Novel Insights into the Molecular Mechanisms of Atherosclerosis
Abstract
1. Introduction
Atherosclerosis
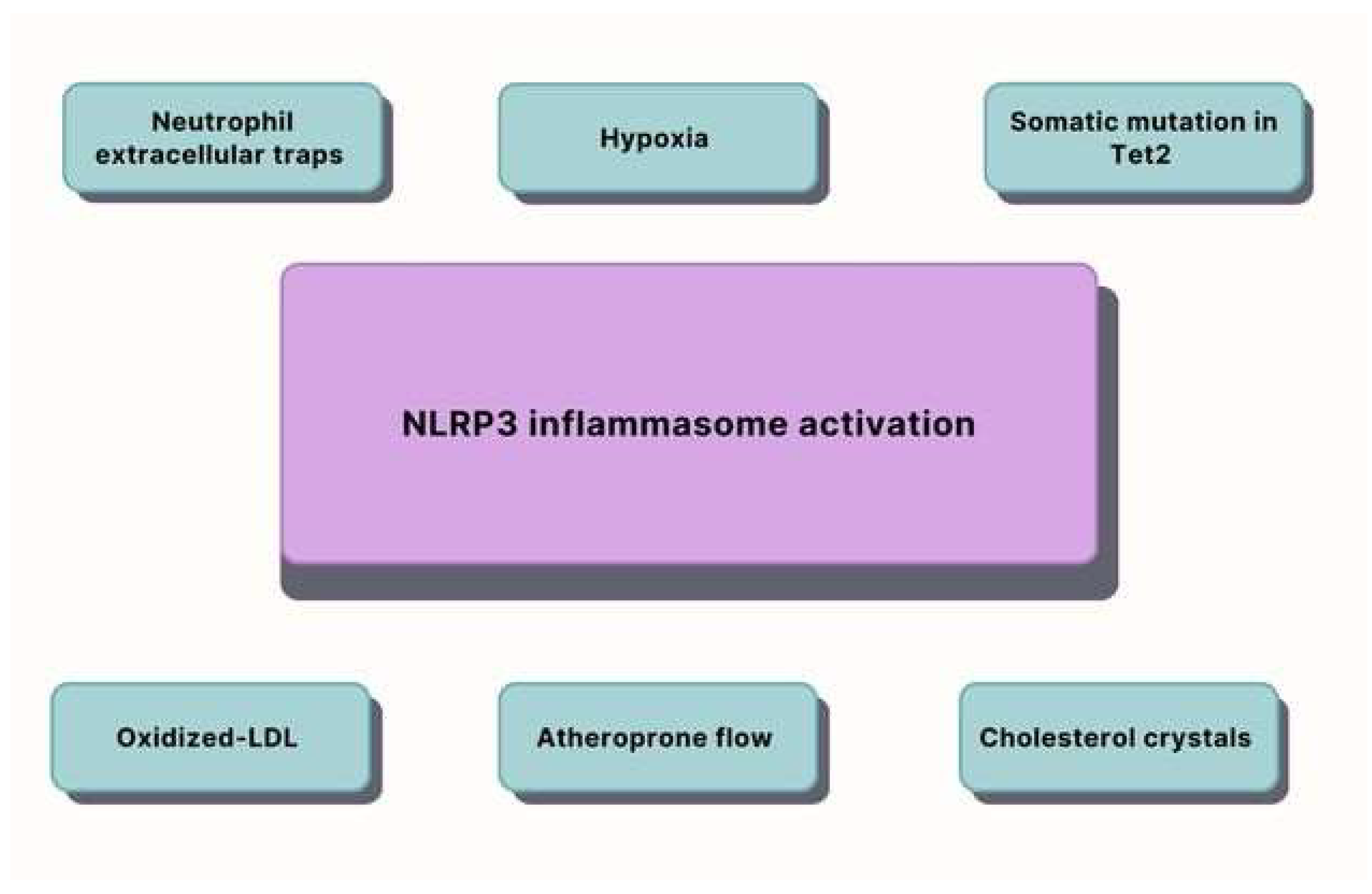
2. Inflammation
3. Aging
- Age increases the aortic plaque burden and the size and severity of the aortic root plaques in the AD-fed ApoE mice independent of the number of weeks on the diet. However, there was no effect from age on the size or severity of oscillatory shear-induced carotid artery atheromas after PCL.
- Treatment with PCSK9 increased the total and LDL cholesterol in the young mice, but the older mice had a larger aortic root atheroma size and morphology grade. Age did not affect the size or degree of the carotid artery lesions in the mice overexpressing PCK9 after PCL. Atherosclerotic plaques will expand spontaneously in the atheroprone regions of the aorta of the AD-fed mice after PCSK9 overexpression by an AAV. The transgenic ApoE mice also overexpressed human PCSK9, demonstrating larger atherosclerotic lesions with greater monocyte infiltration compared with the PCSK9 wildtype ApoE mice [57].
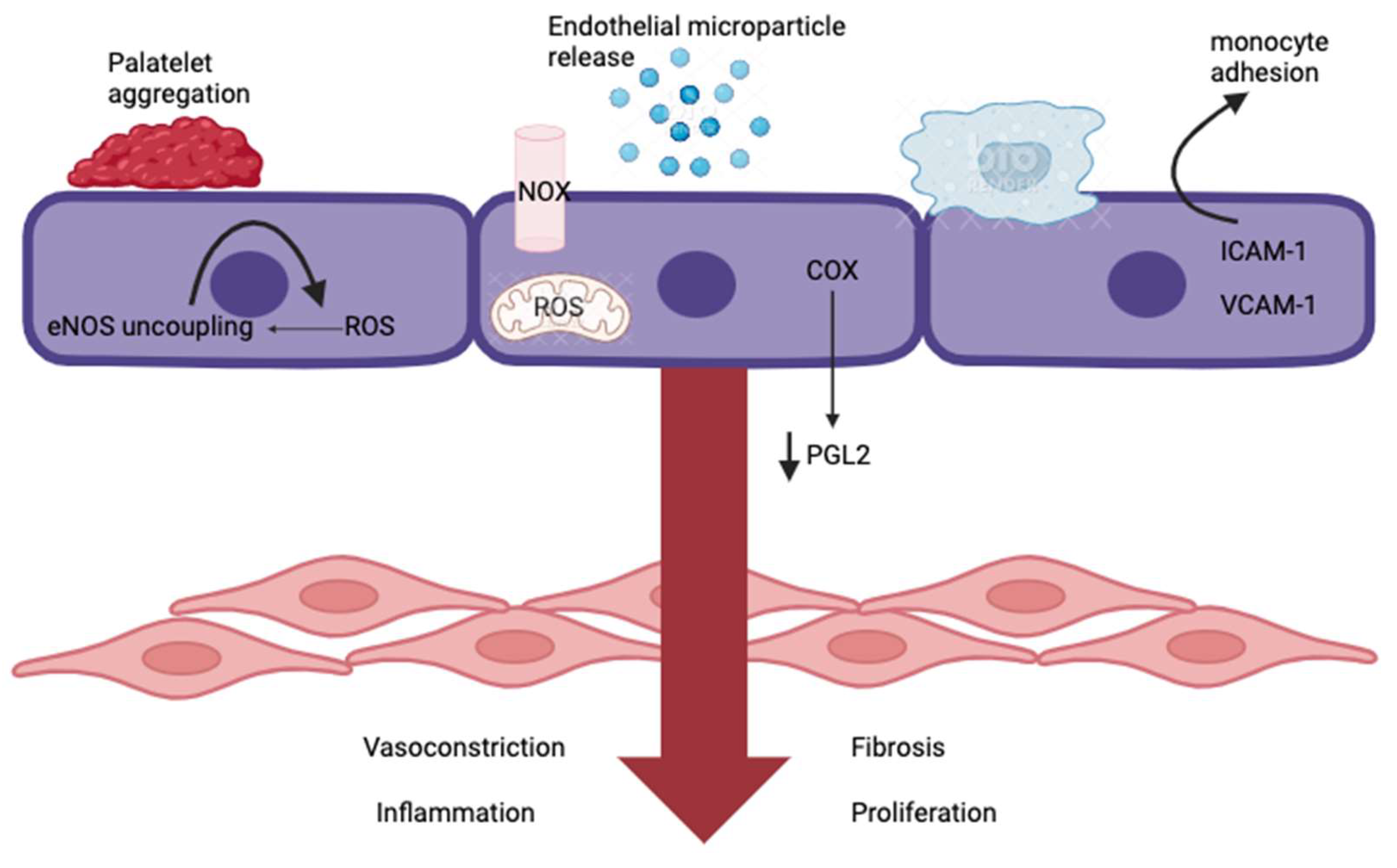
4. Endothelial Dysfunction
5. Uric Acid
6. Vitamin D
6.1. Vitamin D and Its Metabolism
6.2. Vitamin D and Its Impact on Atherosclerosis
7. miRNA Expression
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CVD | cardiovascular disease |
| UA | uric acid |
| Vit. D | vitamin D |
| 1,25(OH)2D | calcitriol, 1,25-dihydroxyvitamin D |
| miRNA | microRNA |
| ACS | acute coronary syndrome |
| CKD | chronic kidney disease |
| LDL | low-density lipoproteins |
| CVDs | cardiovascular diseases |
| CCs | cholesterol-soluble compounds |
| NETs | neutrophil extracellular traps |
| Tet2 | ten-eleven translocation 2 |
| HDL | high-density lipoprotein |
| oxLDLs | oxidized low-density lipoproteins |
| ROS | reactive oxygen species |
| TNFα | tumor necrosis factor α |
| VCAM-1 | vascular cell adhesion molecule-1 |
| RNS | reactive nitrogen species |
| LDL-C | LDL-cholesterol |
| CHIP | clonal hematopoiesis of indeterminate potential |
| VSMCs | vascular smooth muscle cells |
| PCSK9 | proprotein convertase subtilisin/kexin type 9 |
| LDLRs | low-density lipoprotein receptors |
| AAV | adeno-associated virus |
| PCL | partial carotid ligation |
| ACKR3 | atypical chemokine receptor 3 |
| NO | nitrogen oxide |
| RAAS | renin–angiotensin–aldosterone system |
| AngII | angiotensin II |
| XO | xanthine oxidase |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NRF2 | nuclear factor erythroid 2-related factor 2 |
| 25OHD | 25-hydroxyvitamin D |
| VDR | intracellular vitamin D receptor |
| RCT | randomized controlled trial |
| miRNAs | microRNAs |
| MRN A | messenger RNA |
| Sirt1 | silent information regulator |
| pHLIP | pH low-insertion peptides |
References
- WHO. Global Status Report on Noncommunicable Diseases 2010; Alwan, A., Ed.; World Health Organization: Geneva, Switzerland, 2010; pp. 1–176. [Google Scholar]
- Rahman, M.S.; Woollard, K. Atherosclerosis. In The Immunology of Cardiovascular Homeostasis and Pathology; Springer: Cham, Switzerland, 2017; pp. 121–144. [Google Scholar] [CrossRef]
- Pedro-Botet, J.; Climent, E.; Benaiges, D. Atherosclerosis and inflammation. New therapeutic approaches. Med. Clin. 2020, 155, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Andrews, E.S.; Perrenoud, L.; Nowak, K.L.; You, Z.; Pasch, A.; Chonchol, M.; Kendrick, J.; Jalal, D. Examining the effects of uric acid-lowering on markers vascular of calcification and CKD-MBD.; A. post-hoc analysis of a randomized clinical trial. PLoS ONE 2018, 13, e0205831. [Google Scholar] [CrossRef]
- Saghir Afifeh, A.M.; Verdoia, M.; Nardin, M.; Negro, F.; Viglione, F.; Rolla, R.; De Luca, G. Novara Atherosclerosis Study Group (NAS). Determinants of vitamin D activation in patients with acute coronary syndromes and its correlation with inflammatory markers. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Magni, P. The sex-associated burden of atherosclerotic cardiovascular diseases: An update on prevention strategies. Mech. Ageing Dev. 2023, 212, 111805. [Google Scholar] [CrossRef] [PubMed]
- Golub, I.S.; Termeie, O.G.; Kristo, S.; Schroeder, L.P.; Lakshmanan, S.; Shafter, A.M.; Hussein, L.; Verghese, D.; Aldana-Bitar, J.; Manubolu, V.S.; et al. Major global coronary artery calciumguidelines. Cardiovasc. Imaging 2023, 16, 98–117. [Google Scholar]
- Boren, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Ginsberg, H.N.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef]
- Chen, G.; Farris, M.S.; Cowling, T.; Pinto, L.; Rogoza, R.M.; MacKinnon, E.; Champsi, S.; Anderson, T.J. Prevalence of atherosclerotic cardiovascular disease and subsequent major adverse cardiovascular events in Alberta, Canada: A real-world evidence study. Clin. Cardiol. 2021, 44, 1613–1620. [Google Scholar] [CrossRef]
- Lindh, M.; Banefelt, J.; Fox, K.M.; Hallberg, S.; Tai, M.H.; Eriksson, M.; Villa, G.; Svensson, M.K.; Qian, Y. Cardiovascular event rates in a high atherosclerotic cardiovascular disease risk population: Estimates from Swedish population-based register data. Eur. Heart J. Qual. Care Clin. Outcomes 2019, 5, 225–232. [Google Scholar] [CrossRef]
- World Health Organization. Cardiovascular Diseases (CVDs) Fact Sheet. Available online: http://www.who.int/mediacentre/factsheets/fs317/en/ (accessed on 12 April 2023).
- Frostegård, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013, 11, 117. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2023 Update: A Report from the American Heart Association. Circulation 2023, 147, e93–e621, Erratum in: Circulation 2023, 147, e622. [Google Scholar] [CrossRef] [PubMed]
- Jurgens, C.Y.; Lee, C.S.; Aycock, D.M.; Masterson Creber, R.; Denfeld, Q.E.; DeVon, H.A.; Evers, L.R.; Jung, M.; Pucciarelli, G.; Streur, M.M.; et al. American Heart Association Council on Cardiovascular and Stroke Nursing; Council on Hypertension; and Stroke Council. State of the Science: The Relevance of Symptoms in Cardiovascular Disease and Research: A Scientific Statement From the American Heart Association. Circulation 2022, 146, e173–e184. [Google Scholar] [CrossRef] [PubMed]
- DeVon, H.A.; Burke, L.A.; Vuckovic, K.M.; Haugland, T.; Eckhardt, A.L.; Patmon, F.; Rosenfeld, A.G. Symptoms suggestive of acute coronary syndrome: When is sex important? J. Cardiovasc. Nurs. 2017, 32, 383–392. [Google Scholar] [CrossRef]
- Jurgens, C.Y.; Lee, C.S.; Riegel, B. Psychometric analysis of the heart failure somatic perception scale as a measure of patient symptom perception. J. Cardiovasc. Nurs. 2017, 32, 140–147. [Google Scholar] [CrossRef]
- Alpert, C.M.; Smith, M.A.; Hummel, S.L.; Hummel, E.K. Symptom burden in heart failure: Assessment, impact on outcomes, and management. Heart Fail. Rev. 2017, 22, 25–39. [Google Scholar] [CrossRef]
- Berglund, A.; Svensson, L.; Wahlgren, N.; von Euler, M.; HASTA Collaborators. Face Arm Speech Time Test use in the prehospital setting, better in the ambulance than in the emergency medical communication center. Cerebrovasc. Dis. 2014, 37, 212–216. [Google Scholar] [CrossRef]
- Kobiyama, K.; Ley, K. Atherosclerosis. Circ. Res. 2018, 123, 1118–1120. [Google Scholar] [CrossRef]
- Valdivielso, J.M.; Rodríguez-Puyol, D.; Pascual, J.; Barrios, C.; Bermúdez-López, M.; Sánchez-Niño, M.D.; Pérez-Fernández, M.; Ortiz, A. Atherosclerosis in Chronic Kidney Disease: More, Less, or Just Different? Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1938–1966. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus–Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef]
- Lechner, K.; von Schacky, C.; McKenzie, A.L.; Worm, N.; Nixdorff, U.; Lechner, B.; Kränkel, N.; Halle, M.; Krauss, R.M.; Scherr, J. Lifestyle factors and high-risk atherosclerosis: Pathways and mechanisms beyond traditional risk factors. Eur. J. Prev. Cardiol. 2020, 27, 394–406. [Google Scholar] [CrossRef]
- Key, T.J.; Appleby, P.N.; Bradbury, K.E.; Sweeting, M.; Wood, A.; Johansson, I.; Kühn, T.; Steur, M.; Weiderpass, E.; Wennberg, M.; et al. Consumption of Meat, Fish, Dairy Products, and Eggs and Risk of Ischemic Heart Disease. Circulation 2019, 139, 2835–2845. [Google Scholar] [CrossRef] [PubMed]
- Al-Shaar, L.; Satija, A.; Wang, D.D.; Rimm, E.B.; Smith-Warner, S.A.; Stampfer, M.J.; Hu, F.B.; Willett, W.C. Red meat intake and risk of coronary heart disease among US men: Prospective cohort study. BMJ 2020, 371, m4141. [Google Scholar] [CrossRef]
- Zhang, B.; Xiong, K.; Cai, J.; Ma, A. Fish Consumption and Coronary Heart Disease: A Meta-Analysis. Nutrients 2020, 12, 2278. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.L. Effects of eggs on plasma lipoproteins in healthy populations. Food Funct. 2010, 1, 156–160. [Google Scholar] [CrossRef]
- Paoli, A.; Tinsley, G.; Bianco, A.; Moro, T. The Influence of Meal Frequency and Timing on Health in Humans: The Role of Fasting. Nutrients 2019, 11, 719. [Google Scholar] [CrossRef] [PubMed]
- Pem, D.; Jeewon, R. Fruit and Vegetable Intake: Benefits and Progress of Nutrition Education Interventions- Narrative Review Article. Iran J. Public Health 2015, 44, 1309–1321. [Google Scholar] [PubMed]
- Bouchard, J.; Malalgoda, M.; Storsley, J.; Malunga, L.; Netticadan, T.; Thandapilly, S.J. Health Benefits of Cereal Grain- and Pulse-Derived Proteins. Molecules 2022, 27, 3746. [Google Scholar] [CrossRef]
- Soedamah-Muthu, S.S.; Ding, E.L.; Al-delaimy, W.K.; Hu, F.B.; Engberink, M.F.; Willett, W.C.; Geleijnse, J.M. Milk and dairy consumption and incidence of cardiovascular diseases and all-cause mortality: Dose-response meta-analysis of prospective cohort studies. Am. J. Clin. Nutr. 2011, 93, 158–171. [Google Scholar] [CrossRef]
- Tindall, A.M.; Kris-Etherton, P.M.; Petersen, K.S. Replacing Saturated Fats with Unsaturated Fats from Walnuts or Vegetable Oils Lowers Atherogenic Lipoprotein Classes Without Increasing Lipoprotein(a). J. Nutr. 2020, 150, 818–825. [Google Scholar] [CrossRef]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.P.; Hu, F.B. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation 2010, 121, 1356–1364. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Sodium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Tahan, A.C.; Tahan, V. Alcohol is not Safe even at Light Amounts. Balkan Med. J. 2015, 32, 239. [Google Scholar] [CrossRef]
- Franklin, B.A.; Eijsvogels, T.M.H.; Pandey, A.; Quindry, J.; Toth, P.P. Physical activity, cardiorespiratory fitness, and cardiovascular health: A clinical practice statement of the ASPC Part I: Bioenergetics, contemporary physical activity recommendations, benefits, risks, extreme exercise regimens, potential maladaptations. Am. J. Prev. Cardiol. 2022, 12, 100424. [Google Scholar] [CrossRef]
- Kong, P.; Cui, Z.Y.; Huang, X.F.; Zhang, D.D.; Guo, R.J.; Han, M. Inflammation and atherosclerosis: Signaling pathways and therapeutic intervention. Signal Transduct. Target Ther. 2022, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Grebe, A.; Hoss, F.; Latz, E. NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ. Res. 2018, 122, 1722–1740. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 1, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Xie, W.L.; Kong, W.W.; Chen, D.; Qu, P. Expression of the NLRP3 inflammasome in carotid atherosclerosis. J. Stroke Cerebrovasc. Dis. 2015, 24, 2455–2466. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Fu, J. Novel Insights Into the NLRP 3 Inflammasome in Atherosclerosis. J. Am. Heart Assoc. 2019, 8, e012219. [Google Scholar] [CrossRef]
- Heughan, C.; Niinikoski, J.; Hunt, T.K. Oxygen tensions in lesions of experimental atherosclerosis of rabbits. Atherosclerosis 1973, 17, 361–367. [Google Scholar] [CrossRef]
- Bostrom, P.; Magnusson, B.; Svensson, P.A.; Wiklund, O.; Boren, J.; Carlsson, L.M.; Stahlman, M.; Olofsson, S.O.; Hulten, L.M. Hypoxia converts human macrophages into triglyceride-loaded foam cells. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1871–1876. [Google Scholar] [CrossRef]
- Folco, E.J.; Sukhova, G.K.; Quillard, T.; Libby, P. Moderate hypoxia potentiates interleukin-1beta production in activated human macrophages. Circ. Res. 2014, 115, 875–883. [Google Scholar] [CrossRef]
- McKenzie, B.A.; Mamik, M.K.; Saito, L.B.; Boghozian, R.; Monaco, M.C.; Major, E.O.; Lu, J.-Q.; Branton, W.G. Caspase-1 Inhibition Prevents Glial Inflammasome Activation and Pyroptosis in Models of Multiple Sclerosis. Proc. Natl. Acad. Sci. USA 2018, 115, E6065–E6074. [Google Scholar] [CrossRef]
- Klopf, J.; Brostjan, C.; Eilenberg, W.; Neumayer, C. Neutrophil Extracellular Traps and Their Implications in Cardiovascular and Inflammatory Disease. Int. J. Mol. Sci. 2021, 22, 559. [Google Scholar] [CrossRef]
- Malech, H.L.; DeLeo, F.R.; Quinn, M.T. The Role of Neutrophils in the Immune System: An Overview. Methods Mol. Biol. 2020, 2087, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A.; Libby, P.; Soehnlein, O.; Aramburu, I.V.; Papayannopoulos, V.; Silvestre-Roig, C. Neutrophil extracellular traps: From physiology to pathology. Cardiovasc. Res. 2022, 118, 2737–2753. [Google Scholar] [CrossRef] [PubMed]
- Hasler, P.; Giaglis, S.; Hahn, S. Neutrophil extracellular traps in health and disease. Swiss. Med. Wkly. 2016, 146, w14352. [Google Scholar] [CrossRef]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxid. Med. Cell Longev. 2019, 2019, 8563845. [Google Scholar] [CrossRef] [PubMed]
- Bäck, M.; Yurdagul, A., Jr.; Tabas, I.; Öörni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [PubMed]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Tyrrell, D.J.; Blin, M.G.; Song, J.; Wood, S.C.; Zhang, M.; Beard, D.A.; Goldstein, D.R. Age-Associated Mitochondrial Dysfunction Accelerates Atherogenesis. Circ. Res. 2020, 126, 298–314. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Simon, A.; Van Der Meer, J.W.M. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 2012, 11, 633–652. [Google Scholar] [CrossRef]
- Burger, F.; Baptista, D.; Roth, A.; da Silva, R.F.; Montecucco, F.; Mach, F.; Brandt, K.J.; Miteva, K. NLRP3 Inflammasome Activation Controls Vascular Smooth Muscle Cells Phenotypic Switch in Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 340. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Gardner, S.E.; Clarke, M.C. Cell death, damage-associated molecular patterns, and sterile inflammation in cardiovascular disease. Arter. Thromb Vasc Biol. 2011, 31, 2781–2786. [Google Scholar] [CrossRef] [PubMed]
- Gogulamudi, V.R.; Durrant, J.R.; Adeyemo, A.O.; Ho, H.M.; Walker, A.E.; Lesniewski, L.A. Advancing age increases the size and severity of spontaneous atheromas in mouse models of atherosclerosis. Geroscience 2023, 45, 1913–1931. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sano, S.; Yura, Y.; Ke, Z.; Sano, M.; Oshima, K.; Ogawa, H.; Horitani, K.; Min, K.-D.; Miura-Yura, E.; et al. Tet2-mediated clonal hematopoiesis in nonconditioned mice accelerates age-associated cardiac dysfunction. JCI Insight 2020, 5, e135204. [Google Scholar] [CrossRef]
- Jaiswal, S.; Natarajan, P.; Silver, A.J.; Gibson, C.J.; Bick, A.G.; Shvartz, E.; McConkey, M.; Gupta, N.; Gabriel, S.; Ardissino, D.; et al. Clonal hematopoiesis risk of atherosclerotic cardiovascular disease. N. Engl. J. Med. 2017, 377, 111–121. [Google Scholar] [CrossRef]
- Fuster, J.J.; MacLauchlan, S.; Zuriaga, M.A.; Polackal, M.N.; Ostriker, A.C.; Chakraborty, R.; Wu, C.L.; Sano, S.; Muralidharan, S.; Rius, C.; et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 2017, 355, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Bick, A.G.; Pirruccello, J.P.; Griffin, G.K.; Gupta, N.; Gabriel, S.; Saleheen, D.; Libby, P.; Kathiresan, S.; Natarajan, P. Genetic interleukin 6 signaling deficiency attenuates cardiovascular risk in clonal hematopoiesis. Circulation 2020, 141, 124–131. [Google Scholar] [CrossRef]
- Sano, S.; Oshima, K.; Wang, Y.; Katanasaka, Y.; Sano, M.; Walsh, K. CRISPR-Mediated Gene Editing to Assess the Roles of Tet2 and Dnmt3a in Clonal Hematopoiesis and Cardiovascular Disease. Circ. Res. 2018, 123, 335–341. [Google Scholar] [CrossRef]
- Du, W.; Wong, C.; Song, Y.; Shen, H.; Mori, D.; Rotllan, N.; Price, N.; Dobrian, A.D.; Meng, H.; Kleinstein, S.H.; et al. Age-associated vascular inflammation promotes monocytosis during atherogenesis. Aging Cell 2016, 15, 766–777. [Google Scholar] [CrossRef]
- Yu, E.P.K.; Reinhold, J.; Yu, H.; Starks, L.; Uryga, A.K.; Foote, K.; Finigan, A.; Figg, N.; Pung, Y.F.; Logan, A.; et al. Mitochondrial Respiration Is Reduced in Atherosclerosis, Promoting Necrotic Core Formation and Reducing Relative Fibrous Cap Thickness. Arterioscler. Thromb. Vasc. Biol. 2018, 37, 2322–2332, Erratum in Arterioscler. Thromb. Vasc. Biol. 2018, 38, e135. [Google Scholar] [CrossRef]
- Swiader, A.; Nahapetyan, H.; Faccini, J.; D’Angelo, R.; Mucher, E.; Elbaz, M.; Boya, P.; Vindis, C. Mitophagy acts as a safeguard mechanism against human vascular smooth muscle cell apoptosis induced by atherogenic lipids. Oncotarget 2016, 7, 28821–28835. [Google Scholar] [CrossRef]
- Walton, T.A.; Nishtar, S.; Lumb, P.J.; Crook, M.A.; Marber, M.S.; Gill, J.; Wierzbicki, A.S. Pro-protein convertase subtilisin/kexin 9 concentrations correlate with coronary artery disease atheroma burden in a Pakistani cohort with chronic chest pain. Int. J. Clin. Pract. 2015, 69, 738–742. [Google Scholar]
- Melendez, Q.M.; Krishnaji, S.T.; Wooten, C.J.; Lopez, D. Hypercholesterolemia: The role of PCSK9. Arch. Biochem. Biophys. 2017, 625–626, 39–53. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef] [PubMed]
- Mussbacher, M.; Schossleitner, K.; Kral-Pointner, J.B.; Salzmann, M.; Schrammel, A.; Schmid, J.A. More than Just a Monolayer: The Multifaceted Role of Endothelial Cells in the Pathophysiology of Atherosclerosis. Curr. Atheroscler. Rep. 2022, 24, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Gan, Z.; Li, Y.; Zhao, W.; Li, H.; Zheng, J.P.; Ke, Y. REM sleep deprivation induces endothelial dysfunction and hypertension in middle-aged rats: Roles of the eNOS/NO/cGMP pathway and supplementation with L-arginine. PLoS ONE 2017, 12, e0182746. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Patofizjologia Miażdżycy i Choroby Niedokrwiennej Serca/Red. Nauk. Andrzej Beręsewicz; Centrum Medyczne Kształcenia Podyplomowego w Warszawie: Warszawa, Poland, 2011; pp. 187–189.
- Gimbrone, M.A., Jr.; García-Cardeña, G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc. Pathol. 2013, 22, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Gencer, S.; Döring, Y.; Jansen, Y.; Bayasgalan, S.; Yan, Y.; Bianchini, M.; Cimen, I.; Müller, M.; Peters, L.J.F.; Megens, R.T.A.; et al. Endothelial ACKR3 drives atherosclerosis by promoting immune cell adhesion to vascular endothelium. Basic Res. Cardiol. 2022, 117, 30. [Google Scholar] [CrossRef] [PubMed]
- Krams, R.; Bäck, M. The ESC Textbook of Vascular Biology; Oxford University Press: Oxford, UK, 2017; pp. 2017–2103. [Google Scholar]
- Godo, S.; Shimokawa, H. Endothelial Functions. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e108–e114. [Google Scholar] [CrossRef]
- Dimitrievska, S.; Gui, L.; Weyers, A.; Lin, T.; Cai, C.; Wu, W.; Tuggle, C.T.; Sundaram, S.; Balestrini, J.L.; Slattery, D.; et al. New Functional Tools for Antithrombogenic Activity Assessment of Live Surface Glycocalyx. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1847–1853. [Google Scholar] [CrossRef] [PubMed]
- Dzau, V.J. Pathobiology of atherosclerosis and plaque complications. Am. Heart J. 1994, 128 Pt 2, 1300–1304. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Dong, Y.; Somers, V.K.; Peterson, T.E.; Zhang, Y.; Wang, S.; Li, G.; Singh, P. Intermittent hypoxia regulates vasoactive molecules and alters insulin-signaling in vascular endothelial cells. Sci. Rep. 2018, 8, 14110. [Google Scholar] [CrossRef] [PubMed]
- Horio, E.; Kadomatsu, T.; Miyata, K.; Arai, Y.; Hosokawa, K.; Doi, Y.; Ninomiya, T.; Horiguchi, H.; Endo, M.; Tabata, M.; et al. Role of endothelial cell-derived angptl2 in vascular inflammation leading to endothelial dysfunction and atherosclerosis progression. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Bobryshev, Y.V.; Ivanova, E.A.; Chistiakov, D.A.; Nikiforov, N.G.; Orekhov, A.N. Macrophages and Their Role in Atherosclerosis: Pathophysiology and Transcriptome Analysis. Biomed. Res. Int. 2016, 2016, 9582430. [Google Scholar] [CrossRef]
- Hunt, K.J.; Baker, N.L.; Cleary, P.A.; Klein, R.; Virella, G.; Lopes-Virella, M.F. DCCT/EDIC Group of Investigators. Longitudinal Association Between Endothelial Dysfunction, Inflammation, and Clotting Biomarkers With Subclinical Atherosclerosis in Type 1 Diabetes: An Evaluation of the DCCT/EDIC Cohort. Diabetes Care 2015, 38, 1281–1289. [Google Scholar] [CrossRef]
- Wang, B.; Tang, X.; Yao, L.; Wang, Y.; Chen, Z.; Li, M.; Wu, N.; Wu, D.; Dai, X.; Jiang, H.; et al. Disruption of USP9X in macrophages promotes foam cell formation and atherosclerosis. J. Clin. Investig. 2022, 132, e154217. [Google Scholar] [CrossRef]
- Hooglugt, A.; Klatt, O.; Huveneers, S. Vascular stiffening and endothelial dysfunction in atherosclerosis. Curr. Opin. Lipidol. 2022, 33, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef]
- Qu, K.; Yan, F.; Qin, X.; Zhang, K.; He, W.; Dong, M.; Wu, G. Mitochondrial dysfunction in vascular endothelium and its role in atherosclerosis. Front. Physiol. 2022, 13, 1084604. [Google Scholar] [CrossRef]
- Ciccarelli, G.; Conte, S.; Cimmino, G.; Maiorano, P.; Morrione, A.; Giordano, A. Mitochondrial Dysfunction: The Hidden Player in the Pathogenesis of Atherosclerosis? Int. J. Mol. Sci. 2023, 24, 1086. [Google Scholar] [CrossRef]
- Waheed, Y.; Yang, F.; Sun, D. Role of asymptomatic hyperuricemia in the progression of chronic kidney disease and cardiovascular disease. Korean J. Intern. Med. 2021, 36, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.M.; Zhang, N.; Li, J.S.; Yang, Z.H.; Huang, X.L.; Yang, X.F. Purinergic receptors mediate endothelial dysfunction and participate in atherosclerosis. Purinergic Signal. 2023, 19, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Vera, O.D.; Wulff, H.; Braun, A.P. Endothelial KCa channels: Novel targets to reduce atherosclerosis-driven vascular dysfunction. Front. Pharmacol. 2023, 14, 1151244. [Google Scholar] [CrossRef]
- Yuan, D.; Chu, J.; Lin, H.; Zhu, G.; Qian, J.; Yu, Y.; Yao, T.; Ping, F.; Chen, F.; Liu, X. Mechanism of homocysteine-mediated endothelial injury and its consequences for atherosclerosis. Front. Cardiovasc. Med. 2023, 9, 1109445. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Shi, X.; Zhu, Z.; Hao, X.; Chen, L.; Cheng, S.; Foo, S.Y.; Wang, C. Mendelian randomization analysis of 37 clinical factors and coronary artery disease in East Asian and European populations. Genome Med. 2022, 14, 63. [Google Scholar] [CrossRef]
- Zuo, T.; Liu, X.; Jiang, L.; Mao, S.; Yin, X.; Guo, L. Hyperuricemia and coronary heart disease mortality: A meta-analysis of prospective cohort studies. BMC Cardiovasc. Disord. 2016, 16, 207. [Google Scholar] [CrossRef]
- Chiang, K.M.; Tsay, Y.C.; Vincent Ng, T.C.; Yang, H.C.; Huang, Y.T.; Chen, C.H.; Pan, W.H. Is Hyperuricemia, an Early-Onset Metabolic Disorder, Causally Associated with Cardiovascular Disease Events in Han Chinese? J. Clin. Med. 2019, 8, 1202. [Google Scholar] [CrossRef]
- Li, M.; Hu, X.; Fan, Y.; Li, K.; Zhang, X.; Hou, W.; Tang, Z. Hyperuricemia and the risk for coronary heart disease morbidity and mortality a systematic review and dose-response meta-analysis. Sci. Rep. 2016, 6, 19520. [Google Scholar] [CrossRef]
- Jayachandran, M.; Qu, S. Harnessing hyperuricemia to atherosclerosis and understanding its mechanistic dependence. Med. Res. Rev. 2021, 41, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Lu, Y.; Mei, M.; Peng, P.; Zhao, Y.; Fu, G.; Qiu, F.; Jin, C. Correlation Between Serum Uric Acid Levels and Coronary Plaque Characteristics on Optical Coherence Tomography. Int. Heart J. 2022, 63, 806–813. [Google Scholar] [CrossRef]
- Mello, F.M.; Bensenor, I.M.; Santos, I.S.; Bittencourt, M.S.; Lotufo, P.A.; Fuller, R. Serum Uric Acid Levels and Subclinical Atherosclerosis: Results From the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Curr. Probl. Cardiol. 2023, 48, 101525. [Google Scholar] [CrossRef] [PubMed]
- Kramer, F.; Voss, S.; Roessig, L.; Igl, B.W.; Butler, J.; Lam, C.S.; Maggioni, A.P.; Shah, S.J.; Pieske, B. Evaluation of high-sensitivity C-reactive protein and uric acid in vericiguat-treated patients with heart failure with reduced ejection fraction. Eur. J. Heart Fail. 2020, 22, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Demiray, A.; Afsar, B.; Covic, A.; Kuwabara, M.; Ferro, C.J.; Lanaspa, M.A.; Johnson, R.J.; Kanbay, M. The Role of Uric Acid in the Acute Myocardial Infarction: A Narrative Review. Angiology 2022, 73, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.L.; Pei, D.; Lue, K.H.; Chen, Y.L. Uric Acid Levels Can Predict Metabolic Syndrome and Hypertension in Adolescents: A 10-Year Longitudinal Study. PLoS ONE 2015, 10, e0143786. [Google Scholar] [CrossRef]
- Ohno, I. Relationship between hyperuricemia and chronic kidney disease. Nucleosides Nucleotides Nucleic Acids 2011, 30, 1039–1044. [Google Scholar] [CrossRef]
- Jukema, R.A.; de Winter, R.W.; van Diemen, P.A.; Driessen, R.S.; Danser, A.J.; Garrelds, I.M.; Raijmakers, P.R.; van de Ven, P.M.; Knaapen, P.; Danad, I.; et al. The relation of RAAS activity and endothelin-1 levels to coronary atherosclerotic burden and microvascular dysfunction in chest pain patients. Atherosclerosis 2022, 347, 47–54. [Google Scholar] [CrossRef]
- Li, K.; Li, K.; Yao, Q.; Shui, X.; Zheng, J.; He, Y.; Lei, W. The potential relationship of coronary artery disease and hyperuricemia: A cardiometabolic risk factor. Heliyon 2023, 9, e16097. [Google Scholar] [CrossRef]
- Russo, E.; Bussalino, E.; Macciò, L.; Verzola, D.; Saio, M.; Esposito, P.; Leoncini, G.; Pontremoli, R.; Viazzi, F. Non-Haemodynamic Mechanisms Underlying Hypertension-Associated Damage in Target Kidney Components. Int. J. Mol. Sci. 2023, 24, 9422. [Google Scholar] [CrossRef]
- Lu, J.; Sun, M.; Wu, X.; Yuan, X.; Liu, Z.; Qu, X.; Ji, X.; Merriman, T.R.; Li, C. Urate-lowering therapy alleviates atherosclerosis inflammatory response factors and neointimal lesions in a mouse model of induced carotid atherosclerosis. FEBS J. 2019, 286, 1346–1359. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Bakris, G.L.; Borghi, C.; Chonchol, M.B.; Feldman, D.; Lanaspa, M.A.; Merriman, T.R.; Moe, O.W.; Mount, D.B.; Sanchez Lozada, L.G.; et al. Hyperuricemia, Acute and Chronic Kidney Disease, Hypertension, and Cardiovascular Disease: Report of a Scientific Workshop Organized by the National Kidney Foundation. Am. J. Kidney Dis. 2018, 71, 851–865. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhang, X.L.; Fu, C.; Han, R.; Chen, W.; Lu, Y.; Ye, Z. Soluble uric acid increases NALP3 inflammasome and interleukin-1β expression in human primary renal proximal tubule epithelial cells through the Toll-like receptor 4-mediated pathway. Int. J. Mol. Med. 2015, 35, 1347–1354. [Google Scholar] [CrossRef]
- Polito, L.; Bortolotti, M.; Battelli, M.G.; Bolognesi, A. Xanthine oxidoreductase: A leading actor in cardiovascular disease drama. Redox Biol. 2021, 48, 102195. [Google Scholar] [CrossRef]
- Kotozaki, Y.; Satoh, M.; Nasu, T.; Tanno, K.; Tanaka, F.; Sasaki, M. Human Plasma Xanthine Oxidoreductase Activity in Cardiovascular Disease: Evidence from a Population-Based Study. Biomedicines 2023, 11, 754. [Google Scholar] [CrossRef]
- Kimura, Y.; Tsukui, D.; Kono, H. Uric Acid in Inflammation and the Pathogenesis of Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 12394. [Google Scholar] [CrossRef] [PubMed]
- Battelli, M.G.; Polito, L.; Bolognesi, A. Xanthine oxidoreductase in atherosclerosis pathogenesis: Not only oxidative stress. Atherosclerosis 2014, 237, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Sautin, Y.Y.; Nakagawa, T.; Zharikov, S.; Johnson, R.J. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am. J. Physiol. Cell Physiol. 2010, 293, C584–C596, Erratum in Am. J. Physiol. Cell Physiol. 2010, 299, C726. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Lozada, L.G.; Lanaspa, M.A.; Cristóbal-García, M.; García-Arroyo, F.; Soto, V.; Cruz-Robles, D.; Nakagawa, T.; Yu, M.A.; Kang, D.-H.; Johnson, R.J. Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron. Exp. Nephrol. 2012, 121, e71–e78. [Google Scholar] [CrossRef]
- Verzola, D.; Ratto, E.; Villaggio, B.; Parodi, E.L.; Pontremoli, R.; Garibotto, G.; Viazzi, F. Uric acid promotes apoptosis in human proximal tubule cells by oxidative stress and the activation of NADPH oxidase NOX 4. PLoS ONE 2014, 9, e115210. [Google Scholar] [CrossRef]
- Yan, M.; Chen, K.; He, L.; Li, S.; Huang, D.; Li, J. Uric Acid Induces Cardiomyocyte Apoptosis via Activation of Calpain-1 and Endoplasmic Reticulum Stress. Cell Physiol. Biochem. 2018, 45, 2122–2135. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, J.; Li, W.; Wang, C.; Li, G.; Zhou, Y.; Dong, H. Elevated serum uric acid was associated with pre-inflammatory state and impacted the role of HDL-C on carotid atherosclerosis. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Liu, W.; Xie, D.; Wang, Q.; Xu, C.; Zhao, H.; Lv, J.; He, F.; Chen, B.; Yamamoto, T.; et al. High Level of Uric Acid Promotes Atherosclerosis by Targeting NRF2-Mediated Autophagy Dysfunction and Ferroptosis. Oxid. Med. Cell Longev. 2022, 2022, 9304383. [Google Scholar] [CrossRef]
- O’Riordan, J.L.H.; Bijvoet, O.L.M. Rickets before the discovery of vitamin D. BoneKEy Rep. 2014, 3, 478. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Siddiqee, M.H.; Bhattacharjee, B.; Siddiqi, U.R.; MeshbahurRahman, M. High prevalence of vitamin D deficiency among the South Asian adults: A systematic review and meta-analysis. BMC Public Health 2021, 21, 1823. [Google Scholar] [CrossRef]
- Holick, M.F.; Biancuzzo, R.M.; Chen, T.C.; Klein, E.K.; Young, A.; Bibuld, D.; Reitz, R.; Salameh, W.; Ameri, A.; Tannenbaum, A.D. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J. Clin. Endocrinol. Metab. 2008, 93, 677–681. [Google Scholar] [CrossRef]
- Heaney, R.P.; Recker, R.R.; Grote, J.; Horst, R.L.; Armas, L.A.G. Vitamin D3 Is More Potent Than Vitamin D2 in Humans. J. Clin. Endocrinol. Metab. 2011, 96, E447–E452. [Google Scholar] [CrossRef]
- Houghton, L.A.; Vieth, R. The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am. J. Clin. Nutr. 2006, 84, 694–697. [Google Scholar] [CrossRef]
- Latic, N.; Erben, R.G. Vitamin D and Cardiovascular Disease, with Emphasis on Hypertension, Atherosclerosis, and Heart Failure. Int. J. Mol. Sci. 2020, 21, 6483. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Grundmann, S.M.; Schutkowski, A.; Schreier, B.; Rabe, S.; König, B.; Gekle, M.; Stangl, G.I. Vitamin D Receptor Deficiency Does Not Affect Blood Pressure and Heart Function. Front. Physiol. 2019, 10, 1118. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; David, V.; Quarles, L.D. Regulation and function of the FGF23/klotho endocrine pathways. Physiol. Rev. 2012, 92, 131–155. [Google Scholar] [CrossRef]
- Hsu, S.; Hoofnagle, A.N.; Gupta, D.K.; Gutierrez, O.M.; Peralta, C.A.; Shea, S.; Allen, N.B.; Burke, G.; Michos, E.D.; Ix, J.H.; et al. Race, Ancestry, and Vitamin D Metabolism: The Multi-Ethnic Study of Atherosclerosis. J. Clin. Endocrinol. Metab. 2020, 105, e4337–e4550. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Brannon, P.M.; Rosen, C.J.; Taylor, C.L. Vitamin D Deficiency—Is There Really a Pandemic? N. Engl. J. Med. 2016, 375, 1817–1820. [Google Scholar] [CrossRef]
- Scragg, R. Seasonality of Cardiovascular Disease Mortality and the Possible Protective Effect of Ultra-violet Radiation. Int. J. Epidemiol. 1981, 10, 337–341. [Google Scholar] [CrossRef]
- Carbone, F.; Liberale, L.; Libby, P.; Montecucco, F. Vitamin D in atherosclerosis and cardiovascular events. Eur. Heart J. 2023, 44, 2078–2094. [Google Scholar] [CrossRef]
- Kassi, E.; Adamopoulos, C.; Basdra, E.K.; Papavassiliou, A.G. Role of Vitamin D in Atherosclerosis. Circulation 2013, 128, 2517–2531. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.K.; Singh, H.V.; Raizada, A.; Singh, S.K. C-reactive protein, inflammation and coronary heart disease. Egypt Heart J. 2015, 67, 89–97. [Google Scholar] [CrossRef]
- Chen, S.; Swier, V.J.; Boosani, C.S.; Radwan, M.M.; Agrawal, D.K. Vitamin D Deficiency Accelerates Coronary Artery Disease Progression in Swine. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Surdu, A.M.; Pînzariu, O.; Ciobanu, D.M.; Negru, A.G.; Căinap, S.S.; Lazea, C.; Iacob, D.; Săraci, G.; Tirinescu, D.; Borda, I.M.; et al. Vitamin D and Its Role in the Lipid Metabolism and the Development of Atherosclerosis. Biomedicines 2021, 9, 172. [Google Scholar] [CrossRef]
- Kose, M.; Senkal, N.; Tukek, T.; Cebeci, T.; Atalar, S.C.; Altinkaynak, M.; Arici, H.; Genc, S.; Catma, Y.; Kocaaga, M.; et al. Severe vitamin D deficiency is associated with endothelial inflammation in healthy individuals even in the absence of subclinical atherosclerosis. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 7046–7052. [Google Scholar] [CrossRef] [PubMed]
- Kheiri, B.; Abdalla, A.; Osman, M.; Ahmed, S.; Hassan, M.; Bachuwa, G. Vitamin D deficiency and risk of cardiovascular diseases: A narrative review. Clin. Hypertens 2018, 24, 9, Erratum in Clin. Hypertens 2018, 24, 19. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.-Y.; Kim, J.-K.; Kang, J.-H.; Yu, B.-Y.; Kim, S.-J. Relationship between coronary atherosclerosis in coronary computed tomography angiography and serum vitamin D level. Osteoporos. Sarcopenia 2017, 3, 155–158. [Google Scholar] [CrossRef]
- Alfieri, D.F.; Lehmann, M.F.; Oliveira, S.R.; Flauzino, T.; Delongui, F.; de Araújo, M.C.M.; Dichi, I.; Delfino, V.D.; Mezzaroba, L.; Simão, A.N.; et al. Vitamin D deficiency is associated with acute ischemic stroke, C-reactive protein, and short-term outcome. Metab. Brain Dis. 2017, 32, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Wajda, J.; Świat, M.; Owczarek, A.J.; Brzozowska, A.; Olszanecka-Glinianowicz, M.; Chudek, J. Severity of Vitamin D Deficiency Predicts Mortality in Ischemic Stroke Patients. Dis. Markers 2019, 2019, 3652894. [Google Scholar] [CrossRef]
- Dziedzic, E.A.; Smyk, W.; Sowińska, I.; Dąbrowski, M.; Jankowski, P. Serum Level of Vitamin D Is Associated with Severity of Coronary Atherosclerosis in Postmenopausal Women. Biology 2021, 10, 1139. [Google Scholar] [CrossRef] [PubMed]
- Bima, A.I.; Mahdi, A.S.; Al Fayez, F.F.; Khawaja, T.M.; Abo El-Khair, S.M.; Elsamanoudy, A.Z. Cellular Senescence and Vitamin D Deficiency Play a Role in the Pathogenesis of Obesity-Associated Subclinical Atherosclerosis: Study of the Potential Protective Role of Vitamin D Supplementation. Cells 2021, 10, 920. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Vellas, B.; Rizzoli, R.; Kressig, R.W.; Da Silva, J.A.P.; Blauth, M.; Felson, D.T.; McCloskey, E.V.; Watzl, B.; Hofbauer, L.C.; et al. Effect of Vitamin D Supplementation, Omega-3 Fatty Acid Supplementation, or a Strength-Training Exercise Program on Clinical Outcomes in Older Adults: The DO-HEALTH Randomized Clinical Trial. JAMA 2020, 324, 1855–1868. [Google Scholar] [CrossRef]
- Scragg, R.; Stewart, A.W.; Waayer, D.; Lawes, C.M.M.; Toop, L.; Sluyter, J.; Murphy, J.; Khaw, K.T.; Camargo, C.A., Jr. Effect of Monthly High-Dose Vitamin D Supplementation on Cardiovascular Disease in the Vitamin D Assessment Study: A Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 608–616. [Google Scholar] [CrossRef]
- Avenell, A.; MacLennan, G.S.; Jenkinson, D.J.; McPherson, G.C.; McDonald, A.M.; Pant, P.R.; Grant, A.M.; Campbell, M.K.; Anderson, F.H.; Cooper, C.; et al. Long-term follow-up for mortality and cancer in a randomized placebo-controlled trial of vitamin D3 and/or calcium (RECORD trial). J. Clin. Endocrinol. Metab. 2012, 97, 614–622. [Google Scholar] [CrossRef]
- LaCroix, A.Z.; Kotchen, J.; Anderson, G.; Brzyski, R.; Cauley, J.A.; Cummings, S.R.; Gass, M.; Johnson, K.C.; Ko, M.; Larson, J.; et al. Calcium plus vitamin D supplementation and mortality in postmenopausal women: The Women’s Health Initiative calcium-vitamin D randomized controlled trial. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Green, D.; Dalmay, T.; Chapman, T. Microguards and micromessengers of the genome. Heredity 2016, 116, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Citron, F.; Armenia, J.; Franchin, G.; Polesel, J.; Talamini, R.; D’andrea, S.; Sulfaro, S.; Croce, C.M.; Klement, W.; Otasek, D. An integrated approach identifies mediators of local recurrence in head and neck squamous carcinoma. Clin. Cancer Res. 2017, 23, 3769–3780. [Google Scholar] [CrossRef]
- Hill, M.; Tran, N. miRNA interplay: Mechanisms and consequences in cancer. Dis. Model Mech. 2021, 14, dmm047662. [Google Scholar] [CrossRef]
- Lu, Y.; Thavarajah, T.; Gu, W.; Cai, J.; Xu, Q. Impact of miRNA in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2018, 38, e159–e170. [Google Scholar] [CrossRef]
- Suárez, Y.; Fernández-Hernando, C.; Yu, J.; Gerber, S.A.; Harrison, K.D.; Pober, J.S.; Iruela-Arispe, M.L.; Merkenschlager, M.; Sessa, W.C. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 14082–14087. [Google Scholar] [CrossRef]
- Daiber, A.; Xia, N.; Steven, S.; Oelze, M.; Hanf, A.; Kroller-Schon, S.; Munzel, T.; Li, H. New therapeutic implications of endothelial nitric oxide synthase (eNOS) function/dysfunction in cardiovascular disease. Int. J. Mol. Sci. 2019, 20, 187. [Google Scholar] [CrossRef]
- de Yébenes, V.G.; Briones, A.M.; Martos-Folgado, I.; Mur, S.M.; Oller, J.; Bilal, F.; González-Amor, M.; Méndez-Barbero, N.; Silla-Castro, J.C.; Were, F.; et al. Aging-Associated miR-217 Aggravates Atherosclerosis and Promotes Cardiovascular Dysfunction. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2408–2424. [Google Scholar] [CrossRef]
- Canfran-Duque, A.; Lin, C.S.; Goedeke, L.; Suarez, Y.; Fernandez-Hernando, C. Micro-RNAs and High-Density Lipoprotein Metabolism. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, B.; Wang, Z.; Wang, D.; Ni, H.; Zhang, L.; Wang, Y. Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics 2019, 9, 6901–6919. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.R.; Sharma, G.; Bhattacharya, M.; Lee, S.S.; Chakraborty, C. Circulating miRNA in Atherosclerosis: A Clinical Biomarker and Early Diagnostic Tool. Curr. Mol. Med. 2022, 22, 250–262. [Google Scholar] [CrossRef]
- Liu, X.; Guo, J.W.; Lin, X.C.; Tuo, Y.H.; Peng, W.L.; He, S.Y.; Li, Z.Q.; Ye, Y.C.; Yu, J.; Zhang, F.R.; et al. Macrophage NFATc3 prevents foam cell formation and atherosclerosis: Evidence and mechanisms. Eur. Heart J. 2021, 42, 4847–4861. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yang, B.; Yang, H.; Wang, L.; Li, H.; Chen, S.; Lu, X.; Gu, D. MicroRNA-320b Modulates Cholesterol Efflux and Atherosclerosis. J. Atheroscler. Thromb. 2022, 29, 200–220. [Google Scholar] [CrossRef]
- Price, N.L.; Rotllan, N.; Zhang, X.; Canfran-Duque, A.; Nottoli, T.; Suarez, Y.; Fernandez-Hernando, C. Specific disruption of abca1 targeting largely mimics the effects of mir-33 knockout on macrophage cholesterol efflux and atherosclerotic plaque development. Circ. Res. 2019, 124, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Rotllan, N.; Canfrán-Duque, A.; Sun, J.; Toczek, J.; Moshnikova, A.; Malik, S.; Price, N.L.; Araldi, E.; Zhong, W.; et al. Targeted Suppression of miRNA-33 Using pHLIP Improves Atherosclerosis Regression. Circ. Res. 2022, 131, 77–90. [Google Scholar] [CrossRef]
- Sum, H.; Brewer, A.C. Epigenetic modifications as therapeutic targets in atherosclerosis: A focus on DNA methylation and non-coding RNAs. Front. Cardiovasc. Med. 2023, 10, 1183181. [Google Scholar] [CrossRef]
- Aavik, E.; Babu, M.; Yla-Herttuala, S. DNA methylation processes in atheosclerotic plaque. Atherosclerosis 2019, 281, 168–179. [Google Scholar] [CrossRef]
- Tao, J.; Xia, L.; Cai, Z.; Liang, L.; Chen, Y.; Meng, J.; Wang, Z. Interaction Between microRNA and DNA Methylation in Atherosclerosis. DNA Cell Biol. 2021, 40, 101–115. [Google Scholar] [CrossRef]


| Study | Year | Study Design | Findings |
|---|---|---|---|
| Polito et al. [110] | 2021 | Review article | XO plays a significant role in CVDs, including atherosclerosis, through various mechanisms such as oxidative stress, inflammation, endothelial dysfunction, and modulation of purinergic signaling. |
| Kotozaki et al. [111] | 2023 | Population-based study | Higher XO activity in human plasma is associated with an increased risk of CVDs, indicating its potential role as a biomarker and a therapeutic target. |
| Kimura et al. [112] | 2021 | Review article | UA contributes to the pathogenesis of atherosclerosis by promoting inflammation through the activation of immune cells, modulation of vascular function, and induction of oxidative stress. |
| Yan et al. [117] | 2018 | In vitro study (cardiomyocytes) | UA induces cardiomyocyte apoptosis by activating calpain-1 and endoplasmic reticulum stress, suggesting a potential mechanism by which hyperuricemia contributes to cardiac dysfunction. |
| Hu et al. [118] | 2022 | Clinical study | Elevated serum US levels are associated with a pro-inflammatory state and have an impact on the role of HDL-C in promoting carotid atherosclerosis, highlighting the link between UA, inflammation, and atherosclerosis. |
| Yu et al. [119] | 2022 | Experimental study (cellular and animal models) | High levels of UA promote atherosclerosis by impairing autophagy through NRF2-mediated dysfunction and inducing ferroptosis, providing insights into the molecular mechanisms underlying the pro-atherogenic effects of UA. |
| Gene | Cell/Tissue Type | Function or Association with Disease |
|---|---|---|
| miR-1 | blood, VSMCs | Expression is associated with subclinical atherosclerosis. Induces VSMC differentiation. |
| miR-10a | serum, VSMCs | Negative regulator of SMC differentiation. |
| miR-19a | ECs, B cells, VSMCs | Promotion of VSMC proliferation. Suppression of IL-10-mediated immunomodulation. Mediates the effects of laminar flow and cell cycle progression. |
| miR-33a/b | Cells from liver, macrophages, fibroblasts | Regulation of cholesterol homeostasis. |
| miR-146a | VSMCs, EPCs | VSMC proliferation. Neointimal hyperplasia. |
| miR-302a | macrophages | Regulation of cholesterol efflux. |
| miR-221 and miR-222 | VSMCs | VSMC proliferation. Neointimal hyperplasia. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtasińska, A.; Frąk, W.; Lisińska, W.; Sapeda, N.; Młynarska, E.; Rysz, J.; Franczyk, B. Novel Insights into the Molecular Mechanisms of Atherosclerosis. Int. J. Mol. Sci. 2023, 24, 13434. https://doi.org/10.3390/ijms241713434
Wojtasińska A, Frąk W, Lisińska W, Sapeda N, Młynarska E, Rysz J, Franczyk B. Novel Insights into the Molecular Mechanisms of Atherosclerosis. International Journal of Molecular Sciences. 2023; 24(17):13434. https://doi.org/10.3390/ijms241713434
Chicago/Turabian StyleWojtasińska, Armanda, Weronika Frąk, Wiktoria Lisińska, Natalia Sapeda, Ewelina Młynarska, Jacek Rysz, and Beata Franczyk. 2023. "Novel Insights into the Molecular Mechanisms of Atherosclerosis" International Journal of Molecular Sciences 24, no. 17: 13434. https://doi.org/10.3390/ijms241713434
APA StyleWojtasińska, A., Frąk, W., Lisińska, W., Sapeda, N., Młynarska, E., Rysz, J., & Franczyk, B. (2023). Novel Insights into the Molecular Mechanisms of Atherosclerosis. International Journal of Molecular Sciences, 24(17), 13434. https://doi.org/10.3390/ijms241713434







