1. Introduction
The obesity pandemic is one of the most important problems of modern public health [
1] and is characterized by extremely low efficiency of treatment and catastrophic consequences for human health [
2]. The most detrimental effect on health is caused by well-known visceral obesity, associated with metabolic disorders (dyslipidemia, arterial hypertension, carbohydrate metabolism disorders) and cardiovascular diseases [
2]. Thus, there is a need for the identification of early markers that allow one to estimate the risk of emergence of this phenotype of obesity and, whenever possible, methods of prevention of its development. One of the mechanisms of switching the accumulation of excess energy from the subcutaneous fat depot to the visceral one is the activation of fibrosis in the subcutaneous adipose tissue with deterioration of its plasticity and limitation of further expansion [
3]. Brown adipose tissue (BAT) activation is associated with inhibition of profibrotic genes [
4,
5,
6] that can slow down these pathological changes. In recent years, microRNA (miR) assessment has been increasingly used to study the molecular genetic pathways of various processes, including the activity of different types of adipose tissue (AT). MiRs are small non-coding RNA (19–25 nucleotides long) that regulate gene expression by affecting the matrix RNA. Over 1500 representatives of this class of molecules have already been described in humans. Changes in their expression are observed in many pathological conditions, and miRs are involved in various biological processes such as apoptosis, proliferation, and differentiation [
7]. About 60% of the genome is a target of miRs. The expression of miRs is tissue-specific, but in addition to their effects inside the cell, miRs can reach outside the cell and perform their functions in other cells. They are found in various biological fluids: blood (serum, plasma), urine, saliva, cerebrospinal fluid. Thus, exosomal miRs released from AT can regulate gene expression in other tissues. The interest in AT-produced miRs derives from the fact that the AT is both a miR-producing organ and a target of the miRs it secretes, and that miRs entering the circulation from AT are an important source of miRs in plasma [
8,
9]. Subcutaneous AT (SAT), due to its location, represents a successful purpose for research on change in expression of miRs in obese patients. Comparison of changes in the level of miRs in SAT and plasma allows one to reveal those miRs which are plasma markers of the processes occurring in SAT. Given the pronounced influence of the AT on the circulatory profile of miRs, studies simultaneously assessing the dynamics of miRs in the AT and plasma are of particular interest to identify those of miRs that change concomitantly. Recent studies have demonstrated that obesity alters the profile of a number of circulating miRs [
9,
10,
11], including miR-142-3p and miR-378 [
10,
12,
13]. Our interest in these miRs stems from their important and ambiguous roles in the pathogenesis of obesity. To identify key miRs and gene markers in adipose tissue, we analyzed the literature data to generate a panel of miRs that have been shown to play a significant role in adipogenesis, inflammation, and fibrosis in the adipose tissue according to other researchers [
7,
8,
9,
10,
12]. We examined the expression level of selected miRs in SAT from obese patients and compared them with a group of healthy controls. In further analysis, we included those which expression level significantly differed in healthy and obese patients before weight loss and returned to normal values after weight normalization. These were miR-27, miR-93, miR-142, miR-155, and miR-378. Then, we examined the expression level of these miRs in SAT and in plasma from obese patients during sibutramine therapy. Significant changes during sibutramine therapy were found for miR-142 and miR-378. In vitro studies have shown that at the differentiation stage of progenitor cells (3T3-L1 preadipocytes and ST2 mesenchymal progenitor cell lines), the increase in expression of miR-378 stimulates their differentiation into adipocytes [
14], the synthesis and accumulation of triglycerides and fatty acids in vacuoles of white adipose tissue (WAT). These effects are implemented by increasing the expression of fatty acid synthase, enlarging the size of lipid droplets and thus stimulating lipogenesis without affecting β-oxidation of fatty acids [
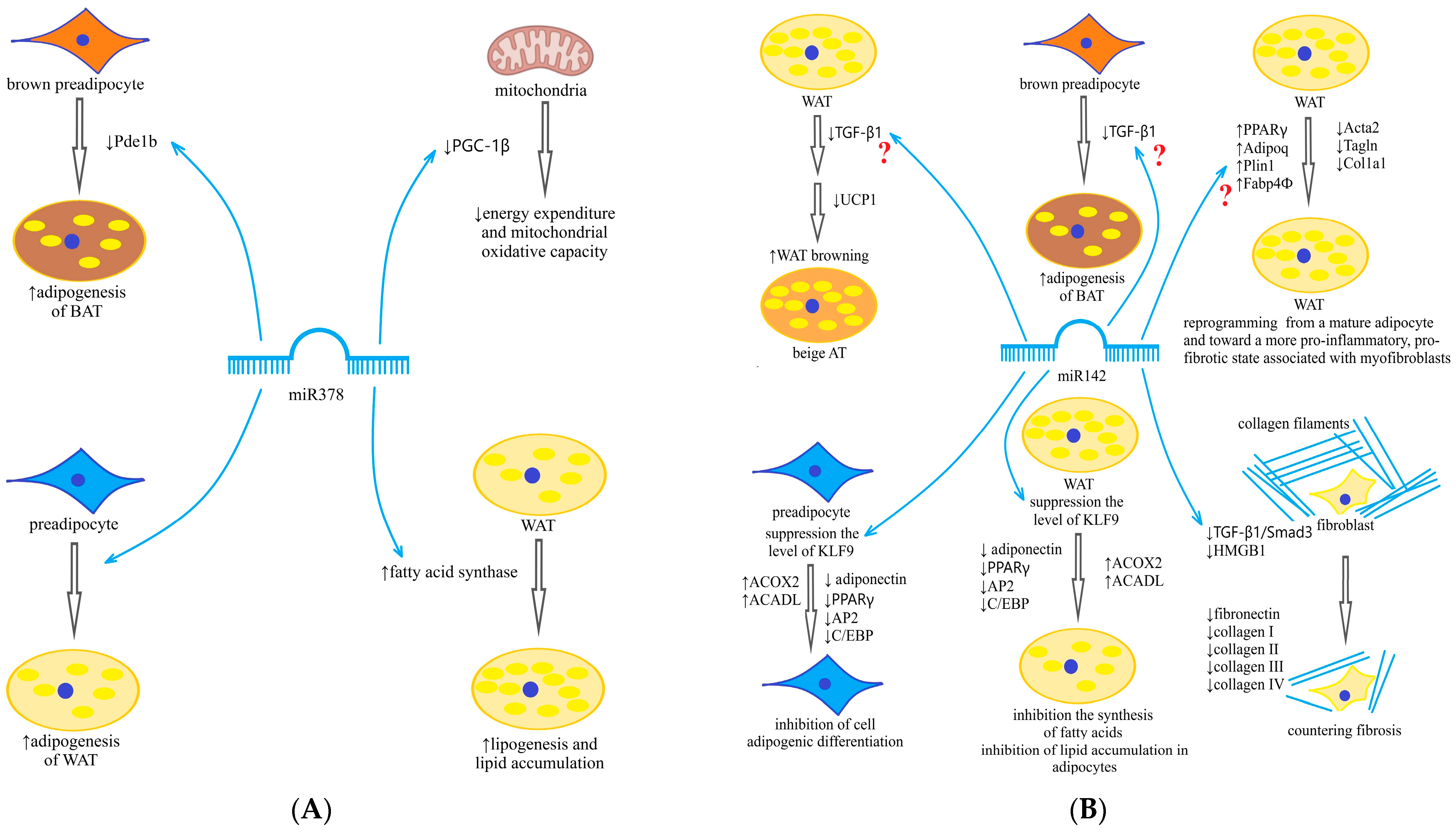
15]. In experiments in obese mice, miR-378 is designated as an inhibitor of insulin signaling, by inhibiting p110a, regulator of glucose and fatty acid metabolism, by counteracting peroxisome proliferator-activated receptor gamma coactivator 1-beta (PGC-1β) [
16] and is potentially associated with the development of insulin resistance (IR), while other studies have demonstrated its association with activation of adipogenesis of BAT and protection from diet-induced obesity [
17,
18]. Phosphodiesterase 1b (PDE1b) is a direct target of miR-378 in BAT but not in WAT [
12,
13]. MiR-378-mediated inhibition of PDE1b regulates the synthesis of cyclic adenosine monophosphate and thus enhances the differentiation of brown adipocytes, increasing the volume of BAT [
18]. Furthermore, miR-378 is involved in the regulation of mitochondrial metabolism and energy homeostasis in mice through a PGC-1β-controlled transcriptional network [
16]. Thus, data from the literature indicate that miR-378 may play various roles under diverse conditions and in different fat depots (
Scheme 1A).
Being expressed in the AT, miR-142 regulates lipid metabolism and adipocyte differentiation and reduces triglyceride accumulation in adipocytes by inhibiting fatty acid synthesis gene expression and enhancing fatty acid oxidation gene expression [
19]. MiR-142 has been shown to be involved in adipogenesis by inhibiting high-mobility group protein (HMG) A1. Some older studies have linked miR-142 overexpression to inflammation activation, although the genetic pathway has not been established. But recent studies have demonstrated involvement of miR-142 in the inhibition pathway of transforming growth factor beta (TGF-β) and TGF-β receptor, antifibrotic effects and a decrease in type 1 collagen formation with its overexpression [
20]. Another mechanism for the antifibrotic effects of miR-142 is the suppression of expression of genes related to the extracellular matrix (COL1A1 encoding the 1-alpha chain of type I collagen and COL3A1 encoding the 1-alpha chain of type III collagen) [
21]. Hyperexpression of miR-142 suppresses profibrotic gene expression in cardiomyocytes by targeting HMGB1 [
22]. Through similar mechanisms, miR-142 is involved in antifibrotic processes in the liver, its expression is dramatically reduced in liver cirrhosis, and ectopic expression of miR-142 in activated stellate liver cells decreases profibrotic markers [
23] (
Scheme 1B). Moreover, we did not find in the available literature any data on the relationship between miR-142 expression in the AT and fibrosis markers.
The continuing expansion of obesity dictates the need to look for new methods of treatment and its personalization depending on various determinants, using existing approaches to therapy. Therapeutic methods that can reduce fibrosis activation are an attractive option in the treatment of obesity. Given the fact that BAT activation is associated with inhibition of profibrotic genes [
4,
5,
6], these changes may be of a concomitant character. Respectively, increased energy expenditure by activation of BAT is the promising directions in the development of treatment of obesity and associated metabolic disorders [
24]. Among the drugs registered in the Russian Federation for the treatment of obesity, such an effect is assumed for the neurotransmitter reuptake inhibitor sibutramine. This drug belongs to a very small group of drugs that, in addition to their central action (serotonin reuptake inhibitor) on appetite, have a peripheral action in the form of catecholaminergic effects, inhibiting norepinephrine (NE) reuptake. NE activates thermogenesis by stimulating β3-adrenoreceptors, increasing energy expenditure. β3-adrenergic stimulation leads to proliferation of preadipocytes through the brown fat cell pathway. This suggests that sibutramine may modulate BAT activity. Previously, the presence of such effects in sibutramine was demonstrated in an experiment [
25]. We did not find similar studies for humans in the available literature. In summary, miR-378 has been described as one of three key miRs (miR-143, miR-145 and miR-378) affecting adipocyte differentiation, a positive regulator of adipogenesis in classical BAT, similar to the miR-193a/b cluster and miR-365, miR-182 and miR-203, and it plays a role in the regulation of ADIPOQ transcriptional activity and mitochondrial biogenesis [
26]. MiR-142 is a serious candidate to be an inhibitor of fibrogenesis in various tissues [
20,
21,
22,
23]. However, under different conditions (plasma or AT, recent obesity or long-term obesity, presence or absence of metabolic disorders), their roles and functions may differ. This leads us to expect that the co-evaluation of their expression and dynamics in obese patients of different severity and duration before and after weight loss on sibutramine therapy will allow us to clarify their effects. In addition, revealing the relationship of their dynamics not only with changes in weight and metabolic status but also with the fact of sibutramine intake may improve our understanding of the mechanisms of action of this drug. Such an analysis is attempted in this article.
3. Discussion
Among the many miRs closely related to the functional activity of the AT and dysregulated in obesity [
8], two that are related to the efficacy of sibutramine therapy attracted our attention: miR-378 and miR-142. We studied their representation both in the SAT and in the circulation. Given the large volume of AT in obesity and its high secretory activity, it is a significant source of miRs in the circulation [
8,
9]. Moreover, the significance of the AT contribution to the circulatory profile of miRs, in our opinion, should be evaluated on a case-by-case basis, especially for those miRs that are also highly expressed in other tissues. This characterization applies in particular to miR-378 and miR-142. To determine whether the circulating levels of these miRs reflect changes in their expression in SAT, we performed a parallel evaluation of miR-378 and miR-142 expression in SAT and their levels in plasma, before and during sibutramine therapy.
According to the literature, miR-378 and miR-142 have multidirectional effects on preadipocyte differentiation into adipocytes and adipogenesis of WAT. Increased expression of miR-378 stimulates differentiation of precursor cells into adipocytes and increases triglyceride deposition therein [
15,
27]. Thus, miR-378 increases both adipocyte hyperplasia and hypertrophy, whereas miR-142 inhibits adipogenic differentiation and autophagy in obesity, targeting Krueppel-like factor 9 [
19].
Considering this, the multidirectional character of the changes in the expression of these miRs, which is the most pronounced in the early stages of obesity, seems very logical. The most pronounced elevation in miR-378 expression and reduction in miR-142 expression exactly at the first stage of obesity can be explained by the fact that at the beginning of obesity there is a maximal activation of mechanisms ensuring the expansion of the SAT volume to store the excess incoming energy. Other researchers have also noted that miR-378 expression increases during normal adipogenesis in humans [
15]. The subsequent “pseudonormalization” of expression at higher degrees of obesity can be explained by the depletion of adaptive mechanisms. Moreover, as the duration of obesity increases, the significance of increased miR-378 expression is lost, whereas the decrease in miR-142 expression becomes more pronounced. As the duration of obesity grows, proinflammatory cytokines and hypoxia factors activate profibrogenic genes and disrupt the activity of miRs that inhibit fibrosis, among which miR-142 occupies an important place. The decreased expression of miR-378 may also be due to prolonged exposure to high levels of proinflammatory cytokines. Tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) have been shown to increase miR-378 expression at short exposure times, but this effect is lost with increasing exposure duration [
28]. The discovery of the role of miR-142 in the modulation of TGF-β activity has prompted the study of the effects of changes in miR-142 expression on fibrosis processes. In recent years, evidence has emerged demonstrating antifibrotic effects of increased miR-142 expression in various tissues: in scleral fibroblasts [
20], in liver star cells (low miR-142 expression was associated with liver cirrhosis) [
23], in lungs [
29] and cardiomyocytes [
22]. Activation of oxidative stress and fibrosis was confirmed in our study by a significant increase in MPO, galectin-3, and PICP levels, and the relationship between miR-142 expression activity in SAT and fibrosis activity was confirmed by a negative correlation between miR-142 expression in SAT and circulating PICP levels. Our results are in agreement with those of Li et al. [
20], who demonstrated that overexpression of miR-142 in scleral fibroblasts reduces TGF-β1 and collagen I expression. A series of studies on pulmonary fibrosis showed the importance of miR determination in plasma, in the miR source organ, and in the target organ. For example, when miR-142-3p was assessed in plasma and saliva of patients with pulmonary fibrosis [
21,
30,
31], its expression was elevated, whereas low miR-142 expression was determined in lung tissue. In these studies, there is a clear analogy with our results, demonstrating decreased expression of miR-142 in SAT in obesity compared to healthy subjects and increased levels of miR-142 in plasma of obese patients.
Circulating miRs are valuable biomarkers of systemic diseases and potential therapeutic targets because of their ease of assessment. Several studies have examined the pattern of miRs in circulation in obesity and their changes after weight loss [
10]. Our findings regarding changes in miR-142 levels are similar to those of Ortega et al., who studied the circulating profile of miRs in 36 men with morbid obesity before and after bariatric treatment [
10]. As in our work, this study showed a marked increase in miR-142-3p (
p < 0.0001) in the circulation. At the same time, surgery-induced (but not diet-induced) weight loss led to a significant decrease in several other miRs but not miR-142 in the circulation. Importantly, this study also assessed levels of transforming growth factor receptor (TGFR), for which circulating levels were associated with those of miR-142-3p. The key targets for miR-142 identified in silico are the transforming growth factor (TGF) beta receptor 1 gene and the leukemia inhibitory factor-α (LIF-α) receptor gene. Members of the TGF-superfamily [
32], like LIF [
33], are known to regulate many aspects of adipocyte development and are involved in the development of obesity and the regulation of energy expenditure. In another study evaluating the circulatory profile of miRs in obese adolescents, there was also a significant increase in miR-142-3p [
32].
The authors of that study noted that miR-142 levels were closely related to body mass index (BMI), adiponectin, leptin, insulin, HOMA-IR, and lipid level in plasma. We did not observe such multiple relationships with metabolic parameters, which may be explained by the lower severity of obesity in the patients in our study and by the fact that some patients received hypolipidemic therapy and the obesity drug. Similarly, another study, also demonstrating increased levels of miR-142 in the circulation, did not find its association with levels of inflammatory markers (CRP, IL-6, TNF-a) in the circulation.
Thus, miR-142 showed consistently elevated levels in the circulation in obesity in multiple studies, which may reflect an attempt to counteract the pro-inflammatory and profibrotic status characterizing obesity [
32,
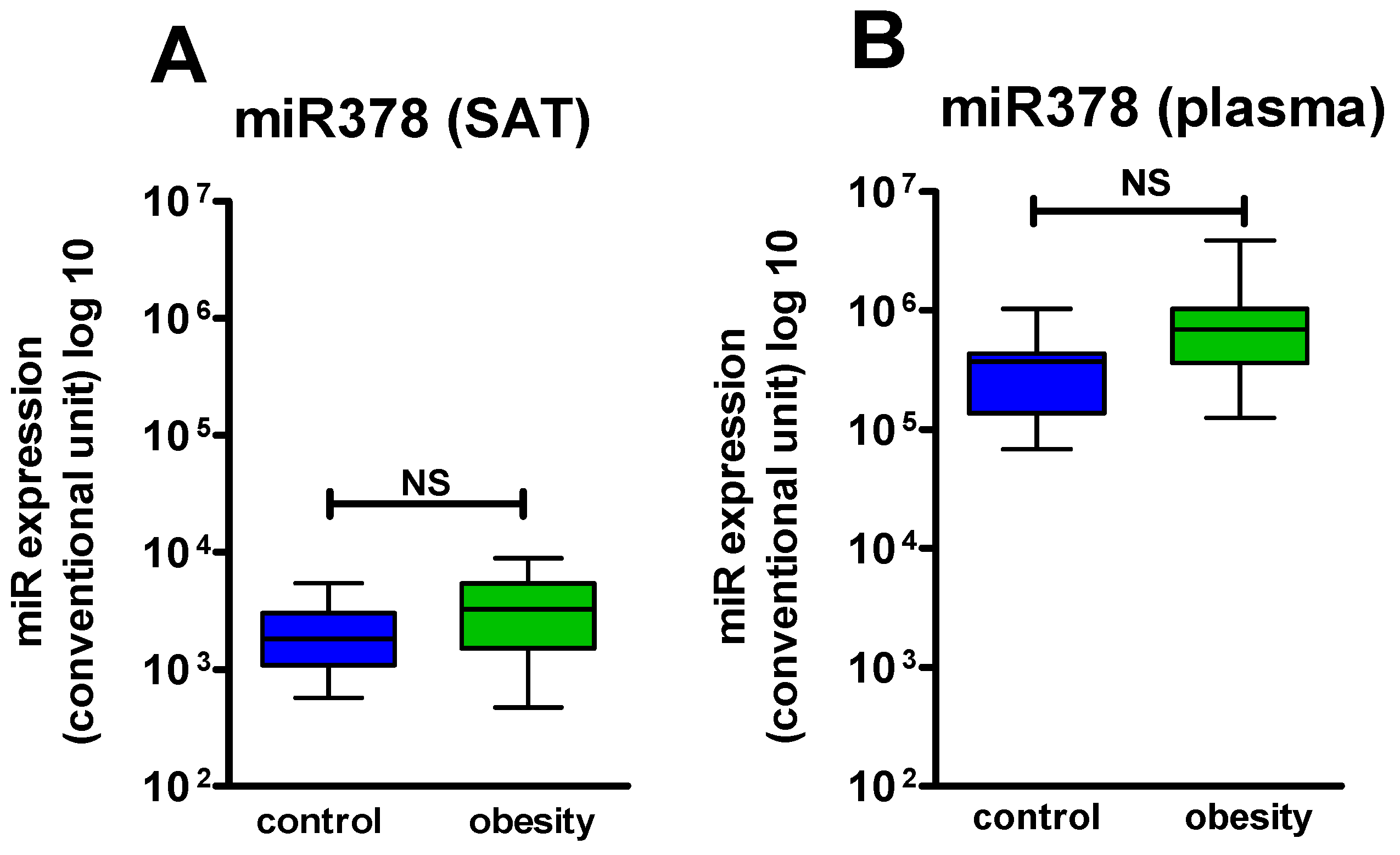
34]. In contrast, miR-378 demonstrated a clear concordance between the changes of expression in SAT and the level in the circulation, indicating that this miR is a good circulatory biomarker, reflecting changes in the activity of several biological processes (adipogenesis, browning, lipolysis) in SAT [
15,
18].
Thus, a feature of our study was the simultaneous assessment of the studied miRs in the SAT and circulation, which allowed us to clarify whether the change in miR levels in the circulation reflects dysregulation of their expression in the AT or systemic changes in obesity. Studies with simultaneous assessment of miRs in the circulation and AT are extremely scarce in the literature. This is supported by a recent meta-analysis [
33] that included 17 studies evaluating the dynamics of miR expression before and after surgical treatment of obesity. Only three of these evaluated miR expression in SAT and not a single one showed a parallel evaluation. This meta-analysis reported that both miR-142 and miR-378 were noted to be differentially expressed in plasma of obese and normal-weight individuals. But, while the results for the direction of change of miR-142 were consistent in the studies presented [
10,
35], decreasing in the postoperative period, the results for miR-378 were divergent. In a study by Alkandari et al. [
36] miR-378 was elevated in plasma before treatment and its level decreased after surgery, while in a study by Lirun et al. [
37], its level was decreased before surgery and increased after. We observed the dynamics of miR-378 levels in plasma similar to those of Lirun et al. [
37].
The next aspect of our study was to assess the dynamics of miR-378 and miR-142 during sibutramine therapy. Changes in miR expression can be a useful tool to clarify the mechanisms of drug action. Thus, Xue et al. [
28] showed a significant increase in miR-378 expression under the influence of rosiglitazone, demonstrating the involvement of the adipocyte differentiation and adipogenesis pathways regulated by it in the mechanisms of action of this drug. Given the known data on the action of sibutramine, including catecholaminergic effects, we expected a more pronounced change in miR-378 expression during therapy. We observed a significant increase in its level in the circulation but only an increasing trend in SAT, which may reflect the systemic action of the drug. Moreover, miR-378 overexpression is associated with the activation of adipogenesis of classical BAT [
18], localized mainly in the supraclavicular region rather than with the browning of SAT in the abdominal region, from where SAT was sampled. Classical BAT is extremely rare and sparsely represented in obese subjects and is unlikely to contribute to changes in circulatory levels of miR-378, which may partially explain our results. At the same time, the expression of miR-142 significantly increased during therapy in SAT but not in plasma, which may indicate the ability of sibutramine to reduce the activity of profibrotic genes and fibrogenesis processes in SAT. This may have important implications for the maintenance of SAT plasticity and metabolic health in obesity. The fact that the expression changes are associated specifically with sibutramine therapy rather than with BMI reduction during therapy is confirmed by the fact that there was no difference in the expression of the studied miRs after therapy in responders and non-responders.
Another interesting finding of our study was the differences in miR-378 and miR-142 expression in patients with good response to sibutramine therapy and in non-responders. Good response to therapy was characterized by those patients in whom miR-378 expression was not elevated (significantly lower than in non-responders), and in contrast, miR-142 expression had a marked decrease. These patients seem to have a point of application for therapy, whereas non-responders, who had no significant deviations in expression from healthy individuals’ parameters, did not. Attempts to use changes in miR expression to predict response to interventions have been made before. For example, in 2013, Milagro et al. studied the expression of miRs in peripheral blood leukocytes of 10 obese women, during an 8-week hypocaloric diet. Patients were divided into responders (>5% weight loss,
n = 5) and non-responders (<5% weight loss,
n = 5) [
38], the same as in our study. Differences in the expression of the five miRs before intervention between the two groups were found. In the non-responder group, miR-935 and miR-4772 were up-regulated, whereas miR-223, 224, and 376b were down-regulated [
38]. Marques-Rocha et al., studied the modulation of the expression of nine inflammation-related miRs in leukocytes in MS after an 8-week hypocaloric diet and found a significant change in two miRs after the intervention: miR-155-3p was strongly decreased and miR-7b was significantly up-regulated [
39]. Several studies have found differences in miR profiles depending on the type of diet [
40,
41]. In a study by Assmann et al., among the miRs that differed in circulation in obese patients and were significantly associated with response to a low-fat diet was miR-142 [
41], and in a study by Giardina et al., miR-378 was among the 13 miRs whose expression was reduced in responders compared with non-responders on both a low-carb and a low-fat diet [
40]. These data on the one hand confirm the importance of the mechanisms determined by these miRs for treatment efficacy but on the other hand require consideration of dietary characteristics. To exclude the influence of dietary characteristics on miR expression changes, all patients in our study were given the same type of dietary and physical activity recommendations, which were strictly monitored.
We found no data on prediction of response to drug therapy for obesity in the available literature. Drug therapy requires high-precision response prediction due to the fact that the fraction of responders undergoing therapy is about 60%, and the cost is quite high. Thus, according to Formichi et al., 19.2% of patients dropped out of the study of glucagon-like peptide 1 receptor agonists (GLP1ra) therapy because of side effects [
42]. According to our data in real clinical practice, 46.1% of patients fully received a 6-month course of sibutramine therapy (20.5% dropped out due to side effects, 33.4%—due to unsatisfactory price/effect ratio), and 48% of patients received GLP1ra liraglutide (16% of patients dropped out due to side effects, 36%—due to unsatisfactory price/effect ratio) [
43]. In a study by Formichi et al. [
42], as in our study, a block of miRs involved in metabolism processes, not AT but glucose and differing in expression levels in diabetes mellitus type 2 with obesity, was selected based on literature data and the miR dynamics in plasma during GLP1ra therapy was evaluated. Given that GLP1ra are also registered as drugs for the treatment of obesity, and considering the significant weight reduction according to this study (
p < 0.05), we present it as an example of an attempt to predict the response to drug therapy for weight loss based on the miRs’ profile. The study analyzed the dynamics of 8 miRs, including miR-378, in circulation, and showed that high baseline levels of miR-375 had the greatest predictive value for achieving a good glycemic effect. However, baseline levels of miR-378-3p also correlated with a reduction in HbA1c by 12 months relative to baseline (
p = 0.002). The effects on weight loss were analyzed separately. As in our study, responders were defined as those who reduced weight by 5% or more. Responders had significantly higher baseline levels of miR-15a-5p than non-responders (
p = 0.03). These results suggest the need to account for glycemic abnormalities when analyzing miR-378 changes. In the available literature, we did not find any studies on the dynamics of miR expression in SAT and plasma in obese patients treated with sibutramine or similar drugs (serotonin receptor agonists), which determines the novelty of our findings on miR-378 and miR-142 expression changes in such patients.
4. Materials and Methods
4.1. Clinical Characteristics of the Examined Patients, Study Design
This study included obese patients who met the following inclusion criteria:
Separate informed consent was signed for the SAT biopsy.
Taking into account catecholaminergic-associated adverse reactions to sibutramine, the inclusion criteria were cardiovascular diseases (ischemic heart disease, congestive heart failure, heart defects, acute myocardial infarction, acute stroke, arrhythmia, tachycardia), uncontrolled arterial hypertension, as well as individual intolerance reactions, anorexia or bulimia, thyrotoxicosis, liver or kidney failure, benign prostatic hyperplasia or glaucoma.
In accordance with these criteria, 64 patients with varying degrees of obesity were included in the study; however, due to the poor quality of the samples, 51 patients were included in the final analysis (BMI 37.3 ± 4.8 (33.8; 40.2) kg/m2, mean age 37.5 ± 10.8 years), and 10 healthy non-obese volunteers (BMI 22.4 ± 1.5 (21.0; 23.6) kg/m2), comparable by age (40.4 ± 9.5 years) and sex ratio, constituted the control group.
Informed consent to perform SAT biopsy was signed by 32 obese patients: the first SAT sampling (before therapy) was performed in all 32 patients, repeated biopsy (after 6 months of sibutramine therapy)—in 13 patients who followed all treatment recommendations for 6 months. Informed consent for SAT biopsy was signed by all 10 subjects from the control group, who also underwent SAT sampling.
The study included two visits, the first one (V1) before the start of sibutramine therapy and the second one (V2) 6 months after initiation of the therapy. At visit V1, after signing the informed consent, the patients underwent an examination that included taking an anamnesis, assessment of anthropometric parameters (weight, height, calculating BMI using the formula: body weight (kg)/height
2 (m)), assessment of the level of arterial BP (
Table 2).
To clarify the reasons for the expression dynamics of the studied miRs (weight loss or drug effect), we compared their dynamics in patients who lost weight (responders) and those who did not lose weight (non-responders). Responders were considered patients whose body weight loss reached 5% or more by the end of the third month of therapy, with subsequent maintenance or increase in weight loss by the sixth month of therapy.
Taking into account the possible influence of nutritional components on the miR profile [
44], after a basal examination, obese patients were given standardized lifestyle recommendations: moderate hypocaloric nutrition with a calorie deficit (300–500 kcal), balanced in the intake of carbohydrates, proteins and fats (carbohydrates 45–55%, proteins 15–20%, fats 20–35%) with the exclusion of easily digestible carbohydrates and the restriction of animal fats (no more than 10%) of total calories and a high intake of coarse fibers (30 g/day), dosed physical activity. Sibutramine was prescribed at a dosage of 10 mg/day (1 capsule 30 min before meals).
Of the laboratory parameters, the following were evaluated: glucose, lipid metabolism, levels of hormones involved in the regulation of fat metabolism (leptin, adiponectin, insulin), HOMA-IR, markers of inflammation (CRP), oxidative stress (MPO and PO-1) and fibrosis (PICP, PIIINP and galectin-3). A similar scope of examination was performed on V2.
4.2. Laboratory Methods
Biochemical parameters were assessed using a Cobas c311 automated biochemistry analyzer (Roche, Basel, Switzerland) and commercial kits (Roche reagent kits, Basel, Switzerland). Reference values for various biochemical parameters: fasting plasma glucose 3.30–6.10 mmol/L (measurement range 0.11–41.1 mmol/L); total cholesterol (TC) (measurement range 0.1–20.7 mmol/L, normal values 3.50–5.00 mmol/L); serum triglycerides (measurement range 0.1–10.0 mmol/L, normal values < 1.77 mmol/L); high-density lipoprotein cholesterol (HDLC) (measurement range 0.08–3.12 mmol/L, normal values for females > 1.2 mmol/L, for males > 1.0 mmol/L). Serum insulin levels were measured using a Cobas e411 fully automated immunochemical electrochemiluminescence analyzer (Roche, Basel, Switzerland) and commercial Cobas Insulin Elecsys kits (Roche, Basel, Switzerland) within a measuring range of 1.39–6945 IU/mL and a normal value of 17.8–173.0 pmol/L. The pmol/L to μU/mL conversion factor was 0.144
The HOMA index (insulin resistance index) was used to calculate the formula:
The levels of leptin in blood serum and adiponectin in blood plasma were assessed by enzyme immunoassay using an automatic analyzer (Bio-Rad 680—monometer, automated analyzer, Bio-Rad, Hercules, CA, USA). For adiponectin (commercial BioVendor kit, Brno, Czech Republic), measurement range 0.026–100 μg/mL, sensitivity coefficient 0.026 μg/mL, for leptin (commercial DBC kit for enzyme immunoassay (enzyme-linked immunosorbent assay)), measurement range from 2.0 to 11.0 ng/mL, sensitivity 0.5 ng/mL. Serum levels of galectin-3 (R&D system, Minneapolis, MN, USA), PICP (USCN Life Science, Wuhan, China), PIIINP (USCN Life Science, Wuhan, China) were assessed by enzyme immunoassay. The study of the level of myeloperoxidase (enzyme-linked immunosorbent assay) was performed with the Human Myeloperoxidase LotP203356 reagent kit, R&DSystems, Minneapolis, MN, USA ng/mL, PO-1 with a Paraoxonase 1 (PON1) reagent kit, LotL190801668, MyBioSource, MyBioSource, San Diego, CA, USA, μg/mL. CRP was assessed using an automatic analyzer (Cobas c311, Roche Diagnostics GmbH, Mannheim, Germany) and commercial kits (reagent kits, Roche, Basel, Switzerland).
4.3. Biopsy of Subcutaneous Adipose Tissue
Biopsy of subcutaneous adipose tissue was carried out by puncture of subcutaneous fat in the area of the anterior abdominal wall. The puncture was performed in the morning, in the fasting state, in the supine position using conventional injection needles according to the “free hand” method. Obtaining aspiration material was carried out without anesthesia, with preliminary treatment of the surgical field with 70% alcohol. The puncture was made in the umbilical region 2–3 cm to the right and below the navel, then the subcutaneous adipose tissue was aspirated with traction movements, with a total volume of not more than 2 mL. After that, for transportation to the biobanking site, the Eppendorfs were immediately placed in liquid nitrogen. Biosamples were stored at a temperature of −80 °C.
4.4. RNA Isolation and miR Quantification
Total RNA was isolated from adipose tissue biopsies and plasma using the ExtractRNA reagent for the isolation of total RNA from biological samples (Evrogen, cat. no. BC032, Moscow, Russia). Reverse transcription was performed using the TaqMan™ MicroRNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s recommendations. The reaction mixture (5 μL total volume) contained 2.33 μL master mix (0.05 μL dNTP Mix 100 mM; 0.33 μL Multi Scribe reverse transcriptase (50 U/μL); 0.063 μL RNase inhibitor 20 units/μL; 0.5 μL 10× RT buffer, 1.386 μL deionized water), 1.66 μL RNA solution and 1 μL miR-specific primer.
In our work, expression levels of the following miRs were analyzed: hsa-miR-378a-3p, hsa-miR-142-3p. The miR reverse-transcription reaction was performed using a 96-well Veriti thermal cycler (Applied Biosystems, Foster City, CA, USA), in which the samples were incubated at 16 °C for 30 min, then 42 °C for 30 min and 85 °C for 5 min.
MiR amplification by qPCR was performed using TaqMan Universal Master Mix II (Life Technologies, Carlsbad, CA, USA; cat. no. 4440040) and the following TaqMan MicroRNA Assays were used to detect microRNAs of interest, namely hsa-miR-378a-3p (Assay ID 000464) and hsa-miR-142a-3p (Assay ID 001314), according to the manufacturer’s recommendation.
To determine the operating range of the detection system for miR analysis, a standard curve was constructed (a plot of threshold cycles versus the logarithm of concentrations in a series of sample dilutions). Synthetic oligoribonucleotides (Synthol) with a known concentration, identical to the analyzed miR, were used to construct a standard curve.
4.5. Statistical Methods
Statistical analysis was performed using STATISTICA 10 (StatSoft Inc., Tulsa, OK, USA) for Windows. Data were presented as mean ± standard deviation. The distribution of the studied variables deviated from normal (normality test was calculated using the Kolmogorov–Smirnov criterion, dispersion was calculated using the Levene’s test). Due to the fact that the distribution in the groups for some parameters is unequal, the use of simple methods of calculation (Student’s t-criterion, etc.) is inappropriate. Therefore, we used non-parametric statistical methods to evaluate the studied groups (Mann–Whitney test was used to compare two independent samples with an interval scale, Wilcoxon t-criterion was used to evaluate the relationship between a factor and a dependent variable, to compare two dependent samples with each other by the level of expression of a feature). The power of the study for 50 patients was 0.8. The critical significance level (p) for testing statistical hypotheses when comparing statistical indicators was less than 0.05.