MAGED2 Depletion Promotes Stress-Induced Autophagy by Impairing the cAMP/PKA Pathway
Abstract
:1. Introduction
2. Results
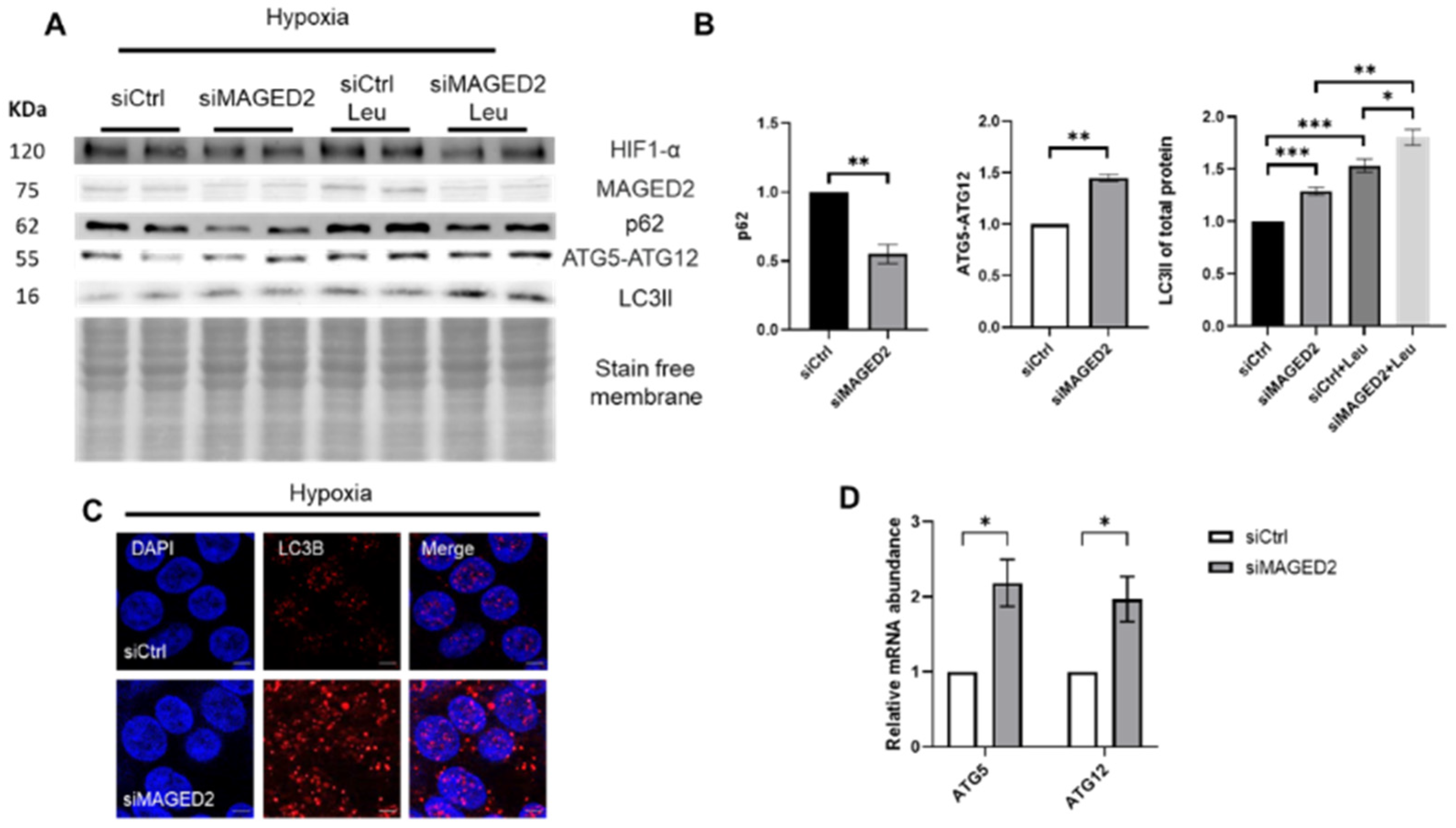
2.1. Induction of Autophagy upon MAGED2 Depletion under Hypoxia
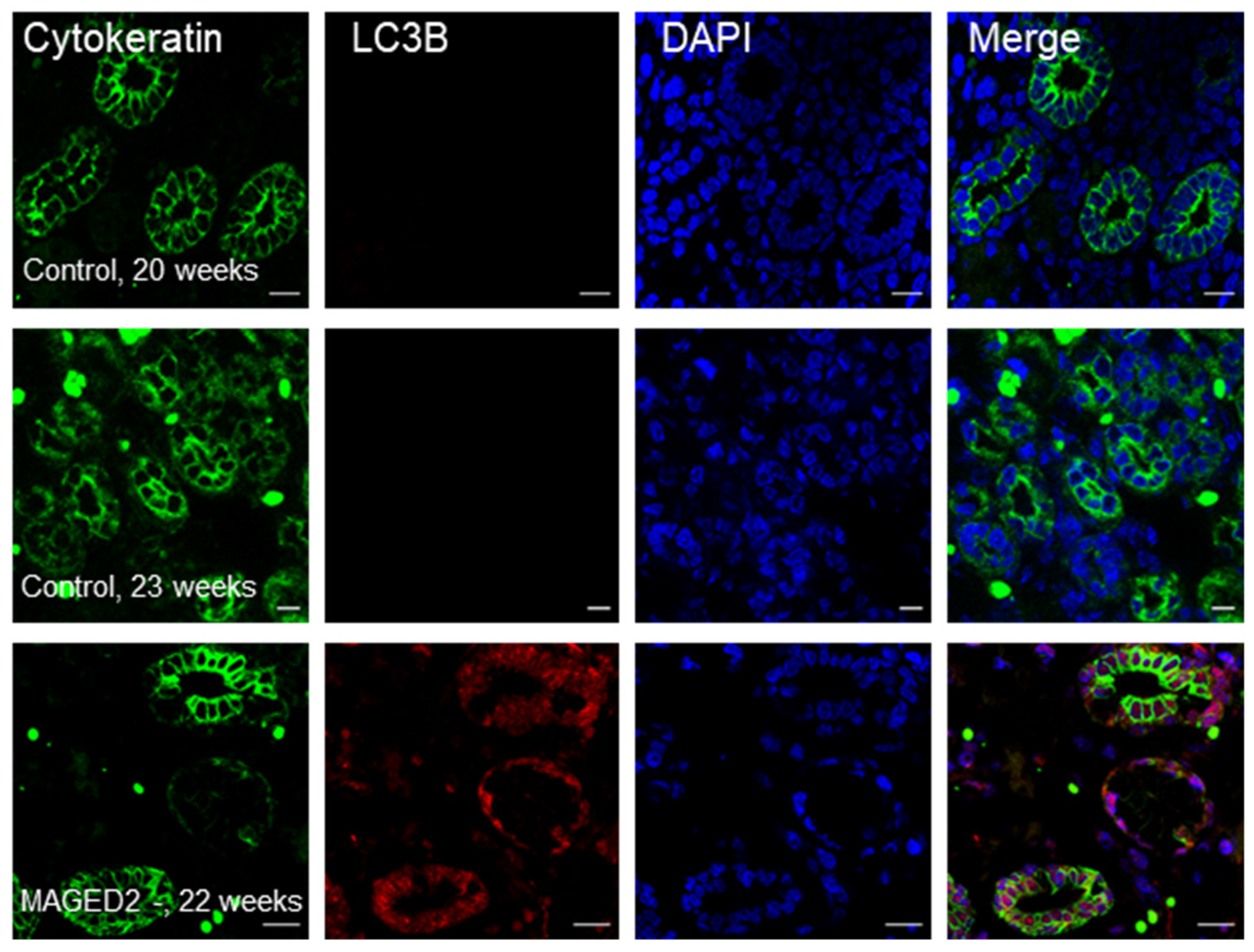
2.2. Increased Abundance of LC3B in a Fetal Kidney from a Patient with a Truncating Mutation in MAGED2
2.3. ER-Stressors Induce Autophagy upon MAGED2 Depletion
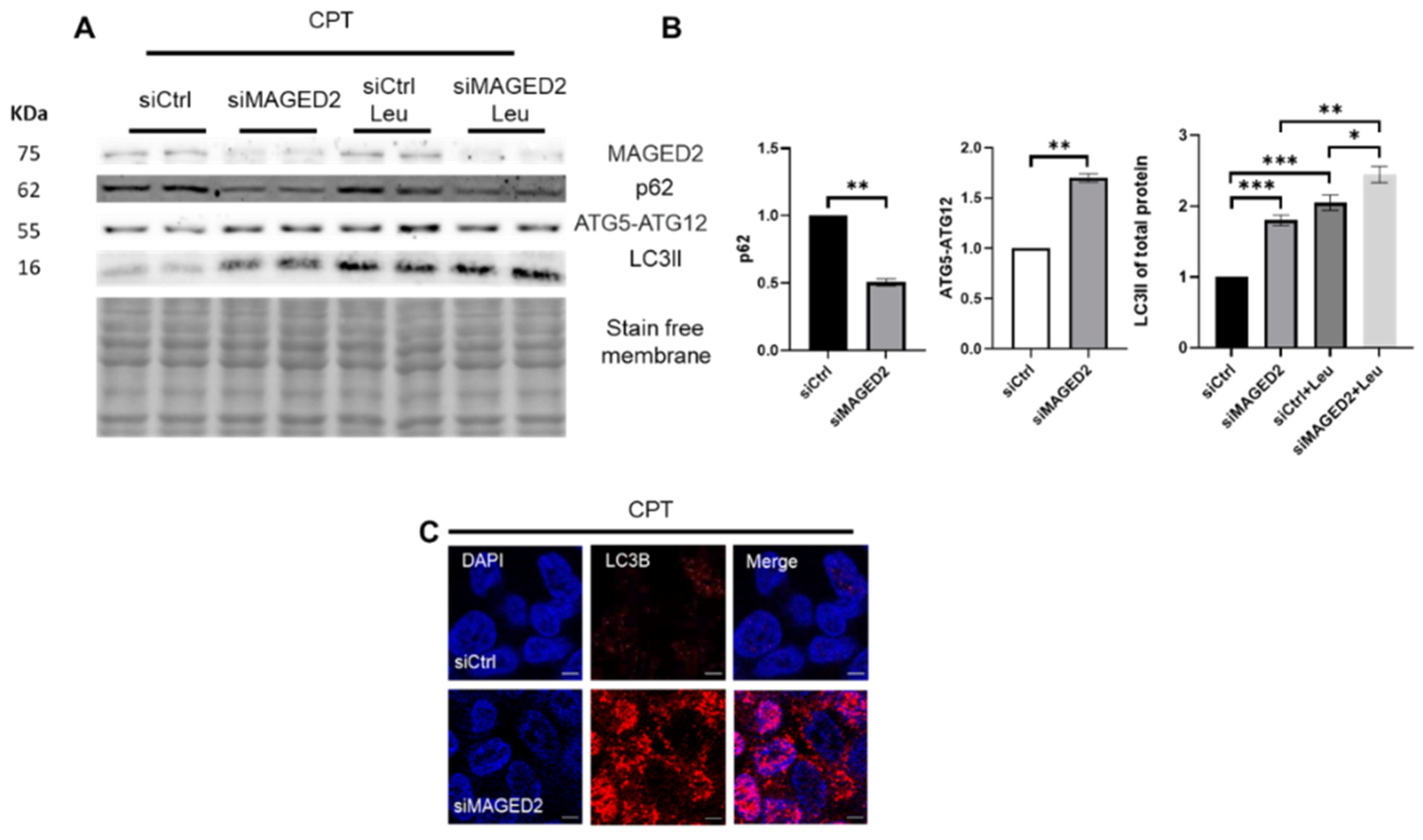
2.4. Genotoxic Stress Promotes Autophagy in MAGED2 Depleted Cells
2.5. Nutritional Stress Promotes Autophagy in MAGED2-Depleted Cells
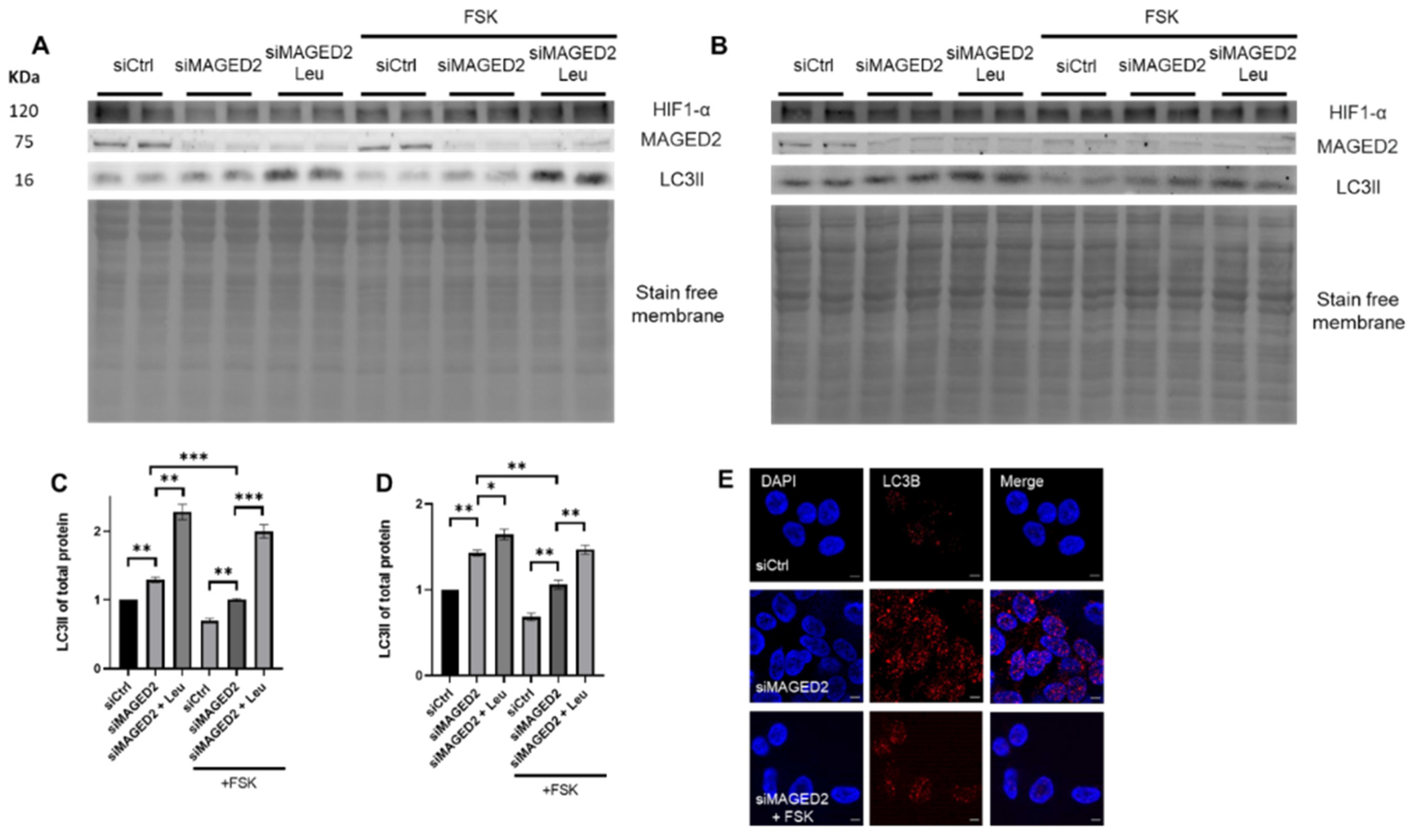
2.6. Activation of cAMP/PKA Pathway Reversed the Stress-Induced Autophagic Machinery upon MAGE2 Depletion
3. Discussion
4. Material and Methods
4.1. Cell Culture
4.2. siRNA (Small Interfering RNA) Knockdown
4.3. Establishment of Cellular Stress
4.4. Immunocytochemistry
4.5. Immunohistochemistry
4.6. Western Blotting
4.7. Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Komhoff, M.; Laghmani, K. MAGED2: A novel form of antenatal Bartter’s syndrome. Curr. Opin. Nephrol. Hypertens. 2018, 27, 323–328. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, E.M.; Jelin, A.C.; Miller, J.L.; Ruano, R.; Atkinson, M.A.; Baschat, A.A.; Jelin, E.B. Amnioinfusions to Treat Early Onset Anhydramnios Caused by Renal Anomalies: Background and Rationale for the Renal Anhydramnios Fetal Therapy Trial. Fetal Diagn. Ther. 2019, 45, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Laghmani, K.; Beck, B.B.; Yang, S.S.; Seaayfan, E.; Wenzel, A.; Reusch, B.; Vitzthum, H.; Priem, D.; Demaretz, S.; Bergmann, K.; et al. Polyhydramnios, Transient Antenatal Bartter’s Syndrome, and MAGED2 Mutations. N. Engl. J. Med. 2016, 374, 1853–1863. [Google Scholar] [CrossRef]
- Legrand, A.; Treard, C.; Roncelin, I.; Dreux, S.; Bertholet-Thomas, A.; Broux, F.; Bruno, D.; Decramer, S.; Deschenes, G.; Djeddi, D.; et al. Prevalence of Novel MAGED2 Mutations in Antenatal Bartter Syndrome. Clin. J. Am. Soc. Nephrol. 2018, 13, 242–250. [Google Scholar] [CrossRef]
- Seaayfan, E.; Nasrah, S.; Quell, L.; Radi, A.; Kleim, M.; Schermuly, R.T.; Weber, S.; Laghmani, K.; Kömhoff, M. Reciprocal Regulation of MAGED2 and HIF-1α Augments Their Expression under Hypoxia: Role of cAMP and PKA Type II. Cells 2022, 11, 3424. [Google Scholar] [PubMed]
- Seaayfan, E.; Nasrah, S.; Quell, L.; Kleim, M.; Weber, S.; Meyer, H.; Laghmani, K.; Kömhoff, M. MAGED2 Is Required under Hypoxia for cAMP Signaling by Inhibiting MDM2-Dependent Endocytosis of G-Alpha-S. Cells 2022, 11, 2546. [Google Scholar] [CrossRef]
- Fenton, R.A.; Knepper, M.A. Mouse models and the urinary concentrating mechanism in the new millennium. Physiol. Rev. 2007, 87, 1083–1112. [Google Scholar] [CrossRef]
- Ecelbarger, C.A.; Yu, S.; Lee, A.J.; Weinstein, L.S.; Knepper, M.A. Decreased renal Na-K-2Cl cotransporter abundance in mice with heterozygous disruption of the G(s)alpha gene. Am. J. Physiol. 1999, 277, F235–F244. [Google Scholar]
- Florke Gee, R.R.; Chen, H.; Lee, A.K.; Daly, C.A.; Wilander, B.A.; Fon Tacer, K.; Potts, P.R. Emerging roles of the MAGE protein family in stress response pathways. J. Biol. Chem. 2020, 295, 16121–16155. [Google Scholar] [CrossRef]
- De Donato, M.; Peters, S.O.; Hussain, T.; Rodulfo, H.; Thomas, B.N.; Babar, M.E.; Imumorin, I.G. Molecular evolution of type II MAGE genes from ancestral MAGED2 gene and their phylogenetic resolution of basal mammalian clades. Mamm. Genome 2017, 28, 443–454. [Google Scholar] [CrossRef]
- Doyle, J.M.; Gao, J.; Wang, J.; Yang, M.; Potts, P.R. MAGE-RING protein complexes comprise a family of E3 ubiquitin ligases. Mol. Cell 2010, 39, 963–974. [Google Scholar] [CrossRef]
- Lee, A.K.; Potts, P.R. A Comprehensive Guide to the MAGE Family of Ubiquitin Ligases. J. Mol. Biol. 2017, 429, 1114–1142. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Shabek, N. Ubiquitin Ligases: Structure, Function, and Regulation. Annu. Rev. Biochem. 2017, 86, 129–157. [Google Scholar] [CrossRef]
- Fon Tacer, K.; Montoya, M.C.; Oatley, M.J.; Lord, T.; Oatley, J.M.; Klein, J.; Ravichandran, R.; Tillman, H.; Kim, M.; Connelly, J.P.; et al. MAGE cancer-testis antigens protect the mammalian germline under environmental stress. Sci. Adv. 2019, 5, eaav4832. [Google Scholar] [CrossRef] [PubMed]
- Pineda, C.T.; Ramanathan, S.; Fon Tacer, K.; Weon, J.L.; Potts, M.B.; Ou, Y.H.; White, M.A.; Potts, P.R. Degradation of AMPK by a cancer-specific ubiquitin ligase. Cell 2015, 160, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, W.M.; Schmitt, R.; Rosenberger, C.; Munchenhagen, P.M.; Grone, H.J.; Frei, U.; Warnecke, C.; Bachmann, S.; Wiesener, M.S.; Willam, C.; et al. Expression of hypoxia-inducible transcription factors in developing human and rat kidneys. Kidney Int. 2006, 69, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Kömhoff, M.; Hollinshead, M.; Tooze, J.; Kern, H.F. Brefeldin A induced dose-dependent changes to Golgi structure and function in the rat exocrine pancreas. Eur. J. Cell Biol. 1994, 63, 192–207. [Google Scholar] [PubMed]
- Pommier, Y. Topoisomerase I inhibitors: Camptothecins and beyond. Nat. Rev. Cancer 2006, 6, 789–802. [Google Scholar] [CrossRef]
- Babitt, J.L.; Eisenga, M.F.; Haase, V.H.; Kshirsagar, A.V.; Levin, A.; Locatelli, F.; Malyszko, J.; Swinkels, D.W.; Tarng, D.C.; Cheung, M.; et al. Controversies in optimal anemia management: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Conference. Kidney Int. 2021, 99, 1280–1295. [Google Scholar] [CrossRef]
- Jayasooriya, R.; Dilshara, M.G.; Karunarathne, W.; Molagoda, I.M.N.; Choi, Y.H.; Kim, G.Y. Camptothecin enhances c-Myc-mediated endoplasmic reticulum stress and leads to autophagy by activating Ca(2+)-mediated AMPK. Food Chem. Toxicol. 2018, 121, 648–656. [Google Scholar] [CrossRef]
- Prasad Tharanga Jayasooriya, R.G.; Dilshara, M.G.; Neelaka Molagoda, I.M.; Park, C.; Park, S.R.; Lee, S.; Choi, Y.H.; Kim, G.Y. Camptothecin induces G(2)/M phase arrest through the ATM-Chk2-Cdc25C axis as a result of autophagy-induced cytoprotection: Implications of reactive oxygen species. Oncotarget 2018, 9, 21744–21757. [Google Scholar] [CrossRef] [PubMed]
- Wick, A.N.; Drury, D.R.; Nakada, H.I.; Wolfe, J.B. Localization of the primary metabolic block produced by 2-deoxyglucose. J. Biol. Chem. 1957, 224, 963–969. [Google Scholar] [CrossRef]
- Chen, W.; Guéron, M. The inhibition of bovine heart hexokinase by 2-deoxy-D-glucose-6-phosphate: Characterization by 31P NMR and metabolic implications. Biochimie 1992, 74, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Kurtoglu, M.; Gao, N.; Shang, J.; Maher, J.C.; Lehrman, M.A.; Wangpaichitr, M.; Savaraj, N.; Lane, A.N.; Lampidis, T.J. Under normoxia, 2-deoxy-D-glucose elicits cell death in select tumor types not by inhibition of glycolysis but by interfering with N-linked glycosylation. Mol. Cancer Ther. 2007, 6, 3049–3058. [Google Scholar] [CrossRef] [PubMed]
- Budovskaya, Y.V.; Stephan, J.S.; Reggiori, F.; Klionsky, D.J.; Herman, P.K. The Ras/cAMP-dependent protein kinase signaling pathway regulates an early step of the autophagy process in Saccharomyces cerevisiae. J. Biol. Chem. 2004, 279, 20663–20671. [Google Scholar] [CrossRef]
- Palorini, R.; Votta, G.; Pirola, Y.; De Vitto, H.; De Palma, S.; Airoldi, C.; Vasso, M.; Ricciardiello, F.; Lombardi, P.P.; Cirulli, C.; et al. Protein Kinase A Activation Promotes Cancer Cell Resistance to Glucose Starvation and Anoikis. PLoS Genet. 2016, 12, e1005931. [Google Scholar] [CrossRef] [PubMed]
- Jewell, J.L.; Fu, V.; Hong, A.W.; Yu, F.X.; Meng, D.; Melick, C.H.; Wang, H.; Lam, W.M.; Yuan, H.X.; Taylor, S.S.; et al. GPCR signaling inhibits mTORC1 via PKA phosphorylation of Raptor. Elife 2019, 8, e43038. [Google Scholar] [CrossRef]
- Cherra, S.J., 3rd; Kulich, S.M.; Uechi, G.; Balasubramani, M.; Mountzouris, J.; Day, B.W.; Chu, C.T. Regulation of the autophagy protein LC3 by phosphorylation. J. Cell Biol. 2010, 190, 533–539. [Google Scholar] [CrossRef]
- Xie, J.; Ponuwei, G.A.; Moore, C.E.; Willars, G.B.; Tee, A.R.; Herbert, T.P. cAMP inhibits mammalian target of rapamycin complex-1 and -2 (mTORC1 and 2) by promoting complex dissociation and inhibiting mTOR kinase activity. Cell Signal 2011, 23, 1927–1935. [Google Scholar] [CrossRef]
- Grisan, F.; Iannucci, L.F.; Surdo, N.C.; Gerbino, A.; Zanin, S.; Di Benedetto, G.; Pozzan, T.; Lefkimmiatis, K. PKA compartmentalization links cAMP signaling and autophagy. Cell Death Differ. 2021, 28, 2436–2449. [Google Scholar] [CrossRef]
- Zhang, T.; Dong, K.; Liang, W.; Xu, D.; Xia, H.; Geng, J.; Najafov, A.; Liu, M.; Li, Y.; Han, X.; et al. G-protein-coupled receptors regulate autophagy by ZBTB16-mediated ubiquitination and proteasomal degradation of Atg14L. Elife 2015, 4, e06734. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, N.; Wu, J.; Zhen, N.; Li, N.; Li, Y.; Li, Y.X. Silencing of NRAGE induces autophagy via AMPK/Ulk1/Atg13 signaling pathway in NSCLC cells. Tumour Biol. 2017, 39, 1010428317709676. [Google Scholar] [CrossRef] [PubMed]
- Kanda, M.; Nomoto, S.; Oya, H.; Takami, H.; Shimizu, D.; Hibino, S.; Hashimoto, R.; Kobayashi, D.; Tanaka, C.; Yamada, S.; et al. The Expression of Melanoma-Associated Antigen D2 Both in Surgically Resected and Serum Samples Serves as Clinically Relevant Biomarker of Gastric Cancer Progression. Ann. Surg. Oncol. 2015, 23, 214–221. [Google Scholar] [CrossRef]
- Chung, F.Y.; Cheng, T.L.; Chang, H.J.; Chiu, H.H.; Huang, M.Y.; Chang, M.S.; Chen, C.C.; Yang, M.J.; Wang, J.Y.; Lin, S.R. Differential gene expression profile of MAGE family in taiwanese patients with colorectal cancer. J. Surg. Oncol. 2010, 102, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.R.; Chong, I.W.; Chen, Y.H.; Yang, M.J.; Sheu, C.C.; Chang, H.C.; Hwang, J.J.; Hung, J.Y.; Lin, S.R. Differential expression profile of MAGE family in non-small-cell lung cancer. Lung Cancer 2007, 56, 185–192. [Google Scholar] [CrossRef]
- Kidd, M.; Modlin, I.M.; Mane, S.M.; Camp, R.L.; Eick, G.; Latich, I. The role of genetic markers--NAP1L1, MAGE-D2, and MTA1--in defining small-intestinal carcinoid neoplasia. Ann. Surg. Oncol. 2006, 13, 253–262. [Google Scholar] [CrossRef]
- Hartleben, B.; Gödel, M.; Meyer-Schwesinger, C.; Liu, S.; Ulrich, T.; Köbler, S.; Wiech, T.; Grahammer, F.; Arnold, S.J.; Lindenmeyer, M.T.; et al. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J. Clin. Investig. 2010, 120, 1084–1096. [Google Scholar] [CrossRef]
- Liu, S.; Hartleben, B.; Kretz, O.; Wiech, T.; Igarashi, P.; Mizushima, N.; Walz, G.; Huber, T.B. Autophagy plays a critical role in kidney tubule maintenance, aging and ischemia-reperfusion injury. Autophagy 2012, 8, 826–837. [Google Scholar] [CrossRef]
- Tang, C.; Livingston, M.J.; Liu, Z.; Dong, Z. Autophagy in kidney homeostasis and disease. Nat. Rev. Nephrol. 2020, 16, 489–508. [Google Scholar] [CrossRef]
- Rosenbaek, L.L.; Rizzo, F.; Wu, Q.; Rojas-Vega, L.; Gamba, G.; MacAulay, N.; Staub, O.; Fenton, R.A. The thiazide sensitive sodium chloride co-transporter NCC is modulated by site-specific ubiquitylation. Sci. Rep. 2017, 7, 12981. [Google Scholar] [CrossRef]
- Lee, J.W.; Chou, C.L.; Knepper, M.A. Deep Sequencing in Microdissected Renal Tubules Identifies Nephron Segment-Specific Transcriptomes. J. Am. Soc. Nephrol. 2015, 26, 2669–2677. [Google Scholar] [CrossRef] [PubMed]
- Valiño-Rivas, L.; Cuarental, L.; Agustin, M.; Husi, H.; Cannata-Ortiz, P.; Sanz, A.B.; Mischak, H.; Ortiz, A.; Sanchez-Niño, M.D. MAGE genes in the kidney: Identification of MAGED2 as upregulated during kidney injury and in stressed tubular cells. Nephrol. Dial. Transplant. 2019, 34, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Wollner, C.; Kurth, T.; Bukowy, J.D.; Cowley, A.W., Jr. Inhibition of Mammalian Target of Rapamycin Complex 1 Attenuates Salt-Induced Hypertension and Kidney Injury in Dahl Salt-Sensitive Rats. Hypertension 2017, 70, 813–821. [Google Scholar] [CrossRef]
- Grahammer, F.; Haenisch, N.; Steinhardt, F.; Sandner, L.; Roerden, M.; Arnold, F.; Cordts, T.; Wanner, N.; Reichardt, W.; Kerjaschki, D.; et al. mTORC1 maintains renal tubular homeostasis and is essential in response to ischemic stress. Proc. Natl. Acad. Sci. USA 2014, 111, E2817–E2826. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Taylor, S.C.; Posch, A. The design of a quantitative western blot experiment. BioMed Res. Int. 2014, 2014, 361590. [Google Scholar] [CrossRef]





| Reagent or Resource | Source | Identifier |
|---|---|---|
| Antibodies | ||
| Anti-MAGED2 rabbit raised against this peptide (QVQENQDTRPKVKAK) | Eurogentec (Cologne, Germany) | |
| Anti-LC3B | Thermo Fisher Scientific (Dreieich, Germany) | PA1-46286 |
| Cytokeratin | Agilent | M0821 |
| ATG5 Antibody | Santa Cruz Biotechnology | sc-133158 |
| P62 Antibody | Santa Cruz Biotechnology | sc-28359 |
| Goat anti-rabbit IgG (H + L), Alexa Fluor Plus 555 | Thermo Fisher Scientific (Dreieich, Germany) | A32732 |
| Goat anti-rabbit IgG (H + L), Alexa Fluor Plus 555 | Thermo Fisher Scientific (Dreieich, Germany) | A32732 |
| StarBright Blue 520 Goat Anti-Rabbit IgG | Bio-rad (Feldkirchen, Germany) | 12005869 |
| StarBright Blue 700 Goat Anti-Mouse IgG | Bio-rad (Feldkirchen, Germany) | 12004158 |
| Chemical stressors | ||
| Cobalt(II) chloride hexahydrate | Sigma Aldrich (Schnelldorf, Germany) | C8661 |
| Tunicamycin | Cayman Chemical (Ann Arbor, MI, USA) | 11445 |
| Camptothecin | Cayman Chemical (Ann Arbor, MI, USA) | 11694 |
| 2-deoxy-D-glucose | TCI (Eschborn, Germany) | D0051 |
| Brefeldin A | Sigma Aldrich (Schnelldorf, Germany) | B6542 |
| Transfection | ||
| DhramaFECT4 | Dharmacon (Cambridge, UK) | T-2004-03 |
| Critical Commercial Assays | ||
| SingleShot Cell Lysis Kit | Bio-rad (Feldkirchen, Germany) | 1725080 |
| iScript Advanced cDNA Synthesis Kit for RT-qPCR | Bio-rad (Feldkirchen, Germany) | 1725038 |
| SsoAdvanced Universal SYBR Green Supermix | Bio-rad (Feldkirchen, Germany) | 1725271 |
| Experimental Models: Cell Lines | ||
| HEK293 | ATCC | CRL1573 |
| HeLa | Gift from Dr. Vijay Renigunta | |
| U2OS | Gift from Prof. Thorsten Stiewe, ZIT, Philipps-University, Marburg | |
| Oligonucleotides | ||
| ON-TARGETplus Non-targeting Control Pool | Dharmacon (Cambridge, UK) | D-001810-10-05 |
| UGGUUUACAUGUCGACUAA | ||
| UGGUUUACAUGUUGUGUGA | ||
| UGGUUUACAUGUUUUCUGA | ||
| UGGUUUACAUGUUUUCCUA | ||
| ON-TARGETplus Human MAGED2 siRNA—SMARTpool | Dharmacon (Cambridge, UK) | L-017284-01-0005 |
| GGACGAAGCUGAUAUCGGA | ||
| GCUAAAGACCAGACGAAGA | ||
| AGGCGAUGGAAGCGGAUUU | ||
| GAAAAGGACAGUAGCUCGA | ||
| ON-TARGETplus Human GNAS siRNA—SMARTpool | Dharmacon (Cambridge, UK) | L-010825-00-0005 |
| GCAAGUGGAUCCAGUGCUU | ||
| GCAUGCACCUUCGUCAGUA | ||
| AUGAGGAUCCUGCAUGUUA | ||
| CAACCAAAGUGCAGGACAU | ||
| GAPD, Human GAPDH, Real-Time PCR Primer Set | Biomol (Hamburg, Germany) | VHPS-3541 |
| TTTTGGCTAAAGACCAGACG | ||
| AATAGCCTGCTCGTTCAATG | ||
| ATG5 | Sigma-Aldric (Schnelldorf, Germany) | |
| AAAGATGTGCTTCGAGATGTGT | ||
| CACTTTGTCAGTTACCAACGTCA | ||
| ATG12 | Sigma-Aldrich (Schnelldorf, Germany) | |
| CTGCTGGCGACACCAAGAAA | ||
| CGTGTTCGCTCTACTGCCC | ||
| Software and Algorithms | ||
| ImageJ | Schneider et al., 2012 [46] | https://imagej.nih.gov/ij/, accessed on 22 July 2022 |
| GraphPad Prism 9 | GraphPad | |
| EndNote X9 | Clarivate Analytics | |
| BioRender | https://www.biorender.com/, accessed on 22 August 2023 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasrah, S.; Radi, A.; Daberkow, J.K.; Hummler, H.; Weber, S.; Seaayfan, E.; Kömhoff, M. MAGED2 Depletion Promotes Stress-Induced Autophagy by Impairing the cAMP/PKA Pathway. Int. J. Mol. Sci. 2023, 24, 13433. https://doi.org/10.3390/ijms241713433
Nasrah S, Radi A, Daberkow JK, Hummler H, Weber S, Seaayfan E, Kömhoff M. MAGED2 Depletion Promotes Stress-Induced Autophagy by Impairing the cAMP/PKA Pathway. International Journal of Molecular Sciences. 2023; 24(17):13433. https://doi.org/10.3390/ijms241713433
Chicago/Turabian StyleNasrah, Sadiq, Aline Radi, Johanna K. Daberkow, Helmut Hummler, Stefanie Weber, Elie Seaayfan, and Martin Kömhoff. 2023. "MAGED2 Depletion Promotes Stress-Induced Autophagy by Impairing the cAMP/PKA Pathway" International Journal of Molecular Sciences 24, no. 17: 13433. https://doi.org/10.3390/ijms241713433





