The Role of Biomarkers, Metabolomics, and COVID-19 in Venous Thromboembolism—A Review of Literature
Abstract
1. Introduction
2. COVID and Venous Thromboembolism
3. Biomarkers in Venous Thromboembolism
3.1. D-Dimer
3.2. Thrombin
3.3. P-Selectin
3.4. Inflammatory Cytokines
3.5. MPs (Microparticles)
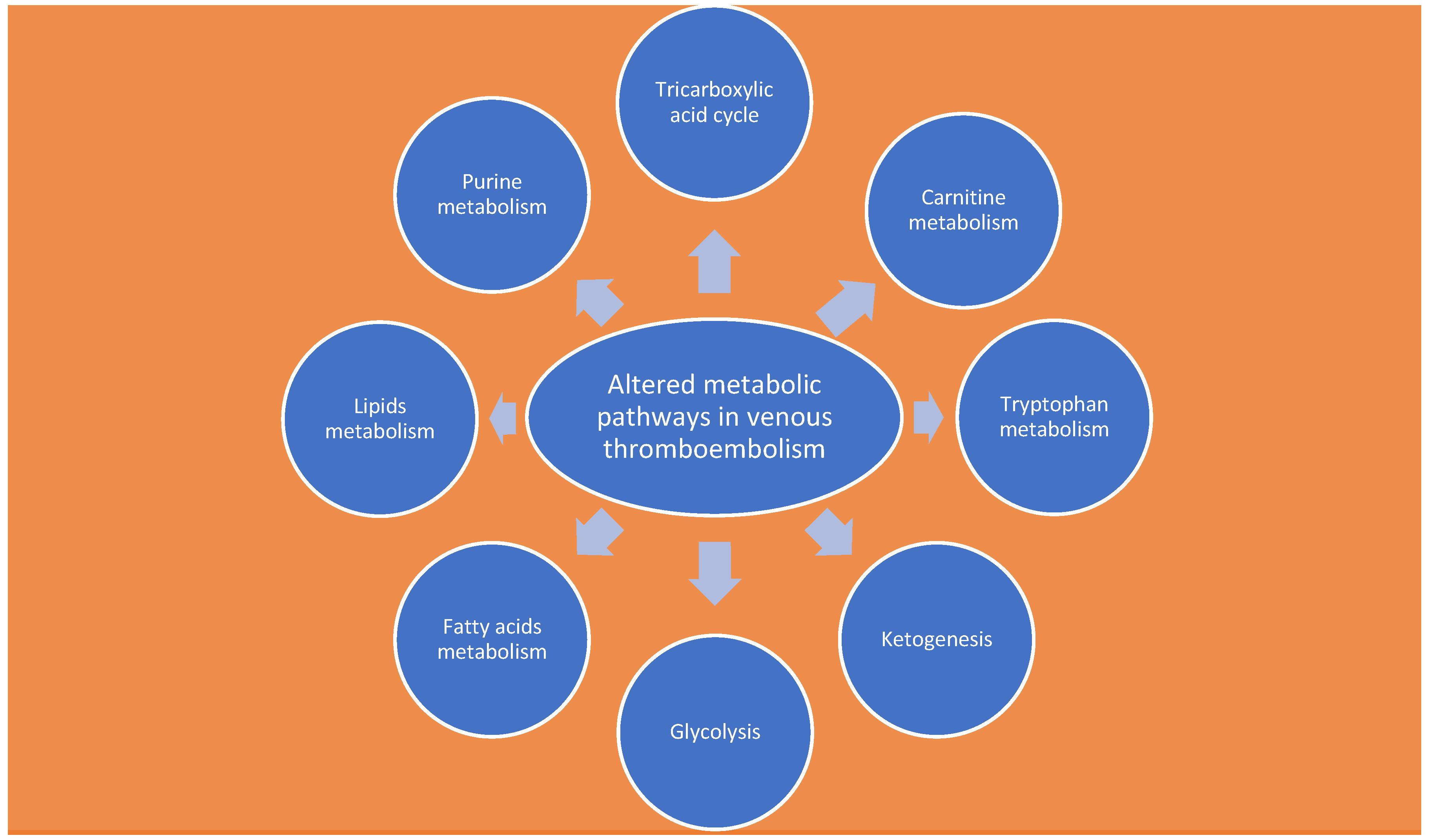
4. Metabolomics
Analysis of Metabolites Involved
5. Treatment of Venous Thromboembolism
- -
- Proximity deep vein thrombosis (DVT) of the lower extremity;
- -
- Symptomatic distal DVT (calf vein);
- -
- Symptomatic upper extremity DVT (axillary-subclavian veins);
- -
- Pulmonary embolism (PE);
- -
- Subsegmental EP in a patient at risk of recurrence;
- -
- Surveillance for subsegmental EP in a patient without proximal DVT and a reduced risk of recurrence.
- -
- Low molecular weight heparin;
- -
- Fondaparinux;
- -
- Unfractionated heparin;
- -
- Oral anticoagulants directed against factor Xa or thrombin inhibitors.
- The initial phase (about 5–21 days after diagnosis): patients, depending on clinical features, receive either treatment initially parenterally and then switch to treatment with vitamin K antagonists (VKAs), or begin treatment with high-dose therapy [11]
- Long-term treatment: therapy with VKA or DOAC 3–6 months after diagnosis [11].
- -
- Prolonged treatment (after the initial 3–6 months): The decision to prolong treatment (beyond the first 3–6 months) is related to the benefit/risk ratio of continuing anticoagulant therapy and must be tailored on every single patient [97].
- -
- Thrombosis, usually employed in patients with acute thromboembolism and hemodynamic instability;
- -
- Vena cava filter in patients for whom anticoagulant therapy is absolutely contraindicated [97];
- -
- Elastic compression and rapid mobilization have also been found to be effective in the treatment of DVT—however, careful caution is needed in patients with severe peripheral arterial disease [97].
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, X.; Zeleznik, O.A.; Lindström, S.; Lasky-Su, J.; Hagan, K.; Clish, C.B.; Eliassen, A.H.; Kraft, P.; Kabrhel, C. Metabolites Associated with the Risk of Incident Venous Thromboembolism: A Metabolomic Analysis. J. Am. Hear. Assoc. 2018, 7, e010317. [Google Scholar] [CrossRef] [PubMed]
- Spencer, F.A.; Emery, C.; Joffe, S.W.; Pacifico, L.; Lessard, D.; Reed, G.; Gore, J.M.; Goldberg, R.J. Incidence rates, clinical profile, and outcomes of patients with venous thromboembolism. The Worcester VTE study. J. Thromb. Thrombolysis 2009, 28, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Wendelboe, A.M.; Raskob, G.E. Global Burden of Thrombosis. Circ. Res. 2016, 118, 1340–1347. [Google Scholar] [CrossRef]
- Khan, F.; Rahman, A.; Carrier, M.; Kearon, C.; Weitz, J.I.; Schulman, S.; Couturaud, F.; Eichinger, S.; Kyrle, P.A.; Becattini, C.; et al. Long term risk of symptomatic recurrent venous thromboembolism after discontinuation of anticoagulant treatment for first unprovoked venous thromboembolism event: Systematic review and meta-analysis. BMJ 2019, 366, l4363. [Google Scholar] [CrossRef] [PubMed]
- Braithwaite, I.; Healy, B.; Cameron, L.; Weatherall, M.; Beasley, R. Venous thromboembolism risk associated with protracted work- and computer-related seated immobility: A case-control study. JRSM Open 2016, 7, 2054270416632670. [Google Scholar] [CrossRef]
- Baglin, T. Inherited and Acquired Risk Factors for Venous Thromboembolism. Semin. Respir. Crit. Care Med. 2012, 33, 127–137. [Google Scholar] [CrossRef]
- Agnelli, G.; Prandoni, P.; Becattini, C.; Silingardi, M.; Taliani, M.R.; Miccio, M.; Imberti, D.; Poggio, R.; Ageno, W.; Pogliani, E.; et al. Extended Oral Anticoagulant Therapy after a First Episode of Pulmonary Embolism. Ann. Intern. Med. 2003, 139, 19–25. [Google Scholar] [CrossRef]
- Murin, S.; Romano, P.S.; White, R.H. Comparison of outcomes after hospitalization for deep venous thrombosis or pulmo-nary embolism. Thromb. Haemost. 2002, 88, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Kushner, A.; West, W.P.; Pillarisetty, L.S. Virchow Triad. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK539697/ (accessed on 30 November 2022).
- Lurie, J.M.; Png, C.M.; Subramaniam, S.; Chen, S.; Chapman, E.; Aboubakr, A.; Marin, M.; Faries, P.; Ting, W. Virchow’s triad in “silent” deep vein thrombosis. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Mazzolai, L.; Aboyans, V.; Ageno, W.; Agnelli, G.; Alatri, A.; Bauersachs, R.; A Brekelmans, M.P.; Büller, H.R.; Elias, A.; Farge, D.; et al. Diagnosis and management of acute deep vein thrombosis: A joint consensus document from the European Society of Cardiology working groups of aorta and peripheral vascular diseases and pulmonary circulation and right ventricular function. Eur. Hear. J. 2017, 39, 4208–4218. [Google Scholar] [CrossRef]
- Kahn, S.R. The post-thrombotic syndrome. Hematology 2016, 2016, 413–418. [Google Scholar] [CrossRef]
- Beckman, M.G.; Hooper, W.C.; Critchley, S.E.; Ortel, T.L. Venous Thromboembolism: A Public Health Concern. Am. J. Prev. Med. 2010, 38 (Suppl. S4), S495–S501. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb. Res. 2020, 191, 148–150. [Google Scholar] [CrossRef]
- Wichmann, D.; Sperhake, J.P.; Lütgehetmann, M.; Steurer, S.; Edler, C.; Heinemann, A.; Heinrich, F.; Mushumba, H.; Kniep, I.; Schröder, A.S.; et al. Autopsy Findings and Venous Thromboembolism in Patients with COVID-19. Ann. Intern. Med. 2020, 173, 268–277. [Google Scholar] [CrossRef]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef]
- Li, M.-Y.; Li, L.; Zhang, Y.; Wang, X.-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; van der Poll, T. Inflammation and coagulation. Crit. Care Med. 2010, 38, S26–S34. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A.M.; Ali, M.A.; Spinler, S.A. COVID-19 and thrombosis: From bench to bedside. Trends Cardiovasc. Med. 2021, 31, 143–160. [Google Scholar] [CrossRef]
- Pulivarthi, S.; Gurram, M.e.K. Effectiveness of D-dimer as a screening test for venous thromboembolism: An update. North Am. J. Med. Sci. 2014, 6, 491–499. [Google Scholar] [CrossRef]
- Righini, M.; Perrier, A.; De Moerloose, P.; Bounameaux, H. D-Dimer for venous thromboembolism diagnosis: 20 years later. J. Thromb. Haemost. 2008, 6, 1059–1071. [Google Scholar] [CrossRef]
- Di Nisio, M.; Squizzato, A.; Rutjes, A.W.S.; Büller, H.R.; Zwinderman, A.H.; Bossuyt, P.M.M. Diagnostic accuracy of D-dimer test for exclusion of venous thromboembolism: A systematic review. J. Thromb. Haemost. 2007, 5, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Verhovsek, M.; Douketis, J.D.; Yi, Q.; Shrivastava, S.; Tait, R.C.; Baglin, T.; Poli, D.; Lim, W. Systematic Review: D-Dimer to Predict Recurrent Disease after Stopping Anticoagulant Therapy for Unprovoked Venous Thromboembolism. Ann. Intern. Med. 2008, 149, 481–490. [Google Scholar] [CrossRef]
- Legnani, C.; Cosmi, B.; Guazzaloca, G.; Pancani, C.; Coccheri, S.; Palareti, G. Risk of Venous Thromboembolism Recurrence: High Negative Predictive Value of D-dimer Performed after Oral Anticoagulation Is Stopped. Thromb. Haemost. 2002, 87, 7–12. [Google Scholar] [CrossRef]
- Eichinger, S.; Hron, G.; Kollars, M.; A Kyrle, P. Prediction of Recurrent Venous Thromboembolism by Endogenous Thrombin Potential and D-Dimer. Clin. Chem. 2008, 54, 2042–2048. [Google Scholar] [CrossRef] [PubMed]
- van Veen, J.J.; Gatt, A.; Makris, M. Thrombin generation testing in routine clinical practice: Are we there yet? Br. J. Haematol. 2008, 142, 889–903. [Google Scholar] [CrossRef] [PubMed]
- Brummel-Ziedins, K.E.; Vossen, C.Y.; Butenas, S.; Mann, K.G.; Rosendaal, F.R. Thrombin generation profiles in deep venous thrombosis. J. Thromb. Haemost. 2005, 3, 2497–2505. [Google Scholar] [CrossRef]
- Lutsey, P.L.; Zakai, N.A. Epidemiology and prevention of venous thromboembolism. Nat. Rev. Cardiol. 2023, 20, 248–262. [Google Scholar] [CrossRef]
- Haas, F.J.L.M.; Schutgens, R.E.G.; Kluft, C.; Biesma, D.H. A thrombin generation assay may reduce the need for compression ultrasonography for the exclusion of deep venous thrombosis in the elderly. Scand. J. Clin. Lab. Investig. 2011, 71, 12–18. [Google Scholar] [CrossRef]
- McEver, R.P. Adhesive interactions of leukocytes, platelets, and the vessel wall during hemostasis and inflammation. Thromb. Haemost. 2001, 86. [Google Scholar] [CrossRef]
- Ley, K. The role of selectins in inflammation and disease. Trends Mol. Med. 2003, 9, 263–268. [Google Scholar] [CrossRef]
- Geng, J.-G.; Bevilacquat, M.P.; Moore, K.L.; Mclntyre, T.M.; Prescott, S.M.; Kim, J.M.; Bliss, G.A.; Zimmerman, G.A.; McEver, R.P. Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature 1990, 343, 757–760. [Google Scholar] [CrossRef]
- Moore, K.L.; Stults, N.L.; Diaz, S.; Smith, D.F.; Cummings, R.D.; Varki, A.; McEver, R.P. Identification of a specific glycoprotein ligand for P-selectin (CD62) on myeloid cells. J. Cell Biol. 1992, 118, 445–456. [Google Scholar] [CrossRef]
- Furie, B.; Furie, B.C. Role of platelet P-selectin and microparticle PSGL-1 in thrombus formation. Trends Mol. Med. 2004, 10, 171–178. [Google Scholar] [CrossRef]
- Théorêt, J.-F.; Yacoub, D.; Hachem, A.; Gillis, M.-A.; Merhi, Y. P-selectin ligation induces platelet activation and enhances microaggregate and thrombus formation. Thromb. Res. 2011, 128, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Palabrica, T.; Lobb, R.; Furie, B.C.; Aronovitz, M.; Benjamin, C.; Hsu, Y.-M.; Sajer, S.A.; Furie, B. Leukocyte accumulation promoting fibrin deposition is mediated in vivo by P-selectin on adherent platelets. Nature 1992, 359, 848–851. [Google Scholar] [CrossRef]
- Miszti-Blasius, K.; Debreceni, I.B.; Felszeghy, S.; Dezső, B.; Kappelmayer, J. Lack of P-selectin glycoprotein ligand-1 protects mice from thrombosis after collagen/epinephrine challenge. Thromb. Res. 2011, 127, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Vandy, F.C.; Stabler, C.; Eliassen, A.M.; Hawley, A.E.; Guire, K.E.; Myers, D.D.; Henke, P.K.; Wakefield, T.W. Soluble P-selectin for the diagnosis of lower extremity deep venous thrombosis. J. Vasc. Surg. Venous Lymphat. Disord. 2013, 1, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Hrachovinová, I.; Cambien, B.; Hafezi-Moghadam, A.; Kappelmayer, J.; Camphausen, R.T.; Widom, A.; Xia, L.; Kazazian, H.H., Jr.; Schaub, R.G.; McEver, R.P.; et al. Interaction of P-selectin and PSGL-1 generates microparticles that correct hemostasis in a mouse model of hemophilia A. Nat. Med. 2003, 9, 1020–1025. [Google Scholar] [CrossRef]
- Rauch, U.; Bonderman, D.; Bohrmann, B.; Badimon, J.J.; Himber, J.; A Riederer, M.; Nemerson, Y. Transfer of tissue factor from leukocytes to platelets is mediated by CD15 and tissue factor. Blood 2000, 96, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Osnes, L.; Westvik, A.B.; Joø, G.-B.; Okkenhaug, C.; Kierulf, P. Inhibition of IL-1 induced tissue factor (TF) synthesis and procoagulant activity (PCA) in purified human monocytes by IL-4, IL-10 and IL-13. Cytokine 1996, 8, 822–827. [Google Scholar] [CrossRef]
- Lutsey, P.L.; Astor, B.C.; Cushman, M.; Folsom, A.R. C-reactive protein and venous thromboembolism. Thromb. Haemost. 2009, 102, 615–619. [Google Scholar] [CrossRef]
- Zacho, J.; Tybjærg-Hansen, A.; Nordestgaard, B.G. C-Reactive Protein and Risk of Venous Thromboembolism in the General Population. Arter. Thromb. Vasc. Biol. 2010, 30, 1672–1678. [Google Scholar] [CrossRef]
- Viel, K.R.; Machiah, D.K.; Warren, D.M.; Khachidze, M.; Buil, A.; Fernstrom, K.; Souto, J.C.; Peralta, J.M.; Smith, T.; Blangero, J.; et al. A sequence variation scan of the coagulation factor VIII (FVIII) structural gene and associations with plasma FVIII activity levels. Blood 2007, 109, 3713–3724. [Google Scholar] [CrossRef]
- Beckers, M.; Ruven, H.; Haas, F.; Doevendans, P.; Cate, H.T.; Prins, M.; Biesma, D. Single nucleotide polymorphisms in inflammation-related genes are associated with venous thromboembolism. Eur. J. Intern. Med. 2010, 21, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Vormittag, R.; Hsieh, K.; Kaider, A.; Minar, E.; Bialonczyk, C.; Hirschl, M.; Mannhalter, C.; Pabinger, I. Interleukin-6 and interleukin-6 promoter polymorphism (−174) G>C in patients with spontaneous venous thromboembolism. Thromb. Haemost. 2006, 95, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, S.C.; Næss, I.A.; Cannegieter, S.C.; Hammerstrøm, J.; Rosendaal, F.R.; Reitsma, P.H. Inflammatory Cytokines as Risk Factors for a First Venous Thrombosis: A Prospective Population-Based Study. PLoS Med. 2006, 3, e334. [Google Scholar] [CrossRef]
- Downing, L.J.; Strieter, R.M.; Kadell, A.M.; Wilke, C.A.; Austin, J.C.; Hare, B.D.; Burdick, M.D.; Greenfield, L.J.; Wakefield, T.W. IL-10 regulates thrombus-induced vein wall inflammation and thrombosis. J. Immunol. 1998, 161, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- Myers, D.D.; Hawley, A.E.; Longo, C.; Henke, P.K.; Guire, K.E.; Schmaier, A.H.; Wakefield, T.W.; Rectenwald, J.E. D-dimer, P-selectin, and microparticles: Novel markers to predict deep venous thrombosis. Thromb. Haemost. 2005, 94, 1312–1317. [Google Scholar] [CrossRef]
- Key, N.S.; Chantrathammachart, P.; Moody, P.W.; Chang, J.-Y. Membrane microparticles in VTE and cancer. Thromb. Res. 2010, 125 (Suppl. S2), S80–S83. [Google Scholar] [CrossRef]
- Campello, E.; Spiezia, L.; Radu, C.M.; Bulato, C.; Castelli, M.; Gavasso, S.; Simioni, P. Endothelial, platelet, and tissue factor-bearing microparticles in cancer patients with and without venous thromboembolism. Thromb. Res. 2011, 127, 473–477. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Heresi, G.A.; Velasquez, H.; Jy, W.; Jimenez, J.J.; Ahn, E.; Horstman, L.L.; Soriano, A.O.; Zambrano, J.P.; Ahn, Y.S. Elevation of Endothelial Microparticles, Platelets, and Leukocyte Activation in Patients with Venous Thromboembolism. J. Am. Coll. Cardiol. 2005, 45, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Fraser, K.; Roy, N.C.; Goumidi, L.; Verdu, A.; Suchon, P.; Leal-Valentim, F.; Trégouët, D.-A.; Morange, P.-E.; Martin, J.-C. Plasma Biomarkers and Identification of Resilient Metabolic Disruptions in Patients with Venous Thromboembolism Using a Metabolic Systems Approach. Arter. Thromb. Vasc. Biol. 2020, 40, 2527–2538. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.; Spagou, K.; Kafeza, M.; Kyriakides, M.; Dharmarajah, B.; Shalhoub, J.; Diaz, J.A.; Wakefield, T.W.; Holmes, E.; Davies, A.H. Deep Vein Thrombosis Exhibits Characteristic Serum and Vein Wall Metabolic Phenotypes in the Inferior Vena Cava Ligation Mouse Model. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 703–713. [Google Scholar] [CrossRef]
- Obi, A.T.; Stringer, K.A.; Diaz, J.A.; Finkel, M.A.; Farris, D.M.; Yeomans, L.; Wakefield, T.; Myers, D.D. 1D-1H-nuclear magnetic resonance metabolomics reveals age-related changes in metabolites associated with experimental venous thrombosis. J. Vasc. Surg. Venous Lymphat. Disord. 2016, 4, 221–230. [Google Scholar] [CrossRef]
- Franczyk, B.; Gluba-Brzózka, A.; Ławiński, J.; Rysz-Górzyńska, M.; Rysz, J. Metabolomic Profile in Venous Thromboembolism (VTE). Metabolites 2021, 11, 495. [Google Scholar] [CrossRef] [PubMed]
- Kirlikaya, B.; Langridge, B.; Davies, A.; Onida, S. Metabolomics as a tool to improve decision making for the vascular surgeon–wishful thinking or a dream come true? Vasc. Pharmacol. 2019, 116, 1–3. [Google Scholar] [CrossRef]
- Maekawa, K.; Sugita, C.; Yamashita, A.; Moriguchi-Goto, S.; Furukoji, E.; Sakae, T.; Gi, T.; Hirai, T.; Asada, Y. Higher lactate and purine metabolite levels in erythrocyte-rich fresh venous thrombus: Potential markers for early deep vein thrombosis. Thromb. Res. 2019, 177, 136–144. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Xie, Z.; Ming, X.; Li, Z.; Kong, Y. A tryptophan derivative TD-26 attenuates thrombus formation by inhibiting both PI3K/Akt signaling and binding of fibrinogen to integrin αIIbβ3. Biochem. Biophys. Res. Commun. 2015, 465, 516–522. [Google Scholar] [CrossRef]
- Voils, S.A.; Shahin, M.H.; Garrett, T.J.; Frye, R.F. Metabolomic association between venous thromboembolism in critically ill trauma patients and kynurenine pathway of tryptophan metabolism. Thromb. Res. 2018, 165, 6–13. [Google Scholar] [CrossRef]
- Bulato, C.; Radu, C.M.; Campello, E.; Gavasso, S.; Spiezia, L.; Tormene, D.; Simioni, P.; Ehrenforth, S.; Prondsinski, M.v.D.; Aygören-Pürsün, E.; et al. New Prothrombin Mutation (Arg596Trp, Prothrombin Padua 2) Associated with Venous Thromboembolism. Arter. Thromb. Vasc. Biol. 2016, 36, 1022–1029. [Google Scholar] [CrossRef]
- Bujak, R.; García-Álvarez, A.; Rupérez, F.J.; Nuño-Ayala, M.; García, A.; Ruiz-Cabello, J.; Fuster, V.; Ibáñez, B.; Barbas, C. Metabolomics Reveals Metabolite Changes in Acute Pulmonary Embolism. J. Proteome Res. 2014, 13, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, H.; Banerjee, Y.; Trauger, S.; Siuzdak, G.; Kalisiak, E.; Fernandez, J.A.; Hoang, L.; Tran, M.; Yegneswaran, S.; Elias, D.J.; et al. Acylcarnitines are anticoagulants that inhibit factor Xa and are reduced in venous thrombosis, based on metabolomics data. Blood 2015, 126, 1595–1600. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Huang, Y.C.; Shultz, T.D.; Mitchell, M. Urinary, plasma, and erythrocyte carnitine concentrations during transition to a lactoovovegetarian diet with vitamin B-6 depletion and repletion in young adult women. Am. J. Clin. Nutr. 1998, 67, 221–230. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Belghasem, M.; Roth, D.; Richards, S.; Napolene, M.A.; Walker, J.; Yin, W.; Arinze, N.; Lyle, C.; Spencer, C.; Francis, J.M.; et al. Metabolites in a mouse cancer model enhance venous thrombogenicity through the aryl hydrocarbon receptor–tissue factor axis. Blood 2019, 134, 2399–2413. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Liu, W.; Le, A.; Hancock, C.; Lane, A.N.; Dang, C.V.; Fan, T.W.-M.; Phang, J.M. Reprogramming of proline and glutamine metabolism contributes to the proliferative and metabolic responses regulated by oncogenic transcription factor c-MYC. Proc. Natl. Acad. Sci. USA 2012, 109, 8983–8988. [Google Scholar] [CrossRef]
- Romano, K.A.; Vivas, E.I.; Amador-Noguez, D.; Rey, F.E. Intestinal Microbiota Composition Modulates Choline Bioavailability from Diet and Accumulation of the Proatherogenic Metabolite Trimethylamine-N-Oxide. mBio 2015, 6, e02481. [Google Scholar] [CrossRef]
- Skye, S.M.; Zhu, W.; Romano, K.A.; Guo, C.-J.; Wang, Z.; Jia, X.; Kirsop, J.; Haag, B.; Lang, J.M.; DiDonato, J.A.; et al. Microbial Transplantation with Human Gut Commensals Containing CutC Is Sufficient to Transmit Enhanced Platelet Reactivity and Thrombosis Potential. Circ. Res. 2018, 123, 1164–1176. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef]
- Königsbrügge, O.; Posch, F.; Riedl, J.; Reitter, E.-M.; Zielinski, C.; Pabinger, I.; Ay, C. Association Between Decreased Serum Albumin with Risk of Venous Thromboembolism and Mortality in Cancer Patients. Oncol. 2016, 21, 252–257. [Google Scholar] [CrossRef]
- Gyamlani, G.; Molnar, M.Z.; Lu, J.L.; Sumida, K.; Kalantar-Zadeh, K.; Kovesdy, C.P. Association of serum albumin level and venous thromboembolic events in a large cohort of patients with nephrotic syndrome. Nephrol. Dial. Transplant. 2017, 32, 157–164. [Google Scholar] [CrossRef]
- Liu, Z.; Mi, J. Serum Albumin and Circulating Metabolites and Risk of Venous Thromboembolism: A Two-Sample Mendelian Randomization Study. Front. Nutr. 2021, 8. [Google Scholar] [CrossRef]
- Morelli, V.M.; Lijfering, W.M.; Bos, M.H.A.; Rosendaal, F.R.; Cannegieter, S.C. Lipid levels and risk of venous thrombosis: Results from the MEGA-study. Eur. J. Epidemiol. 2017, 32, 669–681. [Google Scholar] [CrossRef]
- Voight, B.F.; Peloso, G.M.; Orho-Melander, M.; Frikke-Schmidt, R.; Barbalic, M.; Jensen, M.K.; Hindy, G.; Hólm, H.; Ding, E.L.; Johnson, T.; et al. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet 2012, 380, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Bergner, R.T.B.; Onida, S.B.; Velineni, R.P.; Spagou, K.B.; Gohel, M.S.M.; Bouschbacher, M.; Bohbot, S.; Shalhoub, J.B.; Holmes, E.B.; Davies, A.H.M. Metabolic Profiling Reveals Changes in Serum Predictive of Venous Ulcer Healing. Ann. Surg. 2023, 277, e467–e474. [Google Scholar] [CrossRef]
- Summers, S.A. Ceramides in insulin resistance and lipotoxicity. Prog. Lipid Res. 2006, 45, 42–72. [Google Scholar] [CrossRef]
- Hyde, R.; Hajduch, E.; Powell, D.J.; Taylor, P.M.; Hundal, H.S. Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J. 2005, 19, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Stratford, S.; DeWald, D.B.; Summers, S.A. Ceramide dissociates 3′-phosphoinositide production from pleckstrin homology domain translocation. Biochem. J. 2001, 354, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Cui, W.; Qiu, W.; Zhu, M.; Zhao, R.; Zeng, D.; Dong, C.; Wang, X.; Guo, W.; Xing, W.; et al. Impaired wound healing results from the dysfunction of the Akt/mTOR pathway in diabetic rats. J. Dermatol. Sci. 2015, 79, 241–251. [Google Scholar] [CrossRef]
- Ochsner, A.; DeBakey, M. Therapy of phlebothrombosis and thrombophlebitis. Arch. Surg. 1940, 40, 208–231. [Google Scholar] [CrossRef]
- Artz, C.P.; Pulaski, E.J. The Treatment of Venous Thrombosis with Heparin. Angiology 1953, 4, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, V.V.; Djazaeri, B.; Fok, J.; Fletcher, M.; Scully, M.F.; Westwick, J. Low-molecular-weight heparin and prevention of postoperative deep vein thrombosis. Br. Med. J. (Clin. Res. Ed.) 1982, 284, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.B.; Geske, J.B.; Maguire, J.M.; Zane, N.A.; Carter, R.E.; Morgenthaler, T.I. Early Anticoagulation Is Associated with Reduced Mortality for Acute Pulmonary Embolism. Chest 2010, 137, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Exter, P.L.D.; van Es, J.; Erkens, P.M.G.; van Roosmalen, M.J.G.; Hoven, P.v.D.; Hovens, M.M.C.; Kamphuisen, P.W.; Klok, F.A.; Huisman, M.V. Impact of Delay in Clinical Presentation on the Diagnostic Management and Prognosis of Patients with Suspected Pulmonary Embolism. Am. J. Respir. Crit. Care Med. 2013, 187, 1369–1373. [Google Scholar] [CrossRef]
- Kearon, C.; Akl, E.A.; Comerota, A.J.; Prandoni, P.; Bounameaux, H.; Goldhaber, S.Z.; Nelson, M.E.; Wells, P.S.; Gould, M.K.; Dentali, F.; et al. Antithrombotic Therapy for VTE Disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141 (Suppl. S2), e419S–e496S. [Google Scholar] [CrossRef]
- Stevens, S.M.; Woller, S.C.; Kreuziger, L.B.; Bounameaux, H.; Doerschug, K.; Geersing, G.-J.; Huisman, M.V.; Kearon, C.; King, C.S.; Knighton, A.J.; et al. Antithrombotic Therapy for VTE Disease. Chest 2021, 160, e545–e608. [Google Scholar] [CrossRef]
- Holbrook, A.; Schulman, S.; Witt, D.M.; Vandvik, P.O.; Fish, J.; Kovacs, M.J.; Svensson, P.J.; Veenstra, D.L.; Crowther, M.; Guyatt, G.H. Evidence-Based Management of Anticoagulant Therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141 (Suppl. S2), e152S–e184S. [Google Scholar] [CrossRef]
- Hao, C.; Sun, M.; Wang, H.; Zhang, L.; Wang, W. Low molecular weight heparins and their clinical applications. Prog. Mol. Biol. Transl. Sci. 2019, 163, 21–39. [Google Scholar] [CrossRef]
- Granger, C.B.; Lopes, R.D.; Hanna, M.; Ansell, J.; Hylek, E.M.; Alexander, J.H.; Thomas, L.; Wang, J.; Bahit, M.C.; Verheugt, F.; et al. Clinical events after transitioning from apixaban versus warfarin to warfarin at the end of the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Am. Hear. J. 2015, 169, 25–30. [Google Scholar] [CrossRef]
- Hori, M.; Matsumoto, M.; Tanahashi, N.; Momomura, S.-I.; Uchiyama, S.; Goto, S.; Izumi, T.; Koretsune, Y.; Kajikawa, M.; Kato, M.; et al. Rivaroxaban vs. Warfarin in Japanese Patients with Atrial Fibrillation. Circ. J. 2012, 76, 2104–2111. [Google Scholar] [CrossRef] [PubMed]
- Reilly, P.A.; Lehr, T.; Haertter, S.; Connolly, S.J.; Yusuf, S.; Eikelboom, J.W.; Ezekowitz, M.D.; Nehmiz, G.; Wang, S.; Wallentin, L. The Effect of Dabigatran Plasma Concentrations and Patient Characteristics on the Frequency of Ischemic Stroke and Major Bleeding in Atrial Fibrillation Patients. J. Am. Coll. Cardiol. 2014, 63, 321–328. [Google Scholar] [CrossRef]
- Kato, E.T.; Giugliano, R.P.; Ruff, C.T.; Koretsune, Y.; Yamashita, T.; Kiss, R.G.; Nordio, F.; Murphy, S.A.; Kimura, T.; Jin, J.; et al. Efficacy and Safety of Edoxaban in Elderly Patients with Atrial Fibrillation in the ENGAGE AF–TIMI 48 Trial. J. Am. Hear. Assoc. 2016, 5, e003432. [Google Scholar] [CrossRef] [PubMed]
- Coons, J.C.; Albert, L.; Bejjani, A.; Iasella, C.J. Effectiveness and Safety of Direct Oral Anticoagulants versus Warfarin in Obese Patients with Acute Venous Thromboembolism. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2020, 40, 204–210. [Google Scholar] [CrossRef]
- Raskob, G.E.; van Es, N.; Verhamme, P.; Carrier, M.; Di Nisio, M.; Garcia, D.; Grosso, M.A.; Kakkar, A.K.; Kovacs, M.J.; Mercuri, M.F.; et al. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N. Engl. J. Med. 2018, 378, 615–624. [Google Scholar] [CrossRef]
- Agnelli, G.; Becattini, C.; Meyer, G.; Muñoz, A.; Huisman, M.V.; Connors, J.M.; Cohen, A.; Bauersachs, R.; Brenner, B.; Torbicki, A.; et al. Apixaban for the Treatment of Venous Thromboembolism Associated with Cancer. N. Engl. J. Med. 2020, 382, 1599–1607. [Google Scholar] [CrossRef]
- Onida, S.; Tan, M.K.H.; Kafeza, M.; Bergner, R.T.; Shalhoub, J.; Holmes, E.; Davies, A.H. Metabolic Phenotyping in Venous Disease: The Need for Standardization. J. Proteome Res. 2019, 18, 3809–3820. [Google Scholar] [CrossRef] [PubMed]

| Metabolite | Disease | Directionality and Context of the Changes |
|---|---|---|
| Hypoxanthine | DVT | Raised in jugular vein thrombus, compared to venous blood levels (in rabbit) |
| Serotonin | DVT | Raised in jugular vein thrombus, compared to venous blood levels (in rabbit) |
| Guanine | DVT | Raised in jugular vein thrombus, compared to venous blood levels (in rabbit) |
| Taurine | DVT | Raised in jugular vein thrombus, compared to venous blood levels (in rabbit) |
| AMP | DVT | Raised in jugular vein thrombus, compared to venous blood levels (in rabbit) |
| 3-Hydroxykynurenine | DVT | Raised in jugular vein thrombus, compared to venous blood levels (in rabbit) |
| Lactic acid | DVT | Raised in jugular vein thrombus, compared to venous blood levels (in rabbit) |
| Citric acid | DVT | Reduced in jugular vein thrombus, compared to venous blood levels (in rabbit) |
| Glucose 6-phosphate | DVT | Reduced in jugular vein thrombus, compared to venous blood levels (in rabbit) |
| NADP | DVT | Reduced in jugular vein thrombus, compared to venous blood levels (in rabbit) |
| Tryptophan | DVT | Reduced in jugular vein thrombus, compared to venous blood levels (in rabbit) |
| Methionine sulfoxide | DVT | Reduced in jugular vein thrombus, compared to venous blood levels (in rabbit) |
| Cysteine glutathione disulfide | DVT | Reduced in jugular vein thrombus, compared to venous blood levels (in rabbit) |
| Glutamine | DVT | Raised in whole blood samples of old mice with venous thrombosis (VT) of inferior vena cava, compared with young mice with VT and age-matched controls without VT |
| Phenylalanine | DVT | Raised in whole blood samples of old mice with VT of inferior vena cava, compared with young mice with VT and age-matched controls without VT |
| Proline | DVT | Raised in whole blood samples of old mice with VT of inferior vena cava, compared with young mice with VT and age-matched controls without VT |
| Glycerol | PE | Raised in venous blood samples of pigs with PE compared to pigs without PE. |
| Pyruvic acid | PE | Raised in venous blood samples of pigs with PE compared to pigs without PE. |
| Lactic acid | PE | Raised in venous blood samples of pigs with PE compared to pigs without PE. |
| Palmitic acid | PE | Raised in venous blood samples of pigs with PE compared to pigs without PE. |
| Oleic acid | PE | Raised in venous blood samples of pigs with PE compared to pigs without PE. |
| 3-hydroxybutyric acid | PE | Raised in venous blood samples of pigs with PE compared to pigs without PE. |
| 10:1 Acylcarnitines | PE | Reduced in venous blood samples of patients who had PE 3 months before compared with patients without history of PE. |
| 16:1 Acylcarnitines | PE | Reduced in venous blood samples of patients who had PE 3 months before compared with patients without history of PE. |
| Initial Phase VTE | Dosing | 5–21 Days after Diagnosis |
|---|---|---|
| APIXABAN | 10 mg bid | for the first 7 days |
| RIVAROXABAN | 15 mg bid | for the first 21 days |
| EDOXABAN | 60 mg/day or 30 mg/day with regard to actual functionality by low-molecular-weight heparin | for 5–10 days |
| DABIGATRAN | 150 mg bid preceded by low-molecular-weight heparin | for the first 5–10 days |
| Long-Term Treatment | Dosing | Days after Initial Phase |
| APIXABAN | 5 mg bid/2.5 mg bid | for 3–6 months |
| RIVAROXABAN | 20 mg die | for 3–6 months |
| EDOXABAN | 60 mg/day or 30 mg/day with regard to actual functionality | for 3–6 months |
| DABIGATRAN | 150 mg bid ore 110 mg die | for 3–6 months |
| VKA | Dosing related INR value | for 3–6 months |
| LOW-MOLECULAR-WEIGHT HEPARIN | Dosing is typically weight-based and continued at the same dose used for initial anticoagulation. | for 3–6 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Corte, V.; Riolo, R.; Scaglione, S.; Pecoraro, R.; Tuttolomondo, A. The Role of Biomarkers, Metabolomics, and COVID-19 in Venous Thromboembolism—A Review of Literature. Int. J. Mol. Sci. 2023, 24, 13411. https://doi.org/10.3390/ijms241713411
Della Corte V, Riolo R, Scaglione S, Pecoraro R, Tuttolomondo A. The Role of Biomarkers, Metabolomics, and COVID-19 in Venous Thromboembolism—A Review of Literature. International Journal of Molecular Sciences. 2023; 24(17):13411. https://doi.org/10.3390/ijms241713411
Chicago/Turabian StyleDella Corte, Vittoriano, Renata Riolo, Stefania Scaglione, Rosaria Pecoraro, and Antonino Tuttolomondo. 2023. "The Role of Biomarkers, Metabolomics, and COVID-19 in Venous Thromboembolism—A Review of Literature" International Journal of Molecular Sciences 24, no. 17: 13411. https://doi.org/10.3390/ijms241713411
APA StyleDella Corte, V., Riolo, R., Scaglione, S., Pecoraro, R., & Tuttolomondo, A. (2023). The Role of Biomarkers, Metabolomics, and COVID-19 in Venous Thromboembolism—A Review of Literature. International Journal of Molecular Sciences, 24(17), 13411. https://doi.org/10.3390/ijms241713411







