Extract of Artemisia dracunculus L. Modulates Osteoblast Proliferation and Mineralization
Abstract
1. Introduction
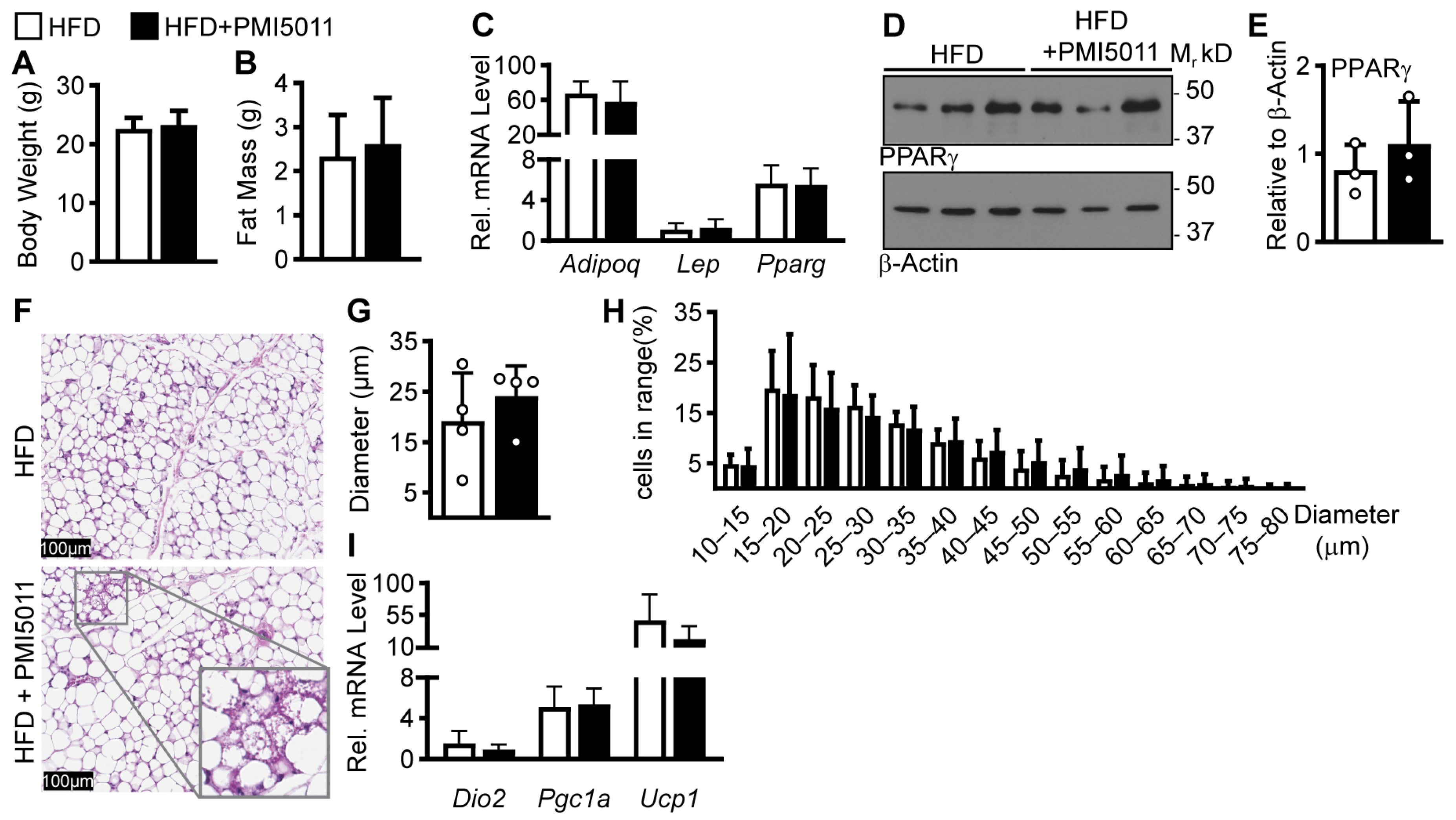
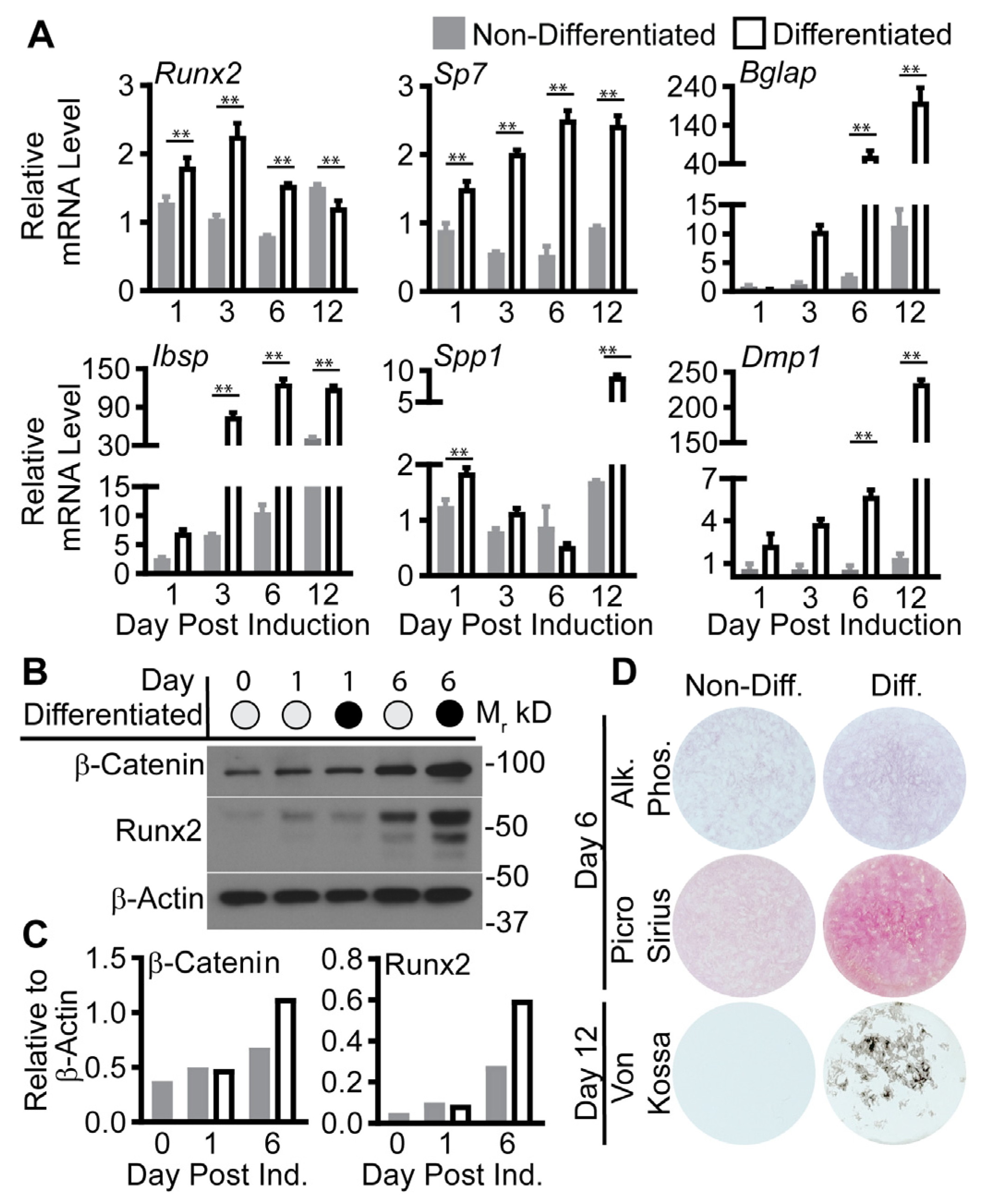
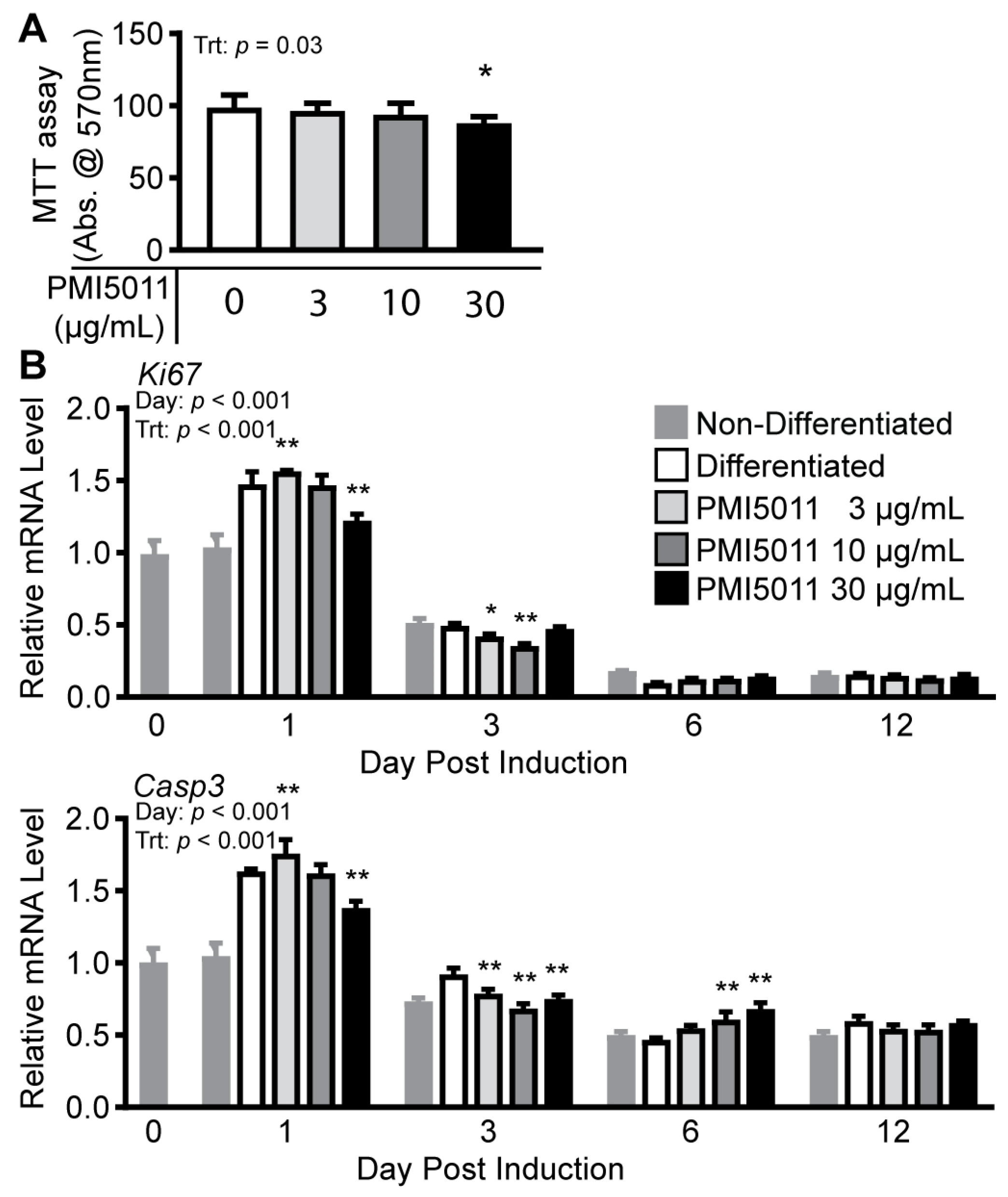
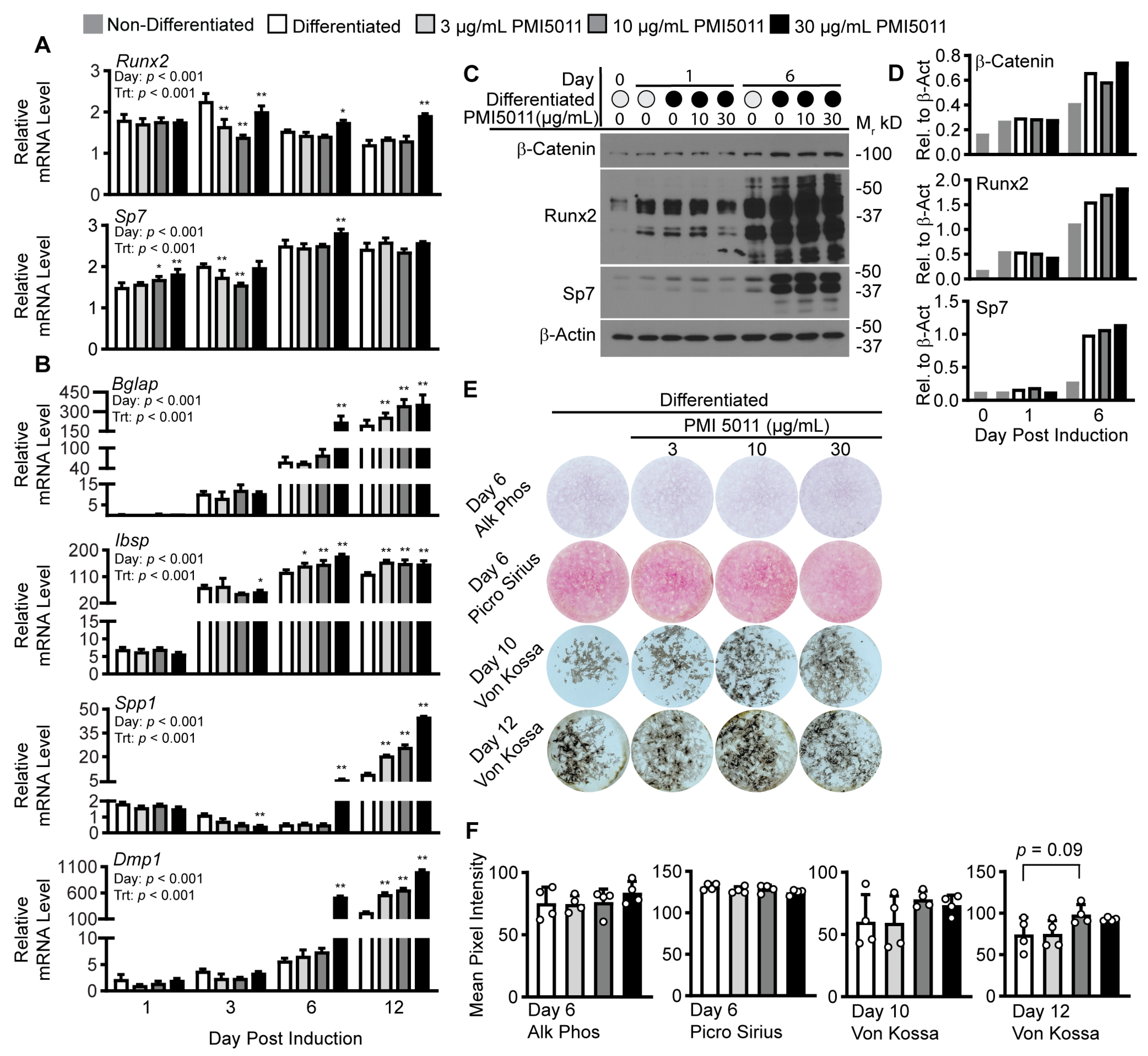
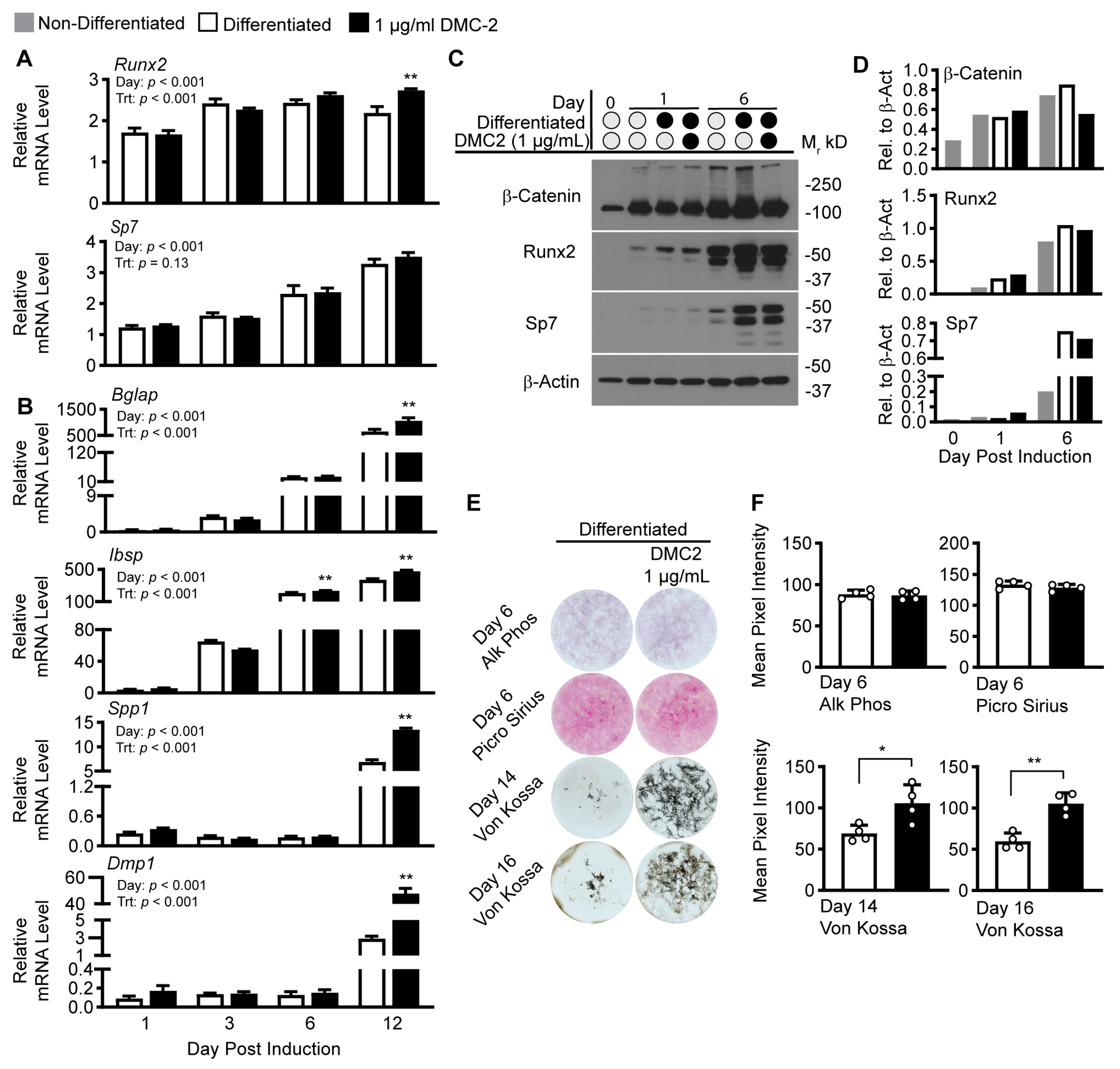
2. Results
3. Discussion
4. Materials and Methods
4.1. PMI5011 and DMC-2 Extracts
4.2. Animal Experiment for Adipose Tissue
4.3. MC3T3-E1 Cell Culture
4.4. Gene Expression
4.5. Protein Expression
4.6. Staining and Image Quantification
4.6.1. H&E Staining, Imaging, and Quantification of iWAT
4.6.2. Cell Culture Staining
4.6.3. Image Capture and Quantification
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klein, S.; Gastaldelli, A.; Yki-Järvinen, H.; Scherer, P.E. Why Does Obesity Cause Diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Salamat, M.R.; Salamat, A.H.; Janghorbani, M. Association between Obesity and Bone Mineral Density by Gender and Menopausal Status. Endocrinol. Metab. 2016, 31, 547. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, L.; Yao, X.; Zhu, Z. The Association between Abdominal Obesity and Femoral Neck Bone Mineral Density in Older Adults. J. Orthop. Surg. Res. 2023, 18, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Piñar-Gutierrez, A.; García-Fontana, C.; García-Fontana, B.; Muñoz-Torres, M. Obesity and Bone Health: A Complex Relationship. Int. J. Mol. Sci. 2022, 23, 8303. [Google Scholar] [CrossRef]
- Compston, J.E.; Watts, N.B.; Chapurlat, R.; Cooper, C.; Boonen, S.; Greenspan, S.; Pfeilschifter, J.; Silverman, S.; Díez-Pérez, A.; Lindsay, R.; et al. Obesity Is Not Protective against Fracture in Postmenopausal Women: GLOW. Am. J. Med. 2011, 124, 1043–1050. [Google Scholar] [CrossRef]
- Rinonapoli, G.; Pace, V.; Ruggiero, C.; Ceccarini, P.; Bisaccia, M.; Meccariello, L.; Caraffa, A. Obesity and Bone: A Complex Relationship. Int. J. Mol. Sci. 2021, 22, 13662. [Google Scholar] [CrossRef]
- Hauner, H. The Mode of Action of Thiazolidinediones. Diabetes/Metab. Res. Rev. 2002, 18 (Suppl. 2), S10–S15. [Google Scholar] [CrossRef]
- Shockley, K.R.; Lazarenko, O.P.; Czernik, P.J.; Rosen, C.J.; Churchill, G.A.; Lecka-Czernik, B. PPARgamma2 Nuclear Receptor Controls Multiple Regulatory Pathways of Osteoblast Differentiation from Marrow Mesenchymal Stem Cells. J. Cell. Biochem. 2009, 106, 232–246. [Google Scholar] [CrossRef]
- Lecka-Czernik, B. Bone Loss in Diabetes: Use of Antidiabetic Thiazolidinediones and Secondary Osteoporosis. Curr. Osteoporos. Rep. 2010, 8, 178–184. [Google Scholar] [CrossRef]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARγ Signaling and Metabolism: The Good, the Bad and the Future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef]
- Bajpeyi, S.; Pasarica, M.; Conley, K.E.; Newcomer, B.R.; Jubrias, S.A.; Gamboa, C.; Murray, K.; Sereda, O.; Sparks, L.M.; Smith, S.R. Pioglitazone-Induced Improvements in Insulin Sensitivity Occur without Concomitant Changes in Muscle Mitochondrial Function. Metabolism 2017, 69, 24–32. [Google Scholar] [CrossRef]
- Park, K.S.; Ciaraldi, T.P.; Abrams-Carter, L.; Mudaliar, S.; Nikoulina, S.E.; Henry, R.R. Troglitazone Regulation of Glucose Metabolism in Human Skeletal Muscle Cultures from Obese Type II Diabetic Subjects. J. Clin. Endocrinol. Metab. 1998, 83, 1636–1643. [Google Scholar] [CrossRef]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr. Physiol. 2020, 10, 785–809. [Google Scholar] [CrossRef] [PubMed]
- Lecka-Czernik, B. Diabetes, Bone and Glucose-Lowering Agents: Basic Biology. Diabetologia 2017, 60, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.V. Diabetes, Bone and Glucose-Lowering Agents: Clinical Outcomes. Diabetologia 2017, 60, 1170–1179. [Google Scholar] [CrossRef]
- Ribnicky, D.M.; Poulev, A.; Watford, M.; Cefalu, W.T.; Raskin, I. Antihyperglycemic Activity of Tarralin, an Ethanolic Extract of Artemisia Dracunculus L. Phytomedicine 2006, 13, 550–557. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Ribnicky, D.; Zhang, X.H.; Zuberi, A.; Raskin, I.; Yu, Y.; Cefalu, W.T. An Extract of Artemisia Dracunculus L. Enhances Insulin Receptor Signaling and Modulates Gene Expression in Skeletal Muscle in KK-A(y) Mice. J. Nutr. Biochem. 2011, 22, 71–78. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Ribnicky, D.; Zhang, X.H.; Raskin, I.; Yu, Y.; Cefalu, W.T. Bioactives of Artemisia Dracunculus L Enhance Cellular Insulin Signaling in Primary Human Skeletal Muscle Culture. Metabolism 2008, 57, S58–S64. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kheterpal, I.; Coleman, L.; Ku, G.; Wang, Z.Q.; Ribnicky, D.; Cefalu, W.T. Regulation of Insulin Action by an Extract of Artemisia Dracunculus L. in Primary Human Skeletal Muscle Culture: A Proteomics Approach. Phytother. Res. 2010, 24, 1278–1284. [Google Scholar] [CrossRef]
- Vandanmagsar, B.; Haynie, K.R.; Wicks, S.E.; Bermudez, E.M.; Mendoza, T.M.; Ribnicky, D.; Cefalu, W.T.; Mynatt, R.L. Artemisia Dracunculus L. Extract Ameliorates Insulin Sensitivity by Attenuating Inflammatory Signalling in Human Skeletal Muscle Culture. Diabetes Obes. Metab. 2014, 16, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Simmler, C.; Kuhn, P.; Poule, A.; Raskin, I.; Ribnicky, D.; Floyd, Z.E.; Pauli, G.F. The DESIGNER Approach Helps Decipher the Hypoglycemic Bioactive Principles of Artemisia Dracunculus (Russian Tarragon). J. Nat. Prod. 2019, 82, 3321–3329. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Mendoza, T.M.; Ribnicky, D.M.; Poulev, A.; Noland, R.C.; Mynatt, R.L.; Raskin, I.; Cefalu, W.T.; Floyd, Z.E. An Extract of Russian Tarragon Prevents Obesity-Related Ectopic Lipid Accumulation. Mol. Nutr. Food Res. 2018, 62, 1700856. [Google Scholar] [CrossRef] [PubMed]
- Vandanmagsar, B.; Yu, Y.; Simmler, C.; Dang, T.N.; Kuhn, P.; Poulev, A.; Ribnicky, D.M.; Pauli, G.F.; Floyd, Z.E. Bioactive Compounds from Artemisia Dracunculus L. Activate AMPK Signaling in Skeletal Muscle. Biomed. Pharmacother. 2021, 143, 112188. [Google Scholar] [CrossRef]
- Schwartz, A.V.; Sellmeyer, D.E. Effect of Thiazolidinediones on Skeletal Health in Women with Type 2 Diabetes. Expert Opin. Drug Saf. 2008, 7, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.W.; Horton, J.A. Variable Osteogenic Performance of MC3T3-E1 Subclones Impacts Their Utility as Models of Osteoblast Biology. Sci. Rep. 2019, 9, 8299. [Google Scholar] [CrossRef]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef]
- Maridas, D.E.; Rendina-Ruedy, E.; Le, P.T.; Rosen, C.J. Isolation, Culture, and Differentiation of Bone Marrow Stromal Cells and Osteoclast Progenitors from Mice. J. Vis. Exp. 2018, 2018, e56750. [Google Scholar] [CrossRef]
- Puchtler, H.; Meloan, S.N.; Waldrop, F.S. Are Picro-Dye Reactions for Collagens Quantitative? Chemical and Histochemical Considerations. Histochemistry 1988, 88, 243–256. [Google Scholar] [CrossRef]
- Schneider, M.R. Von Kossa and His Staining Technique. Histochem. Cell Biol. 2021, 156, 523. [Google Scholar] [CrossRef]
- López-García, J.; Lehocký, M.; Humpolíček, P.; Sáha, P. HaCaT Keratinocytes Response on Antimicrobial Atelocollagen Substrates: Extent of Cytotoxicity, Cell Viability and Proliferation. J. Funct. Biomater. 2014, 5, 43–57. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices-Part 5: Tests for in Vitro Cytotoxicity 2009. International Organization for Standardization: Geneva, Switzerland, 2009.
- Kulterer, B.; Friedl, G.; Jandrositz, A.; Sanchez-Cabo, F.; Prokesch, A.; Paar, C.; Scheideler, M.; Windhager, R.; Preisegger, K.H.; Trajanoski, Z. Gene Expression Profiling of Human Mesenchymal Stem Cells Derived from Bone Marrow during Expansion and Osteoblast Differentiation. BMC Genom. 2007, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Infante, A.; Rodríguez, C.I. Osteogenesis and Aging: Lessons from Mesenchymal Stem Cells. Stem Cell Res. Ther. 2018, 9, 244. [Google Scholar] [CrossRef]
- Miura, M.; Chen, X.-D.; Allen, M.R.; Bi, Y.; Gronthos, S.; Seo, B.-M.; Lakhani, S.; Flavell, R.A.; Feng, X.-H.; Robey, P.G.; et al. A Crucial Role of Caspase-3 in Osteogenic Differentiation of Bone Marrow Stromal Stem Cells. J. Clin. Investig. 2004, 114, 1704–1713. [Google Scholar] [CrossRef]
- Saiwaki, T.; Kotera, I.; Sasaki, M.; Takagi, M.; Yoneda, Y. In Vivo Dynamics and Kinetics of PKi-67: Transition from a Mobile to an Immobile Form at the Onset of Anaphase. Exp. Cell Res. 2005, 308, 123–134. [Google Scholar] [CrossRef]
- Julien, O.; Wells, J.A. Caspases and Their Substrates. Cell Death Differ. 2017, 24, 1380. [Google Scholar] [CrossRef]
- Cohen, G.M. Caspases: The Executioners of Apoptosis. Biochem. J. 1997, 326, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, G.; Li, Y.P. TGF-β and BMP Signaling in Osteoblast, Skeletal Development, and Bone Formation, Homeostasis and Disease. Bone Res. 2016, 4, 1–21. [Google Scholar] [CrossRef]
- Schroeder, T.M.; Jensen, E.D.; Westendorf, J.J. Runx2: A Master Organizer of Gene Transcription in Developing and Maturing Osteoblasts. Birth Defects Res. Part C Embryo Today Rev. 2005, 75, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Ribnicky, D.M.; Kuhn, P.; Poulev, A.; Logendra, S.; Zuberi, A.; Cefalu, W.T.; Raskin, I. Improved Absorption and Bioactivity of Active Compounds from an Anti-Diabetic Extract of Artemisia Dracunculus L. Int. J. Pharm. 2009, 370, 87–92. [Google Scholar] [CrossRef]
- Słupski, W.; Jawień, P.; Nowak, B. Botanicals in Postmenopausal Osteoporosis. Nutrients 2021, 13, 1609. [Google Scholar] [CrossRef]
- Jolly, J.J.; Chin, K.Y.; Alias, E.; Chua, K.H.; Soelaiman, I.N. Protective Effects of Selected Botanical Agents on Bone. Int. J. Environ. Res. Public Health 2018, 15, 963. [Google Scholar] [CrossRef]
- Lebovitz, H.E.; Banerji, M.A. Insulin Resistance and Its Treatment by Thiazolidinediones. Recent Prog. Horm. Res. 2001, 56, 265–294. [Google Scholar] [CrossRef]
- Larson-Meyer, D.E.; Heilbronn, L.K.; Redman, L.M.; Newcomer, B.R.; Frisard, M.I.; Anton, S.; Smith, S.R.; Alfonso, A.; Ravussin, E. Effect of Calorie Restriction With or Without Exercise on Insulin Sensitivity, β-Cell Function, Fat Cell Size, and Ectopic Lipid in Overweight Subjects. Diabetes Care 2006, 29, 1337. [Google Scholar] [CrossRef]
- Zhao, Q.; Khedkar, S.V.; Johnson, K.C. Weight Loss Interventions and Skeletal Health in Persons with Diabetes. Curr. Osteoporos Rep. 2022, 20, 240. [Google Scholar] [CrossRef]
- Kirk-Ballard, H.; Wang, Z.Q.; Acharya, P.; Zhang, X.H.; Yu, Y.; Kilroy, G.; Ribnicky, D.; Cefalu, W.T.; Floyd, Z.E. An Extract of Artemisia Dracunculus L. Inhibits Ubiquitin-Proteasome Activity and Preserves Skeletal Muscle Mass in a Murine Model of Diabetes. PLoS ONE 2013, 8, e57112. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Armamento-Villareal, R. Obesity and Skeletal Fragility. J. Clin. Endocrinol. Metab. 2023, dgad415. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 521497. [Google Scholar] [CrossRef]
- Mukherjee, A.; Rotwein, P. Akt Promotes BMP2-Mediated Osteoblast Differentiation and Bone Development. J. Cell Sci. 2009, 122, 716. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, E.; Ochiai-Shino, H.; Aoki, H.; Onodera, S.; Saito, A.; Saito, A.; Azuma, T. Akt Activation Is Required for TGF-Β1-Induced Osteoblast Differentiation of MC3T3-E1 Pre-Osteoblasts. PLoS ONE 2014, 9, e112566. [Google Scholar] [CrossRef] [PubMed]
- Kasai, T.; Bandow, K.; Suzuki, H.; Chiba, N.; Kakimoto, K.; Ohnishi, T.; Kawamoto, S.I.; Nagaoka, E.; Matsuguchi, T. Osteoblast Differentiation Is Functionally Associated with Decreased AMP Kinase Activity. J. Cell Physiol. 2009, 221, 740–749. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, X.; Yang, Y.; Han, X.; Wang, L.; Qiao, H.; Fan, Q.; Tang, T.; Dai, K. AMPK Promotes Osteogenesis and Inhibits Adipogenesis through AMPK-Gfi1-OPN Axis. Cell Signal. 2016, 28, 1270–1282. [Google Scholar] [CrossRef] [PubMed]
- Pramojanee, S.N.; Phimphilai, M.; Chattipakorn, N.; Chattipakorn, S.C. Possible Roles of Insulin Signaling in Osteoblasts. Endocr. Res. 2014, 39, 144–151. [Google Scholar] [CrossRef]
- Kelley, D.; Mitrakou, A.; Marsh, H.; Schwenk, F.; Benn, J.; Sonnenberg, G.; Arcangeli, M.; Aoki, T.; Sorensen, J.; Berger, M.; et al. Skeletal Muscle Glycolysis, Oxidation, and Storage of an Oral Glucose Load. J. Clin. Investig. 1988, 81, 1563–1571. [Google Scholar] [CrossRef]
- Buehring, B.; Binkley, N. Myostatin--the Holy Grail for Muscle, Bone, and Fat? Curr. Osteoporos. Rep. 2013, 11, 407–414. [Google Scholar] [CrossRef]
- Loiselle, A.E.; Paul, E.M.; Lewis, G.S.; Donahue, H.J. Osteoblast and Osteocyte-Specific Loss of Connexin43 Results in Delayed Bone Formation and Healing during Murine Fracture Healing. J. Orthop. Res. 2013, 31, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Mera, P.; Laue, K.; Wei, J.; Berger, J.M.; Karsenty, G. Osteocalcin Is Necessary and Sufficient to Maintain Muscle Mass in Older Mice. Mol. Metab. 2016, 5, 1042–1047. [Google Scholar] [CrossRef]
- Komori, T. Functions of Osteocalcin in Bone, Pancreas, Testis, and Muscle. Int. J. Mol. Sci. 2020, 21, 7513. [Google Scholar] [CrossRef]
- Mera, P.; Laue, K.; Ferron, M.; Confavreux, C.; Wei, J.; Galán-Díez, M.; Lacampagne, A.; Mitchell, S.J.; Mattison, J.A.; Chen, Y.; et al. Osteocalcin Signaling in Myofibers Is Necessary and Sufficient for Optimum Adaptation to Exercise. Cell Metab. 2016, 23, 1078–1092. [Google Scholar] [CrossRef]
- Lin, X.; Brennan-Speranza, T.C.; Levinger, I.; Yeap, B.B. Undercarboxylated Osteocalcin: Experimental and Human Evidence for a Role in Glucose Homeostasis and Muscle Regulation of Insulin Sensitivity. Nutrients 2018, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Fuller, S.; Yu, Y.; Mendoza, T.; Ribnicky, D.M.; Cefalu, W.T.; Floyd, Z.E. Potential Adverse Effects of Botanical Supplementation in High-Fat-Fed Female Mice. Biol. Sex Differ. 2018, 9, 1–14. [Google Scholar] [CrossRef]
| Gene ID | Gene Name | Accession # | Sequence–Forward | Sequence–Reverse |
|---|---|---|---|---|
| Ppib | Peptidulprolyl isomerase B (cyclophillin B) | NM_011149.2 | 5′-TCC ATC GTG TCA TCA AGG ACT T-3′ | 5′-CTG ATC TGG GAA GCG CTC A-3′ |
| Adipoq | Adiponectin | NM_009605.5 | 5′-CAT GCC GAA GAT GAC GTT ACT A-3′ | 5′-ACG CTG AGC GAT ACA CAT AAG-3′ |
| Lep | Leptin | NM_008493.3 | 5′-GGC TTT GGT CCT ATC TGT CTT ATG-3′ | 5′-CCG ACT GCG TGT GTG AAA T-3′ |
| Pparg | Peroxisome proliferator activated receptor gamma | NM_011146 | 5′-ATG TCA GTA CTG TCG GTT TCA G-3′ | 5′-AGC GGG AAG GAC TTT ATG TAT G-3′ |
| Dio2 | Iodothyronine deiodinase 2 | NM_010050.4 | 5′-CTC CTA GAT GCC TAC AAA CAG G-3′ | 5′-GCA CTG GCA AAG TCA AGA AG-3′ |
| Pgc1a | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha | NM_008904 | 5′-AGC CTC TTT GCC CAG ATC TTC-3′ | 5′-CCA TCT GTC AGT GCA TCA AAT GA-3′ |
| Ucp1 | Uncoupling protein 1 | NM_009463.3 | 5′-GAT CTT CTC AGC CGG AGT TT-3′ | 5′-GCC TTC ACC TTG GAT CTG AA-3′ |
| Rlp13a | Ribosomal protein L13a | NM_009438.5 | 5′-CCA AGA TGC ACT ATC GGA AGA A-3′ | 5′-CTT GAG GAC CTC TGT GAA CTT G-3′ |
| Runx2 | Runt related transcription factor 2 | NM_001146038 | 5′-CCA GGC GTA TTT CAG ATG ATG A-3′ | 5′-GTC CTC AGT GAG GGA TGA AAT G-3′ |
| Sp7 | Transcription factor 7 | NM_130458.4 | 5′-CTC CTC GGT TCT CTC CAT CT-3′ | 5′-GGA GCC ATA GTG AGC TTC TTC-3′ |
| Bglap | Bone gamma-carboxyglutamate protein (osteocalcin) | NM_007541.3 | 5′-CCA AGC AGG AGG GCA ATA A-3′ | 5′-TCG TCA CAA GCA GGG TTA AG-3′ |
| Ibsp | Intergrin binding sialoprotein (bone sialoprotein) | NM_008318.3 | 5′-CTC CAC ACT TTC CAC ACT CTC-3′ | 5′-TTT CTG CAT CTC CAG CCT TC-3′ |
| Spp1 | Secreted phosphoprotein 1 (ostepontin) | NM_001204201.1 | 5′-GGC TGA ATT CTG AGG GAC TAA C-3′ | 5′-GCC ATG TGG CTA TAG GAT CTG-3′ |
| Dmp1 | Dentin matrix protein | NM_001359013.1 | 5′-CAG AGG GAC AGG CAA ATA GTG-3′ | 5′-GTC GTC TTC ATC ATC CTC CTT ATC-3′ |
| Ki67 | Antigen identified by monoclonal antibody Ki67 | NM_001081117.2 | 5′-GAT TCC ATT AAC AAG AGT GAG GGA-3′ | 5′-CTG TGA GTG CCA AGA GAC TTC-3′ |
| Casp3 | Caspase 3 | NM_001284409.1 | 5′-GCA GCT TTG TGT GTG TGA TTC-3′ | 5′-GCA GGC CTG AAT GAT GAA GA-3′ |
| All Primers designed and purchased from Integrated DNA Technologies (Coralville, IA, USA) | ||||
| Anti-: | Host | Manufacturer | Catalogue # |
|---|---|---|---|
| PPARγ | Mouse Monoclonal | Santa Cruz (Santa Cruz, CA, USA) | SC-7273 |
| β-Actin | Rabbit Polyclonal | GeneTex (Irvine, CA, USA) | GTX109639 |
| β-Catenin | Rabbit Polyclonal | Cell Signaling (Danvers, MA, USA) | 9562 |
| Runx2 | Mouse Monoclonal | Santa Cruz (Santa Cruz, CA, USA) | SC-390351 |
| Sp7 | Rabbit Monoclonal | Abcam (Cambridge, UK) | AB209484 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scott, M.C.; Bourgeois, A.; Yu, Y.; Burk, D.H.; Smith, B.J.; Floyd, Z.E. Extract of Artemisia dracunculus L. Modulates Osteoblast Proliferation and Mineralization. Int. J. Mol. Sci. 2023, 24, 13423. https://doi.org/10.3390/ijms241713423
Scott MC, Bourgeois A, Yu Y, Burk DH, Smith BJ, Floyd ZE. Extract of Artemisia dracunculus L. Modulates Osteoblast Proliferation and Mineralization. International Journal of Molecular Sciences. 2023; 24(17):13423. https://doi.org/10.3390/ijms241713423
Chicago/Turabian StyleScott, Matthew C., Aleah Bourgeois, Yongmei Yu, David H. Burk, Brenda J. Smith, and Z. Elizabeth Floyd. 2023. "Extract of Artemisia dracunculus L. Modulates Osteoblast Proliferation and Mineralization" International Journal of Molecular Sciences 24, no. 17: 13423. https://doi.org/10.3390/ijms241713423
APA StyleScott, M. C., Bourgeois, A., Yu, Y., Burk, D. H., Smith, B. J., & Floyd, Z. E. (2023). Extract of Artemisia dracunculus L. Modulates Osteoblast Proliferation and Mineralization. International Journal of Molecular Sciences, 24(17), 13423. https://doi.org/10.3390/ijms241713423






