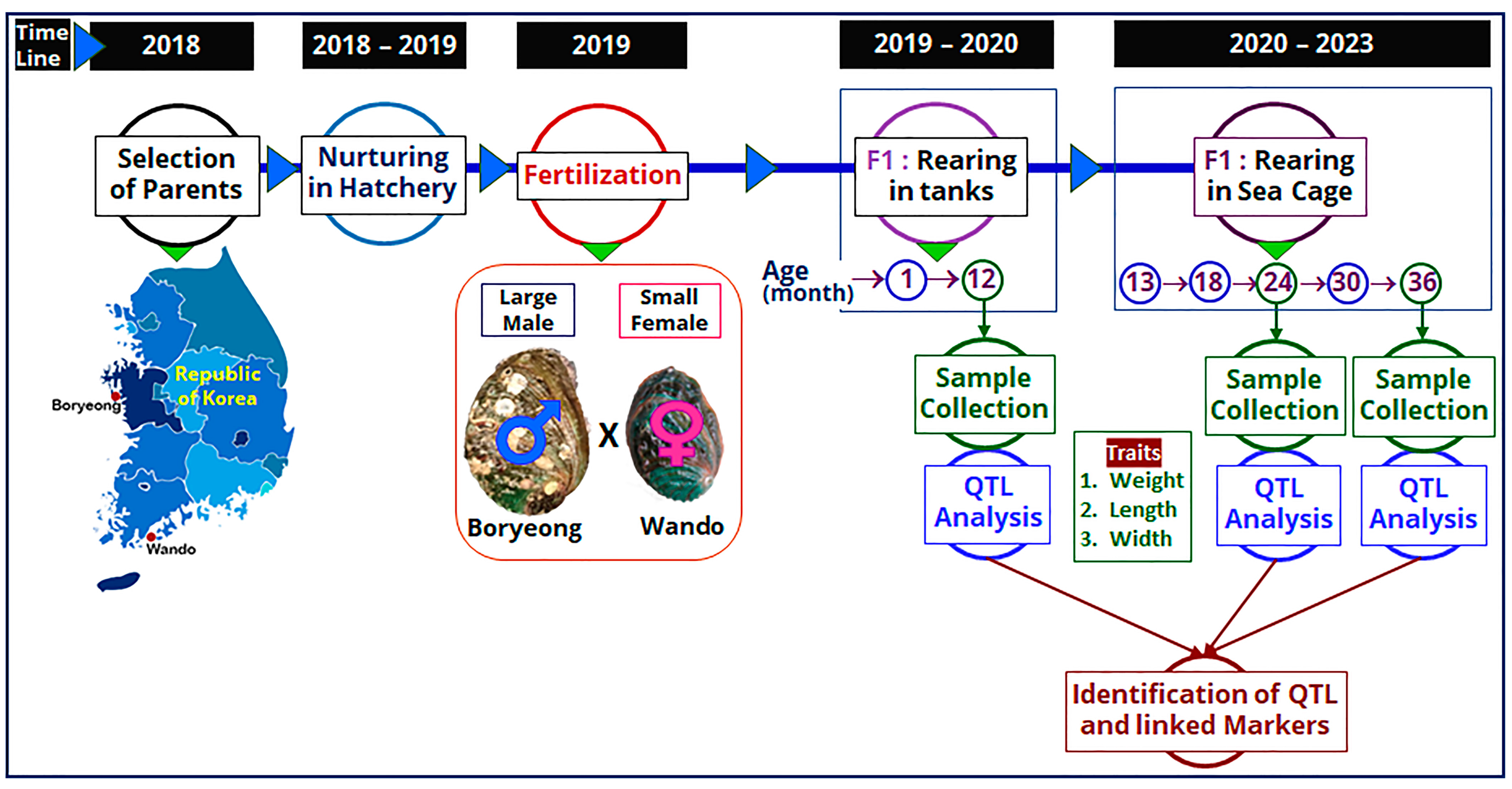
4.1. Collection of Parents and Production of Mapping Family
4.1.1. Collection of Parent Abalone
4.1.2. Production of Mapping Family
4.2. Husbandry of Abalone F1 Population
4.2.1. Husbandry in the Larval Rearing Tank
4.2.2. Husbandry in Sea Cage
4.3. Sample Collection and Trait Measurement
4.4. Genotyping-by-Sequencing (GBS) Library Preparation, Sequencing, and SNP Genotyping
4.4.1. Extraction of Genomic DNA (gDNA)
4.4.2. GBS Library Preparation and Sequencing
4.4.3. Cleaning of Sequences
4.4.4. Detection of Raw SNPs, SNP Filtering, and Genotyping
4.5. Construction of Genetic Linkage Map and QTL Analysis
4.6. QTL Mapping
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gordon, H.R.; Cook, P.A. World abalone supply, markets and pricing: 2011 update. J. Shellfish Res. 2013, 32, 5–7. [Google Scholar] [CrossRef]
- Hossen, S.; Sukhan, Z.P.; Cho, Y.; Lee, W.K.; Kho, K.H. Antioxidant activity and oxidative stress-oriented apoptosis pathway in saccharides supplemented cryopreserved sperm of Pacific abalone, Haliotis discus hannai. Antioxidants 2022, 11, 1303. [Google Scholar] [CrossRef] [PubMed]
- Sukhan, Z.P.; Hossen, S.; Cho, Y.; Lee, W.K.; Kho, K.H. Hdh-tektin-4 regulates motility of fresh and cryopreserved sperm in Pacific abalone, Haliotis discus hannai. Front. Cell Dev. Biol. 2022, 10, 870743. [Google Scholar] [CrossRef]
- Sim, B.R.; Kim, H.C.; Kang, S.; Park, K.D.; Yoon, S.; Hong, S.; Yoon, S.P.; Kim, J.B.; Lee, W.C. Influence of intensive net cage farming on hydrodynamic and geochemical environmental conditions and the mass mortality of abalone in South Korea. Mar. Pollut. Bull. 2021, 169, 112555. [Google Scholar] [CrossRef]
- Hassan, A.L.I.; Mock, T.S.; Searle, K.; Rocker, M.M.; Turchini, G.M.; Francis, D.S. Optimal dietary protein requirement of subadult Australian hybrid abalone (Haliotis rubra × Haliotis laevigata) at different rearing temperatures. Aquac. Res. 2023, 2023, 676340. [Google Scholar] [CrossRef]
- Saunders, T.M.; Connell, S.D.; Mayfield, S. Differences in abalone growth and morphology between locations with high and low food availability: Morphologically fixed or plastic traits? Mar. Biol. 2009, 156, 1255–1263. [Google Scholar] [CrossRef]
- Kho, K.H.; Sukhan, Z.P.; Hossen, S.; Cho, Y.; Kim, S.C.; Sharker, M.R.; Jung, H.J.; Nou, I.S. Construction of a genetic linkage map based on SNP markers, QTL mapping and detection of candidate genes of growth-related traits in Pacific abalone using genotyping-by-sequencing. Front. Mar. Sci. 2021, 8, 713783. [Google Scholar] [CrossRef]
- Liu, Z.J.; Cordes, J.F. DNA marker technologies and their applications in aquaculture genetics. Aquaculture 2004, 238, 1–37. [Google Scholar] [CrossRef]
- Symonds, J.E.; Clarke, S.M.; King, N.; Walker, S.P.; Blanchard, B.; Sutherland, D.; Roberts, R.; Preece, M.A.; Tate, M.; Buxton, P.; et al. Developing successful breeding programs for New Zealand aquaculture: A perspective on progress and future genomic opportunities. Front. Genet. 2019, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, H.; ElHady, M.; Alcivar-Warren, A.; Allen, S.; Al-Tobasei, R.; Bao, L.; Beck, B.; Blackburn, H.; Bosworth, B.; Buchanan, J.; et al. Aquaculture genomics, genetics and breeding in the United States: Current status, challenges, and priorities for future research. BMC Genom. 2017, 18, 191. [Google Scholar] [CrossRef]
- Tan, K.; Zhang, H.; Lim, L.; Zheng, H. Selection breeding program of Nan’ao golden scallop Chlamys nobilis with higher nutritional values and less susceptible to stress. Aquaculture 2020, 517, 734769. [Google Scholar] [CrossRef]
- Andriantahina, F.; Liu, X.; Huang, H. Genetic map construction and quantitative trait locus (QTL) detection of growth-related traits in Litopenaeus vannamei for selective breeding applications. PLoS ONE 2013, 8, e75206. [Google Scholar] [CrossRef]
- Baranski, M.; Moen, T.; Våge, D.I. Mapping of quantitative trait loci for flesh colour and growth traits in Atlantic salmon (Salmo salar). Genet. Sel. Evol. 2010, 42, 17. [Google Scholar] [CrossRef]
- Fuji, K.; Hasegawa, O.; Honda, K.; Kumasaka, K.; Sakamoto, T.; Okamoto, N. Marker-assisted breeding of a lymphocystis disease-resistant Japanese flounder (Paralichthys olivaceus). Aquaculture 2007, 272, 291–295. [Google Scholar] [CrossRef]
- Ozaki, A.; Sakamoto, T.; Khoo, S.; Nakamura, K.; Coimbra, M.R.M.; Akutsu, T.; Okamoto, N. Quantitative trait loci (QTLs) associated with resistance/susceptibility to infectious pancreatic necrosis virus (IPNV) in rainbow trout (Oncorhynchus mykiss). Mol. Genet. Genom. 2001, 265, 23–31. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X.; Zhang, G. Identification of quantitative trait loci for growth-related traits in the Pacific abalone Haliotis discus hannai Ino. Aquac. Res. 2007, 38, 789–797. [Google Scholar] [CrossRef]
- Mackay, T.F.C. The genetic architecture of quantitative traits. Annu. Rev. Genet. 2001, 35, 303–339. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Taniguchi, N. Genetic control of growth in the guppy (Poecilia reticulata). Aquaculture 2002, 204, 393–405. [Google Scholar] [CrossRef]
- Yue, G.H. Recent advances of genome mapping and marker-assisted selection in aquaculture. Fish Fish. 2014, 15, 376–396. [Google Scholar] [CrossRef]
- Powder, K.E. Quantitative trait loci (QTL) mapping. Methods Mol. Biol. 2020, 2082, 211–229. [Google Scholar] [CrossRef]
- Feng, X.; Yu, X.; Fu, B.; Wang, X.; Liu, H.; Pang, M.; Tong, J. A high-resolution genetic linkage map and QTL fine mapping for growth-related traits and sex in the Yangtze River common carp (Cyprinus carpio haematopterus). BMC Genom. 2018, 19, 230. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, Q.; Yu, Y.; Wan, S.; Han, L.; Du, S. Mapping genetic loci for quantitative traits of golden shell color, mineral element contents, and growth-related traits in Pacific oyster (Crassostrea gigas). Mar. Biotechnol. 2018, 20, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Loukovitis, D.; Chatziplis, D.; Batargias, C. Age-dependent QTL affecting body weight in gilthead seabream (Sparus aurata L.). Mediterr. Mar. Sci. 2016, 17, 666–669. [Google Scholar] [CrossRef]
- Hadjipavlou, G.; Bishop, S.C. Age-dependent quantitative trait loci affecting growth traits in Scottish Blackface sheep. Anim. Genet. 2009, 40, 165–175. [Google Scholar] [CrossRef]
- Emebiri, L.C.; Devey, M.E.; Matheson, A.C.; Slee, M.U. Age-related changes in the expression of QTLs for growth in radiata pine seedlings. Theor. Appl. Genet. 1998, 97, 1053–1061. [Google Scholar] [CrossRef]
- Ren, P.; Peng, W.; You, W.; Huang, Z.; Guo, Q.; Chen, N.; He, P.; Ke, J.; Gwo, J.C.; Ke, C. Genetic mapping and quantitative trait loci analysis of growth-related traits in the small abalone Haliotis diversicolor using restriction-site-associated DNA sequencing. Aquaculture 2016, 454, 163–170. [Google Scholar] [CrossRef]
- Baranski, M.; Rourke, M.; Loughnan, S.; Hayes, B.; Austin, C.; Robinson, N. Detection of QTL for growth rate in the blacklip abalone (Haliotis rubra Leach) using selective DNA pooling. Anim. Genet. 2008, 39, 606–614. [Google Scholar] [CrossRef]
- Nie, H.; Yan, X.; Huo, Z.; Jiang, L.; Chen, P.; Liu, H.; Ding, J.; Yang, F. Construction of a high-density genetic map and quantitative trait locus mapping in the manila clam Ruditapes philippinarum. Sci. Rep. 2017, 7, 229. [Google Scholar] [CrossRef]
- Guo, X.; Li, Q.; Wang, Q.Z.; Kong, L.F. Genetic mapping and QTL analysis of growth-related traits in the Pacific oyster. Mar. Biotechnol. 2012, 14, 218–226. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, X.; Zhang, H.; Zhang, G.; Guo, X. Genetic mapping of size-related quantitative trait loci (QTL) in the bay scallop (Argopecten irradians) using AFLP and microsatellite markers. Aquaculture 2007, 272, 281–290. [Google Scholar] [CrossRef]
- Fraimout, A.; Li, Z.; Sillanpää, M.J.; Merilä, J. Age-dependent genetic architecture across ontogeny of body size in sticklebacks. Proc. Biol. Sci. 2022, 289, 20220352. [Google Scholar] [CrossRef] [PubMed]
- Podisi, B.K.; Knott, S.A.; Burt, D.W.; Hocking, P.M. Comparative analysis of quantitative trait loci for body weight, growth rate and growth curve parameters from 3 to 72 weeks of age in female chickens of a broiler-layer cross. BMC Genet. 2013, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Zheng, X.; Kuang, Y.; Cao, D.; Yan, Y.; Sun, X. QTL variations for growth-related traits in eight distinct families of common carp (Cyprinus carpio). BMC Genet. 2016, 17, 65. [Google Scholar] [CrossRef]
- Neuschl, C.; Brockmann, G.A.; Knott, S.A. Multiple-trait QTL mapping for body and organ weights in a cross between NMRI8 and DBA/2 mice. Genet. Res. 2007, 89, 47–59. [Google Scholar] [CrossRef]
- Souza, L.M.; Gazaffi, R.; Mantello, C.C.; Silva, C.C.; Garcia, D.; Le Guen, V.; Cardoso, S.E.; Garcia, A.A.; Souza, A.P. QTL mapping of growth-related traits in a full-sib family of rubber tree (Hevea brasiliensis) evaluated in a sub-tropical climate. PLoS ONE 2013, 8, e61238. [Google Scholar] [CrossRef] [PubMed]
- Atchley, W.R. Ontogeny, timing of development, and genetic variance-covariances structure. Am. Nat. 1984, 123, 519–540. [Google Scholar] [CrossRef]
- Wentzell, A.M.; Boeye, I.; Zhang, Z.; Kliebenstein, D.J. Genetic networks controlling structural outcome of glucosinolate activation across development. PLoS Genet. 2008, 4, e1000234. [Google Scholar] [CrossRef]
- Wellenreuther, M.; Hansson, B. Detecting polygenic evolution: Problems, pitfalls, and promises. Trends Genet. 2016, 32, 155–164. [Google Scholar] [CrossRef]
- Vasemägi, A.; Kahar, S.; Ozerov, M.Y. Genes that affect Atlantic salmon growth in hatchery do not have the same effect in the wild. Funct. Ecol. 2016, 30, 1687–1695. [Google Scholar] [CrossRef]
- Yang, J.; Guo, B.; Shikano, T.; Liu, X.; Merilä, J. Quantitative trait locus analysis of body shape divergence in nine-spined sticklebacks based on high-density SNP-panel. Sci. Rep. 2016, 6, 26632. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.H.; Lin, G.; He, X.; Yunping, B.; Liu, P.; Liu, F.; Sun, F.; Tu, R.; Yue, G.H. Mapping quantitative trait loci for omega-3 fatty acids in Asian seabass. Mar. Biotechnol. 2014, 16, 1–9. [Google Scholar] [CrossRef]
- Sakamoto, T.; Danzmann, R.G.; Gharbi, K.; Howard, P.; Ozaki, A.; Khoo, S.K.; Woram, R.A.; Okamoto, N.; Ferguson, M.M.; Holm, L.E.; et al. A microsatellite linkage map of rainbow trout (Oncorhynchus mykiss) characterized by large sex-specific differences in recombination rates. Genetics 2000, 155, 1331–1345. [Google Scholar] [CrossRef]
- Anderson, J.L.; Rodríguez Marí, A.; Braasch, I.; Amores, A.; Hohenlohe, P.; Batzel, P.; Postlethwait, J.H. Multiple sex-associated regions and a putative sex chromosome in zebrafish revealed by RAD mapping and population genomics. PLoS ONE 2012, 7, e40701. [Google Scholar] [CrossRef]
- Fraslin, C.; Dechamp, N.; Bernard, M.; Krieg, F.; Hervet, C.; Guyomard, R.; Esquerré, D.; Barbieri, J.; Kuchly, C.; Duchaud, E.; et al. Quantitative trait loci for resistance to Flavobacterium psychrophilum in rainbow trout: Effect of the mode of infection and evidence of epistatic interactions. Genet. Sel. Evol. 2018, 50, 60. [Google Scholar] [CrossRef]
- Meuwissen, T.H.E.; Goddard, M.E. The use of marker haplotypes in animal breeding schemes. Genet. Sel. Evol. 1996, 28, 161–176. [Google Scholar] [CrossRef]
- Sukhan, Z.P.; Cho, Y.; Sharker, M.R.; Hossen, S.; Rha, S.J.; Kho, K.H. Effective accumulative temperature affects gonadal maturation by controlling expression of GnRH, GnRH receptor, serotonin receptor and APGWamide gene in Pacific abalone, Haliotis discus hannai during broodstock conditioning in hatcheries. J. Therm. Biol. 2021, 100, 103037. [Google Scholar] [CrossRef] [PubMed]
- Leighton, P. Abalone Hatchery Manual; Abalone File, No. 25, Aquaculture Explained; Robinson, G., McGowan, N., Eds.; Aquaculture Technical Section, Aquaculture Development Division: Dublin, Ireland, 2008; p. 77. [Google Scholar]
- Li, Y.; He, M. Genetic mapping and QTL analysis of growth-related traits in Pinctada fucata using restriction-site associated DNA sequencing. PLoS ONE 2014, 9, e111707. [Google Scholar] [CrossRef] [PubMed]
- Arseneau, J.R.; Steeves, R.; Laflamme, M. Modified lowsalt CTAB extraction of high-quality DNA from contaminant-rich tissues. Mol. Ecol. Resour. 2017, 17, 686–693. [Google Scholar] [CrossRef]
- Mascher, M.; Wu, S.; Amand, P.S.; Stein, N.; Poland, J. Application of genotyping-by-sequencing on semiconductor sequencing platforms: A comparison of genetic and reference-based marker ordering in barley. PLoS ONE 2013, 8, e76925. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Cox, M.P.; Peterson, D.A.; Biggs, P.J. SolexaQA: At-a-glance quality assessment of Illumina second-generation sequencing data. BMC Bioinform. 2010, 11, 485. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Oh, S.K.; Lee, J.H.; Lee, B.M.; Jo, S.H. Genome-wide SNP calling using next generation sequencing data in tomato. Mol. Cells 2014, 37, 36–42. [Google Scholar] [CrossRef]
- Van Ooijen, J.W. JoinMapr4, Software for the Calculation of Genetic Linkage Maps in Experimental Populations; Kyazma BV: Wageningen, The Netherlands, 2006; p. 33. [Google Scholar]
- Grattapaglia, D.; Sederoff, R. Genetic linkage maps of Eucalyptus grandis and Eucalyptus urophylla using a pseudo-testcross: Mapping strategy and RAPD markers. Genetics 1994, 137, 1121–1137. [Google Scholar] [CrossRef]
- Kosambi, D.D. The Estimation of map distances from recombination values. In D.D. Kosambi: Selected Works in Mathematics and Statistics; Ramaswamy, R., Ed.; Springer: New Delhi, India, 2016; pp. 125–130. [Google Scholar] [CrossRef]
- Broman, K.W.; Wu, H.; Sen, S.; Churchill, G.A. R/qtl: QTL mapping in experimental crosses. Bioinformatics 2003, 19, 889–890. [Google Scholar] [CrossRef]
| Age | Body Weight (g) | Total Length (mm) | Total Width (mm) | |||
|---|---|---|---|---|---|---|
| Mean | SD * | Mean | SD | Mean | SD | |
| First Year | 15.28 | 6.82 | 48.95 | 9.42 | 33.13 | 6.13 |
| Second Year | 33.86 | 12.96 | 67.03 | 10.00 | 44.96 | 7.72 |
| Third Year | 72.03 | 21.50 | 83.31 | 10.03 | 55.58 | 6.97 |
| Traits | BW1 | BW2 | BW3 | SL1 | SL2 | SL3 | SW1 | SW2 | SW3 |
|---|---|---|---|---|---|---|---|---|---|
| BW1 | 1 | ||||||||
| BW2 | 0.738 ** | 1 | |||||||
| BW3 | 0.290 | 0.156 | 1 | ||||||
| SL1 | 0.961 ** | 0.745 ** | 0.157 | 1 | |||||
| SL2 | 0.695 ** | 0.937 ** | 0.074 | 0.726 ** | 1 | ||||
| SL3 | 0.437 ** | 0.469 ** | 0.260 * | 0.508 ** | 0.434 ** | 1 | |||
| SW1 | 0.949 ** | 0.702 ** | 0.135 | 0.964 ** | 0.680 ** | 0.463 ** | 1 | ||
| SW2 | 0.625 ** | 0.776 ** | 0.010 | 0.672 ** | 0.753 ** | 0.482 ** | 0.631 ** | 1 | |
| SW3 | 0.313 ** | 0.375 ** | 0.195 | 0.371 ** | 0.334 ** | 0.669 ** | 0.337 ** | 0.633 ** | 1 |
| Linkage Group | First Year * | Second Year | Third Year | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Mapped Markers | Length (cM) | Ave. Marker Interval (cM) | No. of Mapped Markers | Length (cM) | Ave. Marker Interval (cM) | No. of Mapped Markers | Length (cM) | Ave. Marker Interval (cM) | |
| LG01 | 206 | 71.768 | 0.35 | 161 | 82.487 | 0.51 | 113 | 68.163 | 0.60 |
| LG02 | 152 | 81.334 | 0.54 | 134 | 95.684 | 0.71 | 92 | 56.473 | 0.61 |
| LG03 | 222 | 80.119 | 0.36 | 237 | 67.995 | 0.29 | 194 | 58.785 | 0.30 |
| LG04 | 212 | 131.385 | 0.62 | 167 | 97.662 | 0.58 | 190 | 101.311 | 0.53 |
| LG05 | 167 | 108.524 | 0.65 | 184 | 111.115 | 0.60 | 136 | 62.984 | 0.46 |
| LG06 | 160 | 68.593 | 0.43 | 176 | 72.623 | 0.41 | 124 | 79.907 | 0.64 |
| LG07 | 148 | 79.892 | 0.54 | 151 | 74.229 | 0.49 | 103 | 68.240 | 0.66 |
| LG08 | 142 | 107.082 | 0.75 | 123 | 104.333 | 0.85 | 97 | 79.879 | 0.82 |
| LG09 | 112 | 95.413 | 0.85 | 76 | 131.223 | 1.73 | 54 | 76.833 | 1.42 |
| LG10 | 233 | 92.076 | 0.40 | 239 | 92.379 | 0.39 | 183 | 87.043 | 0.48 |
| LG11 | 225 | 148.86 | 0.66 | 189 | 80.865 | 0.43 | 153 | 77.477 | 0.51 |
| LG12 | 180 | 104.492 | 0.58 | 98 | 85.009 | 0.87 | 59 | 93.447 | 1.58 |
| LG13 | 297 | 130.62 | 0.44 | 264 | 106.487 | 0.40 | 243 | 130.929 | 0.54 |
| LG14 | 196 | 97.554 | 0.50 | 204 | 87.166 | 0.43 | 91 | 55.392 | 0.61 |
| LG15 | 191 | 123.956 | 0.65 | 210 | 122.722 | 0.58 | 159 | 90.525 | 0.57 |
| LG16 | 176 | 89.218 | 0.51 | 186 | 104.947 | 0.56 | 157 | 115.88 | 0.74 |
| LG17 | 197 | 63.172 | 0.32 | 99 | 61.642 | 0.62 | 73 | 64.643 | 0.89 |
| LG18 | 98 | 72.965 | 0.74 | 73 | 70.342 | 0.96 | 58 | 93.075 | 1.60 |
| Total | 3314 | 1747.023 | 0.55 | 2971 | 1648.910 | 0.63 | 2279 | 1460.986 | 0.75 |
| Age | QTL | Linkage Group | Nearest Marker | Position (cM) | LOD *1 | PVE *2 | He *3 | PIC *4 | No. of Genes *5 |
|---|---|---|---|---|---|---|---|---|---|
| First Year *6 | |||||||||
| 1yBW01 | LG10 | HDSC01365_168769 | 0.000 | 25.962 | 0.499 | 0.375 | 0.305 | 3 | |
| 1yBW02 | LG10 | HDSC01726_79417 | 8.104 | 29.006 | 0.570 | 0.386 | 0.311 | 1 | |
| Second Year | |||||||||
| 2yBW01 | LG01 | HDSC00729_240731 | 47.456 | 3.005 | 0.137 | 0.337 | 0.280 | 8 | |
| 2yBW02 | LG01 | HDSC00308_125778 | 47.598 | 3.201 | 0.145 | 0.500 | 0.375 | 4 | |
| 2yBW03 | LG01 | HDSC02043_384173 | 57.900 | 3.144 | 0.143 | 0.380 | 0.308 | 7 | |
| 2yBW04 | LG02 | HDSC00624_44315 | 63.182 | 3.156 | 0.143 | 0.500 | 0.375 | 4 | |
| 2yBW05 | LG02 | HDSC00677_150547 | 63.714 | 3.806 | 0.170 | 0.385 | 0.311 | 1 | |
| 2yBW06 | LG02 | HDSC00607_271588 | 63.759 | 3.823 | 0.171 | 0.331 | 0.276 | 5 | |
| 2yBW07 | LG02 | HDSC00650_12582 | 64.208 | 3.070 | 0.140 | 0.370 | 0.301 | 1 | |
| 2yBW08 | LG07 | HDSC00922_267573 | 19.045 | 3.272 | 0.148 | 0.404 | 0.322 | 1 | |
| 2yBW09 | LG07 | HDSC00134_290236 | 19.118 | 3.220 | 0.146 | 0.395 | 0.317 | 2 | |
| 2yBW10 | LG07 | HDSC00537_99197 | 38.002 | 3.030 | 0.138 | 0.433 | 0.339 | 3 | |
| Third Year | |||||||||
| 3yBW01 | LG03 | HDSC00618_342024 | 41.025 | 2.211 | 0.103 | 0.348 | 0.288 | 4 | |
| 3yBW02 | LG04 | HDSC00222_3802 | 117.623 | 2.972 | 0.136 | 0.375 | 0.305 | 1 | |
| 3yBW03 | LG06 | HDSC01200_100472 | 64.852 | 2.211 | 0.103 | 0.392 | 0.315 | 6 | |
| 3yBW04 | LG08 | HDSC00069_17588 | 28.499 | 2.194 | 0.102 | 0.340 | 0.282 | 2 | |
| 3yBW05 | LG11 | HDSC01529_541097 | 34.812 | 2.242 | 0.104 | 0.499 | 0.375 | 2 | |
| 3yBW06 | LG11 | HDSC01433_80411 | 35.054 | 2.326 | 0.108 | 0.500 | 0.375 | 2 | |
| 3yBW07 | LG12 | HDSC03732_121812 | 70.449 | 3.415 | 0.154 | 0.287 | 0.246 | 4 | |
| 3yBW08 | LG12 | HDSC02262_492017 | 71.907 | 3.204 | 0.145 | 0.379 | 0.307 | 2 | |
| 3yBW09 | LG12 | HDSC01679_243975 | 74.531 | 3.208 | 0.145 | 0.291 | 0.248 | 5 | |
| 3yBW10 | LG12 | HDSC00532_295313 | 77.767 | 3.283 | 0.149 | 0.297 | 0.253 | 1 | |
| 3yBW11 | LG13 | HDSC00999_240075 | 45.318 | 2.027 | 0.095 | 0.392 | 0.315 | 2 | |
| 3yBW12 | LG13 | HDSC00040_875405 | 66.641 | 2.177 | 0.101 | 0.498 | 0.374 | 5 | |
| 3yBW13 | LG13 | HDSC00123_226127 | 66.679 | 2.255 | 0.105 | 0.258 | 0.225 | 5 | |
| 3yBW14 | LG13 | HDSC00705_579444 | 67.158 | 2.503 | 0.115 | 0.356 | 0.293 | 1 | |
| 3yBW15 | LG13 | HDSC00023_1297306 | 67.53 | 2.096 | 0.098 | 0.364 | 0.298 | 1 | |
| 3yBW16 | LG13 | HDSC00539_43988 | 67.537 | 2.093 | 0.097 | 0.348 | 0.288 | 3 | |
| 3yBW17 | LG13 | HDSC00706_279669 | 75.196 | 2.000 | 0.093 | 0.496 | 0.373 | 7 | |
| Age | QTL | Linkage Group | Nearest Marker | Position (cM) | LOD *1 | PVE *2 | He *3 | PIC *4 | No. of Genes *5 |
|---|---|---|---|---|---|---|---|---|---|
| First Year *6 | |||||||||
| 1ySL01 | LG10 | HDSC01365_168769 | 0.000 | 13.490 | 0.794 | 0.375 | 0.305 | 3 | |
| 1ySL02 | LG10 | HDSC01726_79417 | 8.104 | 16.513 | 0.823 | 0.386 | 0.311 | 1 | |
| Second Year | |||||||||
| 2ySL01 | LG02 | HDSC00677_150547 | 63.714 | 3.470 | 0.156 | 0.385 | 0.311 | 1 | |
| 2ySL02 | LG02 | HDSC00607_271588 | 63.759 | 3.473 | 0.156 | 0.331 | 0.276 | 5 | |
| 2ySL03 | LG06 | HDSC00436_228138 | 50.751 | 3.003 | 0.136 | 0.408 | 0.325 | 2 | |
| 2ySL04 | LG07 | HDSC00922_267573 | 19.045 | 3.436 | 0.155 | 0.404 | 0.322 | 1 | |
| 2ySL05 | LG07 | HDSC00134_290236 | 19.118 | 3.380 | 0.152 | 0.395 | 0.317 | 2 | |
| Third Year | |||||||||
| 3ySL01 | LG04 | HDSC00222_3802 | 117.623 | 2.991 | 0.136 | 0.375 | 0.305 | 1 | |
| 3ySL02 | LG06 | HDSC01300_285668 | 74.819 | 2.575 | 0.119 | 0.405 | 0.323 | 5 | |
| 3ySL03 | LG06 | HDSC00412_566595 | 79.907 | 2.887 | 0.132 | 0.227 | 0.201 | 1 | |
| 3ySL04 | LG08 | HDSC00069_17588 | 28.499 | 2.753 | 0.126 | 0.340 | 0.282 | 2 | |
| 3ySL05 | LG10 | HDSC00130_23920 | 17.46 | 3.188 | 0.145 | 0.379 | 0.307 | 2 | |
| 3ySL06 | LG10 | HDSC02582_220949 | 17.985 | 2.776 | 0.127 | 0.405 | 0.323 | 4 | |
| 3ySL07 | LG11 | HDSC01448_29264 | 34.477 | 2.533 | 0.117 | 0.496 | 0.373 | 2 | |
| 3ySL08 | LG11 | HDSC01529_541097 | 34.812 | 2.865 | 0.131 | 0.499 | 0.375 | 2 | |
| 3ySL09 | LG11 | HDSC01433_80411 | 35.054 | 2.808 | 0.129 | 0.500 | 0.375 | 2 | |
| 3ySL10 | LG12 | HDSC03732_121812 | 70.449 | 3.690 | 0.165 | 0.287 | 0.246 | 4 | |
| 3ySL11 | LG12 | HDSC02262_492017 | 71.907 | 3.731 | 0.167 | 0.379 | 0.307 | 2 | |
| 3ySL12 | LG12 | HDSC01679_243975 | 74.531 | 3.757 | 0.168 | 0.291 | 0.248 | 5 | |
| 3ySL13 | LG12 | HDSC00532_295313 | 77.767 | 3.851 | 0.172 | 0.297 | 0.253 | 1 | |
| Age | QTL | Linkage Group | Nearest Marker | Position (cM) | LOD *1 | PVE *2 | He *3 | PIC *4 | No. of Genes *5 |
|---|---|---|---|---|---|---|---|---|---|
| First Year *6 | |||||||||
| 1ySW01 | LG10 | HDSC01365_168769 | 0.000 | 30.886 | 0.735 | 0.375 | 0.305 | 3 | |
| 1ySW02 | LG10 | HDSC01726_79417 | 8.104 | 33.883 | 0.773 | 0.386 | 0.311 | 1 | |
| Second Year | |||||||||
| 2ySW01 | LG02 | HDSC00677_150547 | 63.714 | 4.741 | 0.207 | 0.385 | 0.311 | 1 | |
| 2ySW02 | LG02 | HDSC00607_271588 | 63.759 | 4.763 | 0.208 | 0.331 | 0.276 | 5 | |
| Third Year | |||||||||
| 3ySW01 | LG02 | HDSC01658_164332 | 26.222 | 2.593 | 0.119 | 0.356 | 0.293 | 1 | |
| 3ySW02 | LG02 | HDSC02578_525042 | 26.367 | 2.561 | 0.118 | 0.379 | 0.307 | 3 | |
| 3ySW03 | LG04 | HDSC00222_3802 | 117.623 | 2.928 | 0.134 | 0.375 | 0.305 | 1 | |
| 3ySW04 | LG06 | HDSC00412_566595 | 79.907 | 2.583 | 0.119 | 0.227 | 0.201 | 1 | |
| 3ySW05 | LG10 | HDSC00130_23920 | 17.460 | 2.906 | 0.133 | 0.379 | 0.307 | 2 | |
| 3ySW06 | LG10 | HDSC02582_220949 | 17.985 | 2.544 | 0.117 | 0.405 | 0.323 | 4 | |
| 3ySW07 | LG11 | HDSC01448_29264 | 34.477 | 2.843 | 0.130 | 0.496 | 0.373 | 2 | |
| 3ySW08 | LG11 | HDSC01529_541097 | 34.812 | 3.160 | 0.143 | 0.499 | 0.375 | 2 | |
| 3ySW09 | LG11 | HDSC01433_80411 | 35.054 | 3.098 | 0.141 | 0.500 | 0.375 | 2 | |
| 3ySW10 | LG12 | HDSC03732_121812 | 70.449 | 3.800 | 0.170 | 0.287 | 0.246 | 4 | |
| 3ySW11 | LG12 | HDSC02262_492017 | 71.907 | 3.817 | 0.171 | 0.379 | 0.307 | 2 | |
| 3ySW12 | LG12 | HDSC01679_243975 | 74.531 | 3.769 | 0.169 | 0.291 | 0.248 | 5 | |
| 3ySW13 | LG12 | HDSC00532_295313 | 77.767 | 3.764 | 0.168 | 0.297 | 0.253 | 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kho, K.H.; Sukhan, Z.P.; Hossen, S.; Cho, Y.; Lee, W.-K.; Nou, I.-S. Age-Dependent Growth-Related QTL Variations in Pacific Abalone, Haliotis discus hannai. Int. J. Mol. Sci. 2023, 24, 13388. https://doi.org/10.3390/ijms241713388
Kho KH, Sukhan ZP, Hossen S, Cho Y, Lee W-K, Nou I-S. Age-Dependent Growth-Related QTL Variations in Pacific Abalone, Haliotis discus hannai. International Journal of Molecular Sciences. 2023; 24(17):13388. https://doi.org/10.3390/ijms241713388
Chicago/Turabian StyleKho, Kang Hee, Zahid Parvez Sukhan, Shaharior Hossen, Yusin Cho, Won-Kyo Lee, and Ill-Sup Nou. 2023. "Age-Dependent Growth-Related QTL Variations in Pacific Abalone, Haliotis discus hannai" International Journal of Molecular Sciences 24, no. 17: 13388. https://doi.org/10.3390/ijms241713388
APA StyleKho, K. H., Sukhan, Z. P., Hossen, S., Cho, Y., Lee, W.-K., & Nou, I.-S. (2023). Age-Dependent Growth-Related QTL Variations in Pacific Abalone, Haliotis discus hannai. International Journal of Molecular Sciences, 24(17), 13388. https://doi.org/10.3390/ijms241713388







