Differentiation and Identification of Endophytic Bacteria from Populus Based on Mass Fingerprints and Gene Sequences
Abstract
:1. Introduction
2. Results
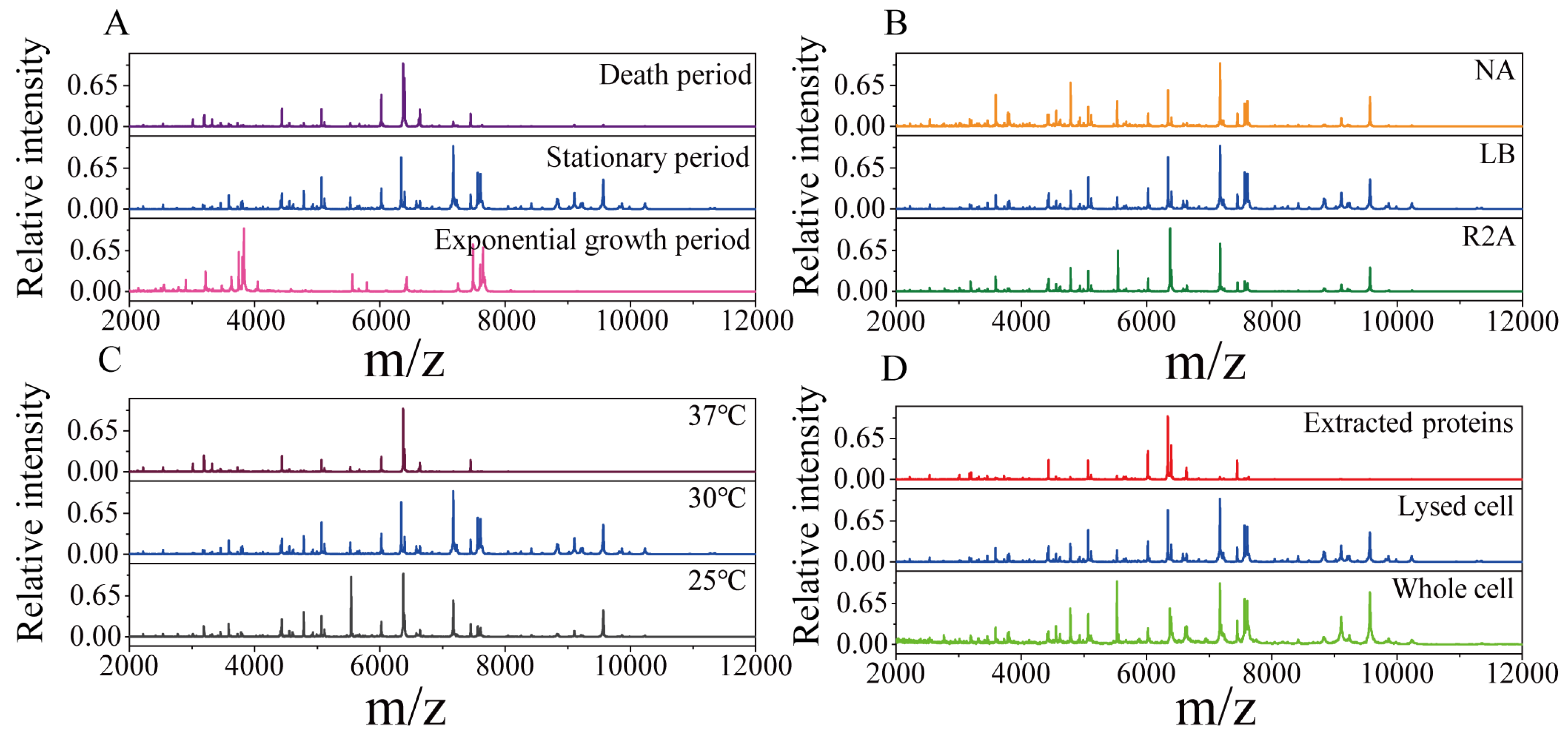
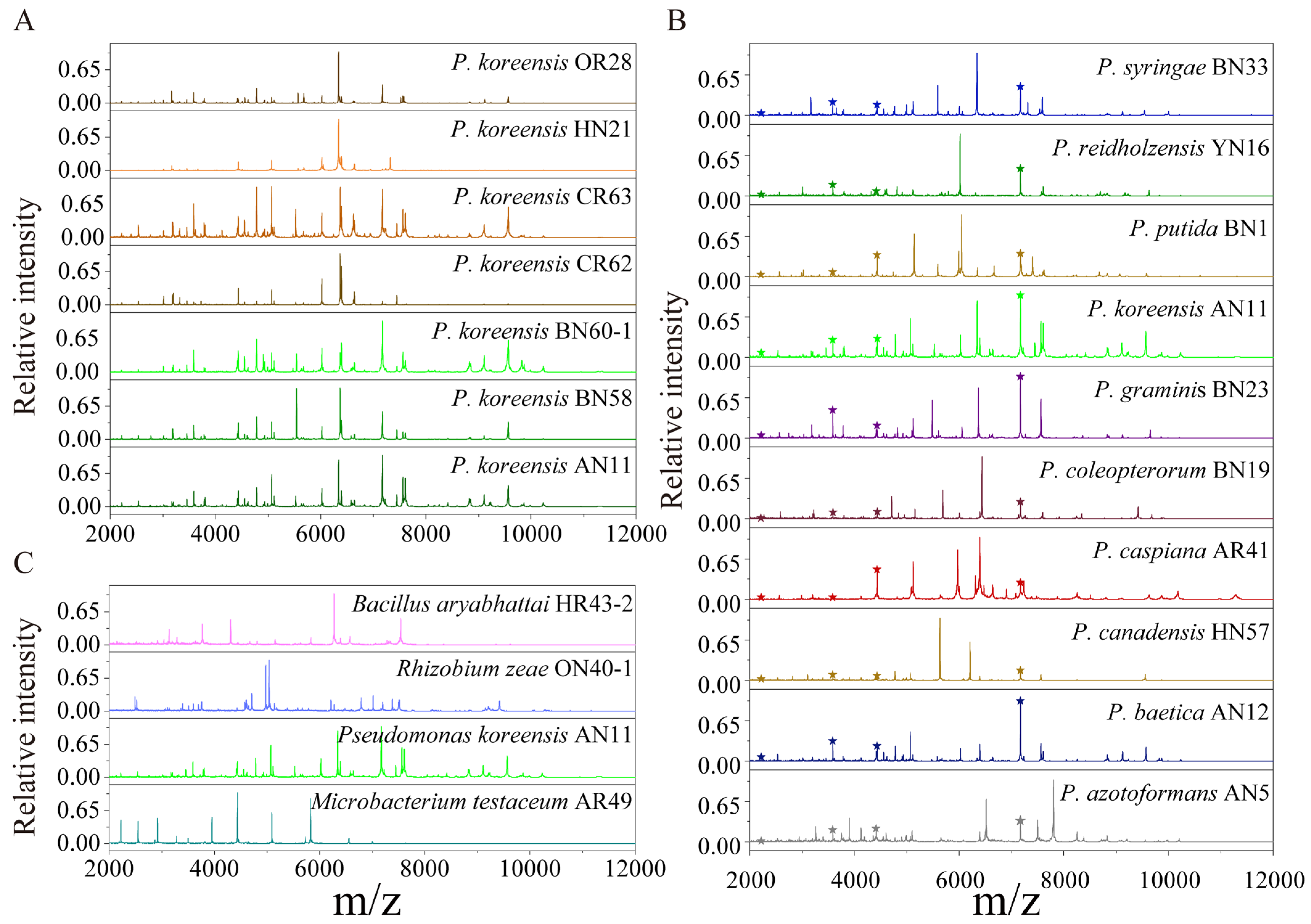
2.1. Protein Mass Fingerprints of Endophytic Bacteria
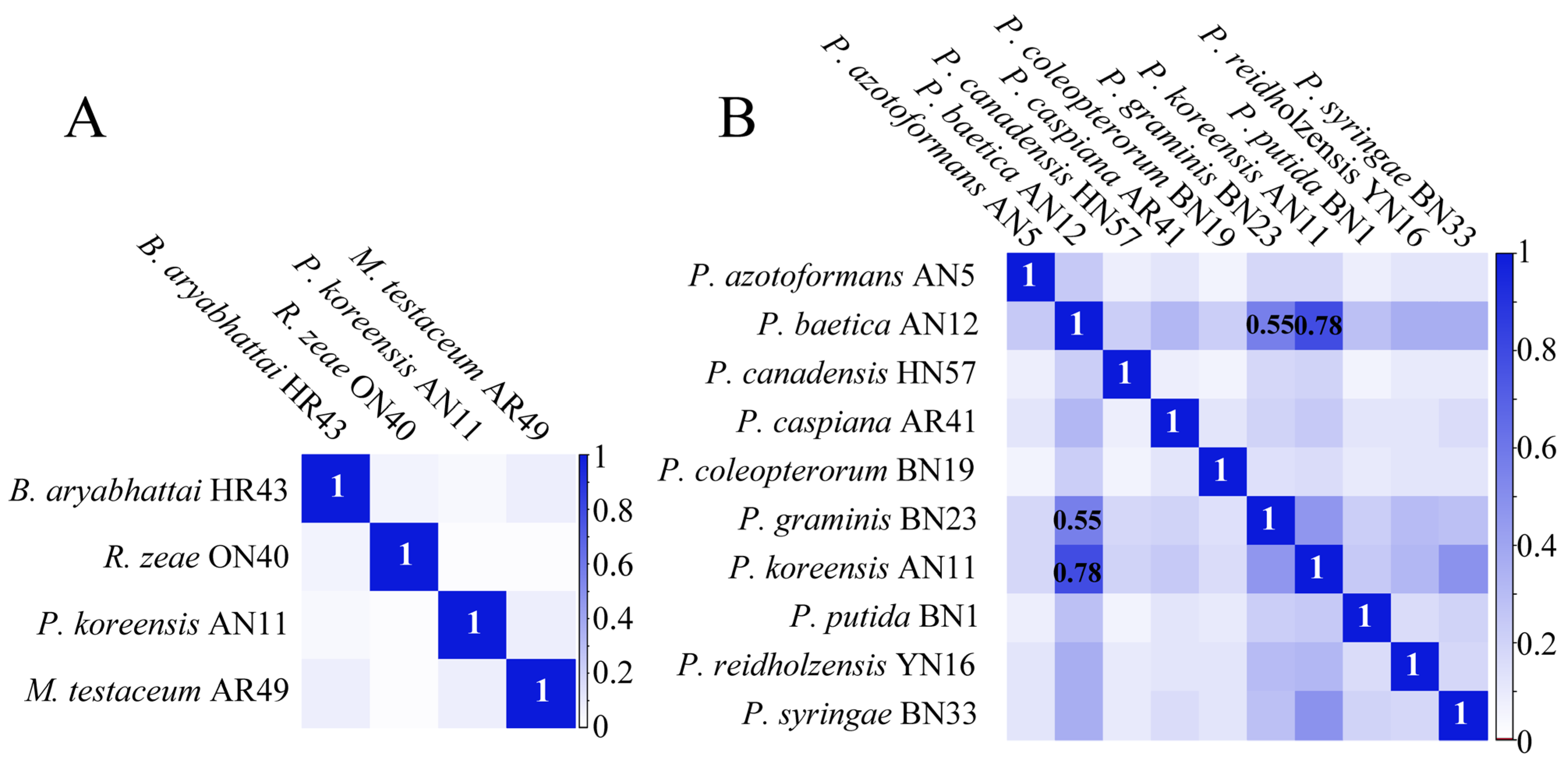
2.2. Composite Correlation Index (CCI) Matrix for Protein Mass Fingerprints
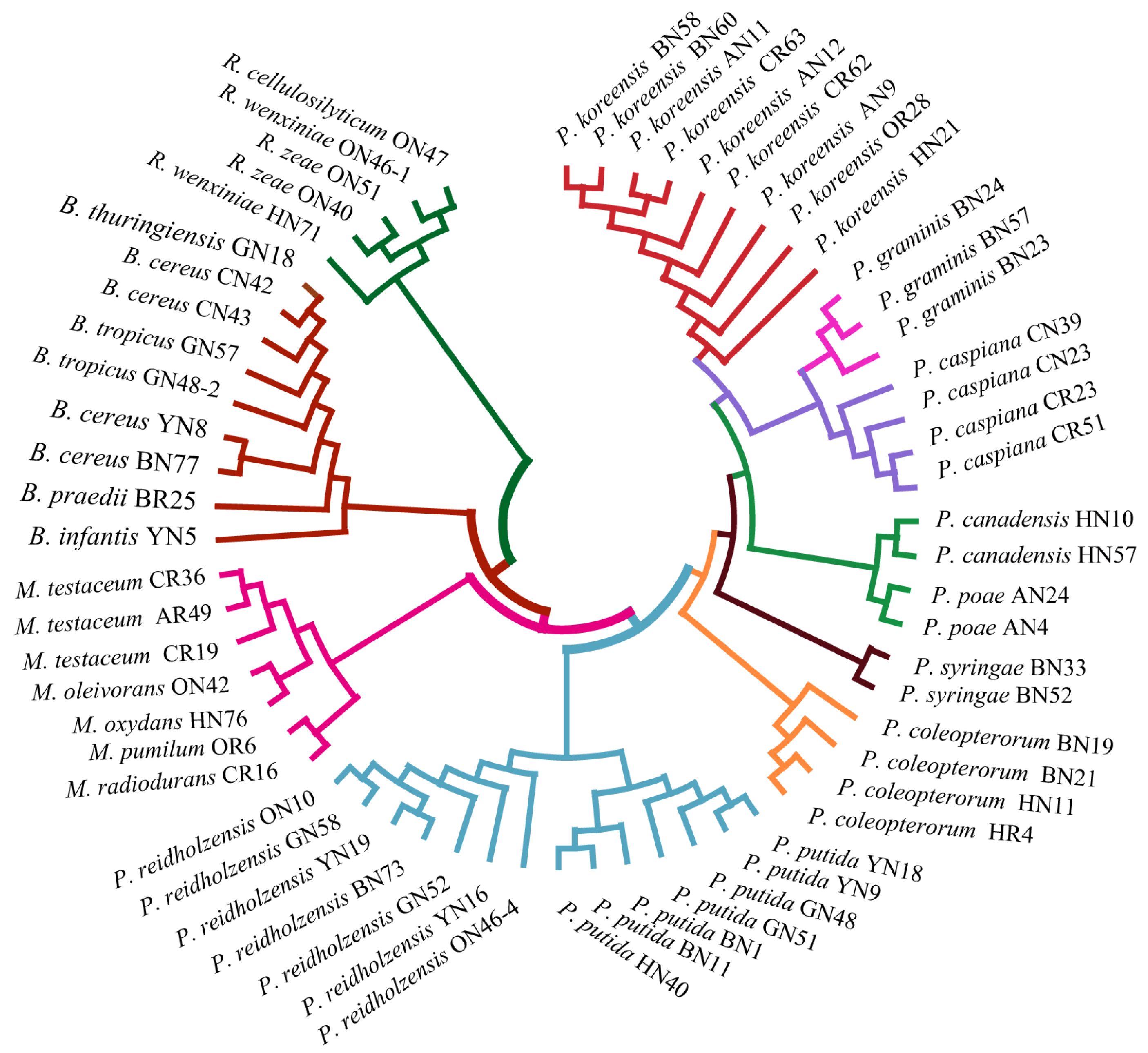
2.3. PCoA to Verify the Differentiation Capability of MALDI-TOF MS
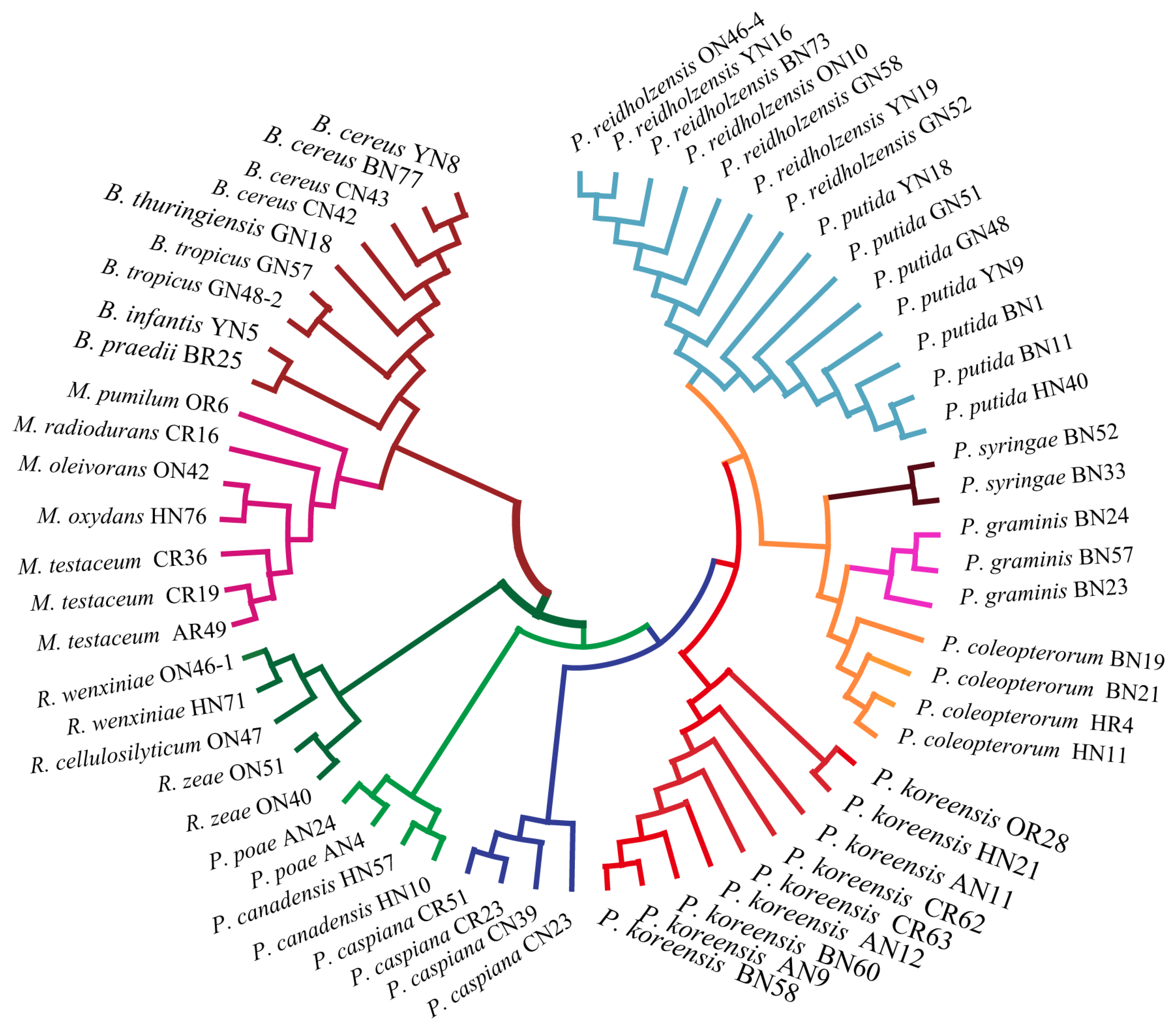
2.4. Evaluation of Relatedness Based on MS Profiles
3. Discussion
3.1. The Effects of Bacterial Pretreatment and Culture Conditions on MS Profiles
3.2. Bacterial Classification Based on MALDI-TOF MS
3.3. Protein Mass Fingerprints vs. 16S rRNA Gene Sequence
4. Methods and Materials
4.1. Populus’s Endophytic Bacterial Strains
4.2. MALDI-TOF MS Sample Preparation and Detection
4.3. Mass Spectra Data Processing
4.4. 16S rRNA Gene Sequence
4.5. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ryan, R.P.; Germaine, K.; Franks, A.; Ryan, D.J.; Dowling, D.N. Bacterial endophytes: Recent developments and applications. FEMS Microbiol. Lett. 2008, 278, 1–9. [Google Scholar] [CrossRef]
- Partida-Martinez, L.P.; Heil, M. The microbe-free plant: Fact or artifact? Front. Plant Sci. 2011, 2, 100. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, B.S.; Babalola, O.O.; Glick, B.R. Plant growth-promoting root-colonizing bacterial endophytes. Rhizosphere 2021, 20, 100433. [Google Scholar] [CrossRef]
- Etesami, H.; Alikhani, H.A.; Hosseini, H.M. Indole-3-acetic acid (IAA) production trait, a useful screening to select endophytic and rhizosphere competent bacteria for rice growth promoting agents. MethodsX 2015, 2, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Dudeja, S.S.; Suneja-Madan, P.; Paul, M.; Maheswari, R.; Kothe, E. Bacterial endophytes: Molecular interactions with their hosts. J. Basic Microbiol. 2021, 61, 475–505. [Google Scholar] [CrossRef] [PubMed]
- Bolivar-Anillo, H.J.; Gonzalez-Rodriguez, V.E.; Cantoral, J.M.; Garcia-Sanchez, D.; Collado, I.G.; Garrido, C. Endophytic Bacteria Bacillus subtilis, Isolated from Zea mays, as Potential Biocontrol Agent against Botrytis cinerea. Biology 2021, 10, 492. [Google Scholar] [CrossRef]
- Goodwin, P.H. The Endosphere Microbiome of Ginseng. Plants 2022, 11, 415. [Google Scholar] [CrossRef]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef]
- Xie, Z.; Chu, Y.; Zhang, W.; Lang, D.; Zhang, X. Bacillus pumilus alleviates drought stress and increases metabolite accumulation in Glycyrrhiza uralensis Fisch. Environ. Exp. Bot. 2019, 158, 99–106. [Google Scholar] [CrossRef]
- Sim, C.S.F.; Tan, W.S.; Ting, A.S.Y. Endophytes from Phragmites for metal removal: Evaluating their metal tolerance, adaptive tolerance behaviour and biosorption efficacy. Desalin. Water Treat. 2016, 57, 6959–6966. [Google Scholar] [CrossRef]
- Wani, Z.A.; Ashraf, N.; Mohiuddin, T.; Riyaz-Ul-Hassan, S. Plant-endophyte symbiosis, an ecological perspective. Appl. Microbiol. Biotechnol. 2015, 99, 2955–2965. [Google Scholar] [CrossRef] [PubMed]
- Sarsaiya, S.; Shi, J.S.; Chen, J.S. A comprehensive review on fungal endophytes and its dynamics on Orchidaceae plants: Current research, challenges, and future possibilities. Bioengineered 2019, 10, 316–334. [Google Scholar] [CrossRef] [PubMed]
- Franco-Duarte, R.; Cernakova, L.; Kadam, S.; Kaushik, K.S.; Salehi, B.; Bevilacqua, A.; Corbo, M.R.; Antolak, H.; Dybka-Stepien, K.; Leszczewicz, M. Advances in Chemical and Biological Methods to Identify Microorganisms—From Past to Present. Microorganisms 2019, 7, 130. [Google Scholar] [CrossRef]
- Bazzi, A.M.; Rabaan, A.A.; Fawarah, M.M.; Al-Tawfiq, J.A. Direct identification and susceptibility testing of positive blood cultures using high speed cold centrifugation and Vitek II system. J. Infect. Public Health 2017, 10, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Breitwieser, F.P.; Lu, J.; Salzberg, S.L. A review of methods and databases for metagenomic classification and assembly. Brief Bioinform. 2019, 20, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Heather, J.M.; Chain, B. The sequence of sequencers: The history of sequencing DNA. Genomics 2016, 107, 1–8. [Google Scholar] [CrossRef]
- Vetrovsky, T.; Baldrian, P. The variability of the 16S rRNA gene in bacterial genomes and its consequences for bacterial community analyses. PLoS ONE 2013, 8, e57923. [Google Scholar] [CrossRef]
- Pace, N.R. A molecular view of microbial diversity and the biosphere. Science 1997, 276, 734–740. [Google Scholar] [CrossRef]
- Stackebrandt, E.; Goebel, B.M. Taxonomic Note: A Place for DNA-DNA Reassociation and 16s rRNA Sequence Analysis in the Present Species Definition in Bacteriology. Int. J. Syst. Bacteriol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Woese, C.R.; Kandler, O.; Wheelis, M. Towards a natural system of organisms: Proposal for the domains Archaea, Bacteria, and Eucarya. Proc. Nat. Acad. Sci. USA 1990, 87, 4576–4579. [Google Scholar] [CrossRef]
- Van Dijk, E.L.; Jaszczyszyn, Y.; Thermes, C. Library preparation methods for next-generation sequencing: Tone down the bias. Exp. Cell Res. 2014, 322, 12–20. [Google Scholar] [CrossRef]
- Karas, M.; Bachmann, D.; Hillenkamp, F. Influence of the wavelength in high irradiance ultraviolet laser desorption mass spectrometry of organic molecules. Anal. Chem. 1985, 57, 2935–2939. [Google Scholar] [CrossRef]
- Sala-Comorera, L.; Blanch, A.R.; Vilaro, C.; Galofre, B.; Garcia-Aljaro, C. Heterotrophic monitoring at a drinking water treatment plant by matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry after different drinking water treatments. J. Water Health 2017, 15, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Uchida-Fujii, E.; Niwa, H.; Kinoshita, Y.; Nukada, T. Construction and Application of an In-House Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS) Database Specific to Bacteria from Horses. J. Equine Vet. Sci. 2021, 103, 103664. [Google Scholar] [CrossRef] [PubMed]
- Krakova, L.; Soltys, K.; Otlewska, A.; Pietrzak, K.; Purkrtova, S.; Savicka, D.; Puskarova, A.; Buckova, M.; Szemes, T.; Budis, J. Comparison of methods for identification of microbial communities in book collections: Culture-dependent (sequencing and MALDI-TOF MS) and culture-independent (Illumina MiSeq). Int. Biodeterior. Biodegrad. 2018, 131, 51–59. [Google Scholar] [CrossRef]
- Wei, L.W.; Shao, J.; Song, Y.G.; Wan, Z.; Yao, L.M.; Wang, H.; Yu, J. Performance of Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry for Identification of Scedosporium, Acremonium-Like, Scopulariopsis, and Microascus Species. Front. Microbiol. 2022, 13, 841286. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Wang, Q.; Wei, L.; Wan, Z.; Li, R.; Yu, J. Limitations of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry for the identification of Aspergillus species. Med. Mycol. 2022, 60, myab084. [Google Scholar] [CrossRef] [PubMed]
- Zvezdanova, M.E.; Escribano, P.; Guinea, J.; Munoz, P.; Rodriguez-Temporal, D.; Rodriguez-Sanchez, B. Evaluation of the Vitek Ms system for the identification of filamentous fungi. Med. Mycol. 2022, 60, myac027. [Google Scholar] [CrossRef]
- Fraser, M.; Brown, Z.; Houldsworth, M.; Borman, A.M.; Johnson, E.M. Rapid identification of 6328 isolates of pathogenic yeasts using MALDI-ToF MS and a simplified, rapid extraction procedure that is compatible with the Bruker Biotyper platform and database. Med. Mycol. 2016, 54, 80–88. [Google Scholar] [CrossRef]
- Lukacova, A.; Beck, T.; Trnkova, K.; Trnikova, M.; Krajcovic, J.; Vesteg, M. Discrimination of Euglena gracilis strains Z and bacillaris by MALDI-TOF MS. J. Appl. Microbiol. 2022, 130, 930–942. [Google Scholar] [CrossRef]
- Kristina, R.; Tara, W.; Cecilia, J. Rapid Subtyping of Yersinia enterocolitica by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS) for Diagnostics and Surveillance. J. Clin. Microbiol. 2013, 51, 4200–4203. [Google Scholar] [CrossRef]
- Claydon, M.A.; Davey, S.N.; Edwards-Jones, V.; Gordon, D.B. The rapid identification of intact microorganisms using mass spectrometry. Nat. Biotechnol. 1996, 14, 1584–1586. [Google Scholar] [CrossRef]
- Unger, M.S.; Blank, M.; Enzlein, T.; Hopf, C. Label-free cell assays to determine compound uptake or drug action using MALDI-TOF mass spectrometry. Nat. Protoc. 2021, 16, 5533–5558. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, S.; Nakayama, T. MALDI-Based Mass Spectrometry in Clinical Testing: Focus on Bacterial Identification. Appl. Sci. 2022, 12, 2814. [Google Scholar] [CrossRef]
- Ghyselinck, J.; Velivelli, S.L.S.; Heylen, K.; O’Herlihy, E.; Franco, J.; Rojas, M.; De Vos, P.; Prestwich, B.D. Bioprospecting in potato fields in the Central Andean Highlands: Screening of rhizobacteria for plant growth-promoting properties. Syst. Appl. Microbiol. 2012, 36, 116–127. [Google Scholar] [CrossRef]
- LaMontagne, M.G.; Tran, P.L.; Benavidez, A.; Morano, L.D. Development of an inexpensive matrix-assisted laser desorption-time of flight mass spectrometry method for the identification of endophytes and rhizobacteria cultured from the microbiome associated with maize. PeerJ 2021, 9, e11359. [Google Scholar] [CrossRef]
- Ashfaq, M.Y.; Da’na, D.A.; Al-Ghouti, M.A. Application of MALDI-TOF MS for identification of environmental bacteria: A review. J. Environ. Manag. 2021, 305, 114359. [Google Scholar] [CrossRef] [PubMed]
- Strejcek, M.; Smrhova, T.; Junkova, P.; Uhlik, O. Whole-Cell MALDI-TOF MS Versus 16S rRNA Gene Analysis for Identification and Dereplication of Recurrent Bacterial Isolates. Front. Microbiol. 2018, 9, 1294. [Google Scholar] [CrossRef]
- Cobo, F.; Pérez-Carrasco, V.; Martín-Hita, L.; García-Salcedo, J.A.; Navarro-Marí, J.M. Comparative evaluation of MALDI-TOF MS and 16S rRNA gene sequencing for the identification of clinically relevant anaerobic bacteria. Critical evaluation of discrepant results. Anaerobe 2023, 82, 102754. [Google Scholar] [CrossRef]
- Chaturvedi, H.; Singh, V. Potential of bacterial endophytes as plant growth promoting factors. J. Plant Pathol. Microbiol. 2016, 7, 1000376. [Google Scholar] [CrossRef]
- Yue, H.; Zhao, L.; Yang, D.; Zhang, M.; Wu, J.; Zhao, Z.; Xing, X.; Zhang, L.; Qin, Y.; Guo, F. et, al. Comparative Analysis of the Endophytic Bacterial Diversity of Populus euphratica Oliv. in Environments of Different Salinity Intensities. Microbiol. Spectr. 2022, 10, e0050022. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, W.; Ding, C.; Zhang, B.; Huang, Q.; Huang, R.; Su, X. Endophytic Communities of Transgenic Poplar Were Determined by the Environment and Niche Rather Than by Transgenic Events. Front. Microbiol. 2019, 10, 588. [Google Scholar] [CrossRef]
- Balazova, T.; Makovcova, J.; Sedo, O.; Slany, M.; Faldyna, M.; Zdrahal, Z. The influence of culture conditions on the identification of Mycobacterium species by MALDI-TOF MS profiling. FEMS Microbiol. Lett. 2014, 353, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, M.F.; Sorrentino, A.; Gaita, M.; Cacace, G.; Di Stasio, M.; Facchiano, A.; Comi, G.; Malorni, A.; Siciliano, R.A. Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry for the Discrimination of Food-Borne Microorganisms. Appl. Environ. Microbiol. 2006, 72, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, X.; Guo, H.; Wang, Y.; Yuan, Z. Comparative proteomic analysis of Escherichia coli during different growth phases. J. Dairy Sci. 2017, 100, 1928–1940. [Google Scholar]
- Ryzhov, V.; Fenselau, C. Characterization of the Protein Subset Desorbed by MALDI from Whole Bacterial Cells. Anal. Chem. 2001, 73, 746–750. [Google Scholar] [CrossRef]
- Sedlacek, I.; Pantucek, R.; Zeman, M.; Holochova, P.; Sedo, O.; Stankova, E.; Svec, P.; Kralova, S.; Videnska, P.; Micenkova, L.; et al. Hymenobacter terrestris sp. nov. and Hymenobacter lapidiphilus sp. nov., isolated from regoliths in Antarctica. Int. J. Syst. Evol. Microbiol. 2020, 70, 6364–6372. [Google Scholar] [CrossRef] [PubMed]
- Sauget, M.; Valot, B.; Bertrand, X.; Hocquet, D. Can MALDI-TOF Mass Spectrometry Reasonably Type Bacteria? Trends Microbiol. 2017, 25, 447–455. [Google Scholar] [CrossRef]
- Shao, J.; Wan, Z.; Li, R.; Yu, J. Species identification and delineation of pathogenic mucorales by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry. J. Clin. Microbiol. 2018, 56, e01886-17. [Google Scholar] [CrossRef]
- Yang, Z.; Rannala, B. Molecular phylogenetics: Principles and practice. Nat. Rev. Genet. 2012, 13, 303–314. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttila, A.M.; Compant, S.; Campisano, A.; Doring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defifining functioning of microbial endophytes. Microbiol. Mol. Biol. R 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wu, G.; Han, S.; Yang, J.; He, X.; Li, H. Differentiation and Identification of Endophytic Bacteria from Populus Based on Mass Fingerprints and Gene Sequences. Int. J. Mol. Sci. 2023, 24, 13449. https://doi.org/10.3390/ijms241713449
Wang X, Wu G, Han S, Yang J, He X, Li H. Differentiation and Identification of Endophytic Bacteria from Populus Based on Mass Fingerprints and Gene Sequences. International Journal of Molecular Sciences. 2023; 24(17):13449. https://doi.org/10.3390/ijms241713449
Chicago/Turabian StyleWang, Xia, Guanqi Wu, Shuo Han, Jingjing Yang, Xiangwei He, and Haifang Li. 2023. "Differentiation and Identification of Endophytic Bacteria from Populus Based on Mass Fingerprints and Gene Sequences" International Journal of Molecular Sciences 24, no. 17: 13449. https://doi.org/10.3390/ijms241713449
APA StyleWang, X., Wu, G., Han, S., Yang, J., He, X., & Li, H. (2023). Differentiation and Identification of Endophytic Bacteria from Populus Based on Mass Fingerprints and Gene Sequences. International Journal of Molecular Sciences, 24(17), 13449. https://doi.org/10.3390/ijms241713449




