Abstract
Two undescribed ent-kaurene diterpenes, named guidongnins I (1) and J (2), were isolated from the medicinal plant Isodon rubescens. Compound 1 was determined to contain an unprecedented 23 carbons in the skeleton by bearing an extra isopropyl group at C-17 out of the diterpenoid parent structure, and compound 2 was the first example of 6,7-seco-7,20-olide-ent-kaurenes with two fused-tetrahydrofuran rings formed between C-6 and C-19/C-20 through oxygen bridges. Their structures, including their absolute configurations, were determined using the analyses of the spectroscopic and X-ray diffraction data. Guidongnins I (1) and J (2) were assessed for their anti-cancer activities against the growth of various cancer cell lines, and 2 displayed cytotoxic potency against HepG2 at IC50 27.14 ± 3.43 μM.
1. Introduction
The Isodon genus, belonging to the Labiatae family, is renowned for its abundant diterpenes featuring diverse carbon frameworks, which exhibit a wide array of biological activities. Over 1200 new diterpenes have been identified from the genus previously [1,2,3,4,5]. Approximately 30 species in this genus were constantly used as Chinese folk medicines. Among them, I. rubescens was found to possess multiple pharmacological effects that have been used to treat respiratory problems, gastrointestinal inflammation, bacterial infected diseases, and cancer [5], and its refined extract has been developed as a Chinese patent medicine to treat sore throat, bacterial infection, and cancer [6]. Many interesting diterpenes have been isolated from this plant [5,7,8,9,10,11]. Apart from the common ent-kaurane diterpenoids, 6,7-seco-ent-kaurane diterpenoids are constantly found in this species and they have been demonstrated to show significant antiproliferative activities against various human tumor cell lines. Acetylexidonin [12] and lushanrubescensin H [13] are the two examples that displayed potent antitumor activities. To further explore the novel bioactive diterpenoids bearing this skeleton from I. rubescens, our group has systematically investigated the phytochemicals of the plant species collected from Guizhou. A number of novel diterpenoids, such as rubesanolides A-G, have been identified from this plant in our previous studies [7,8,9], which revealed that I. rubescens is a special Isodon plant that could richly produce diterpene metabolites with unique structures. As part of our ongoing efforts to find new bioactive diterpenoids, we isolated guidongnins I (1) and J (2) as two novel ent-kaurane diterpenoids from this species (Figure 1). Compound 1 was found to be the first example of a novel C23-carbon skeleton bearing a 6,7-seco diterpene framework conjugated with an isopropyl group at C-17, and 2 contained a complex ring system with two unprecedented tetrahydrofurans fused with a δ-lactone ring. The two compounds could be derived from a commonly occurring ent-kaurane diterpene via a series of enzyme-catalyzed biosynthetic conversions outlined in Scheme 1. This new discovery further illuminates the chemical diversity of ent-kaurene diterpenoids in this species. By evaluating their bioactivities against cancer cells A-549, HL-60, MCF-7, HepG2, and COLO-205, 2 was found to be specifically active against the HepG2 (liver) with an IC50 value of 27.14 ± 3.43 μM. Therefore, we report the isolation, structure elucidation, biosynthetic pathways, along with the bioactivities of two new diterpenoids (1–2).
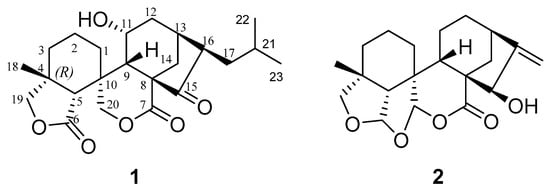
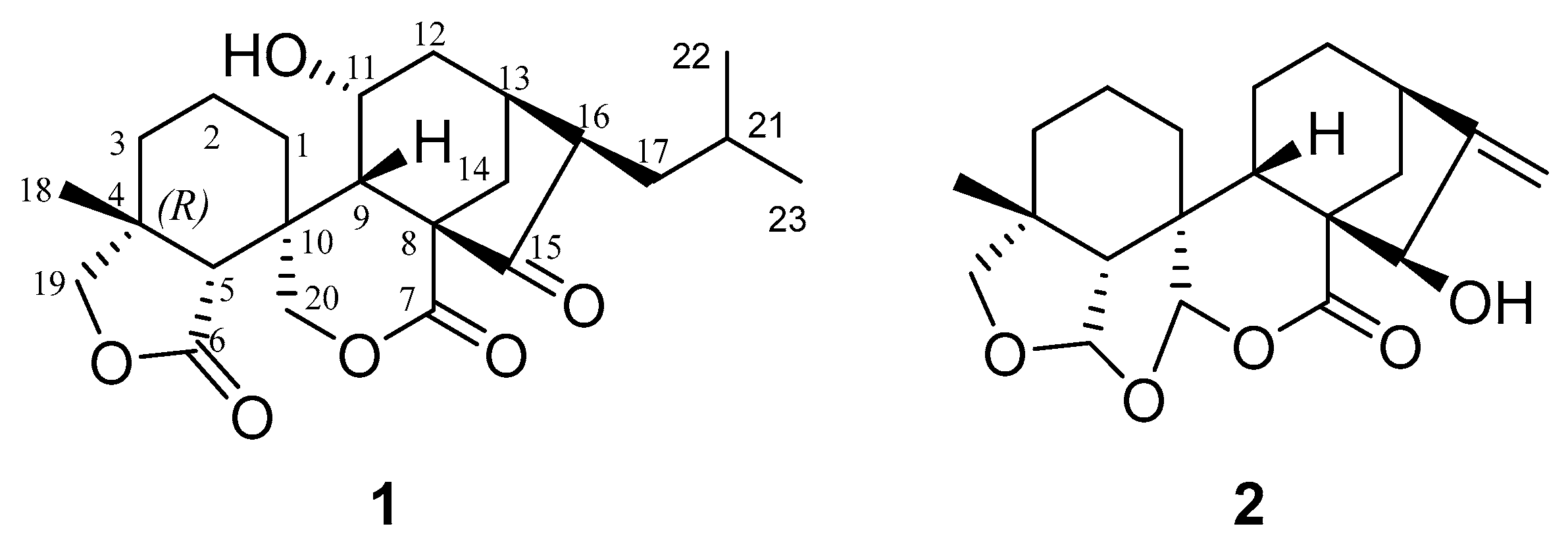
Figure 1.
Structures of compounds 1–2.
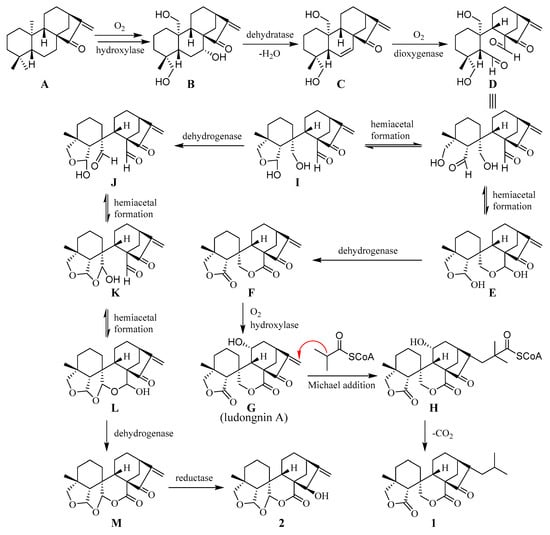
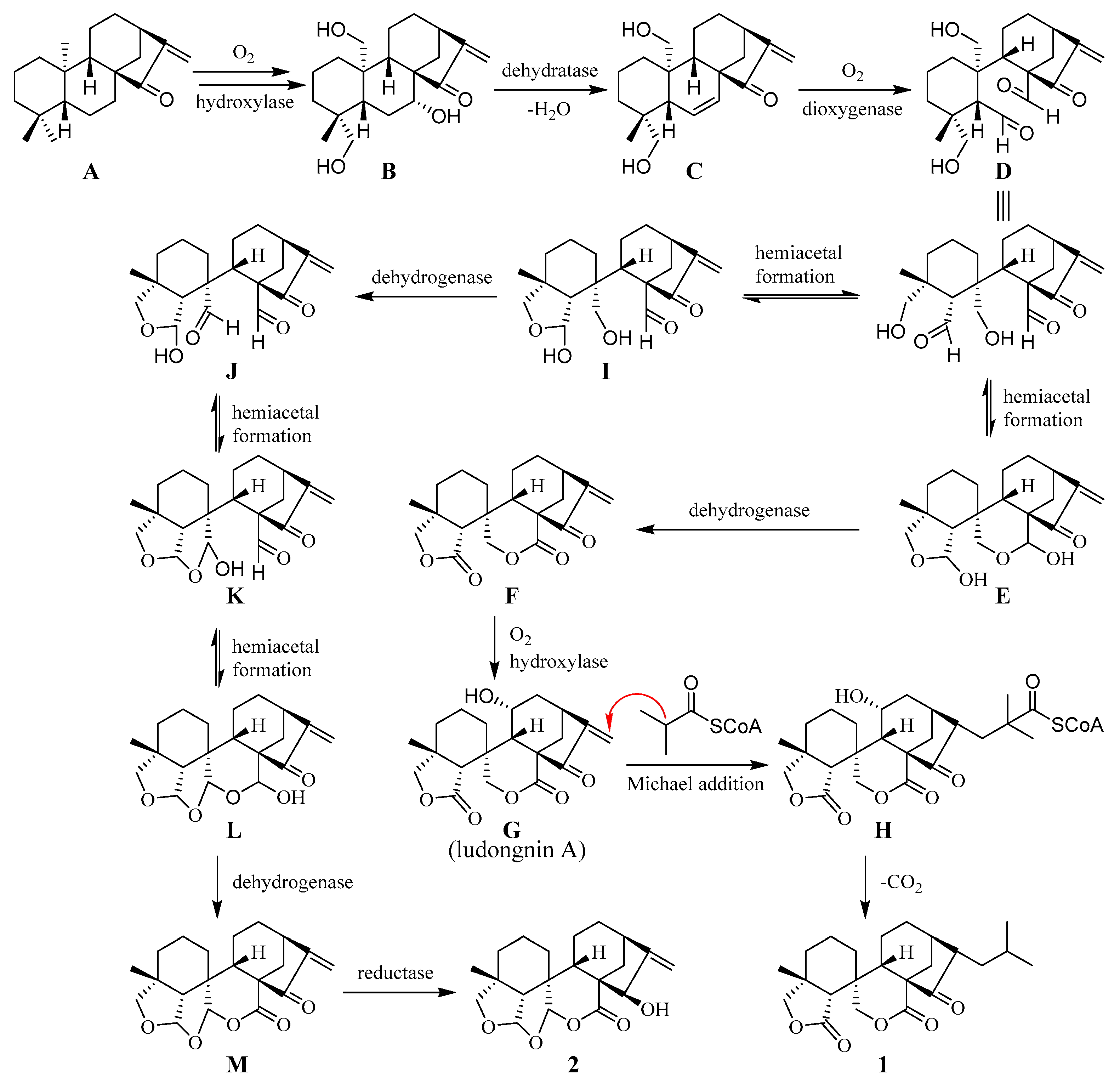
Scheme 1.
Proposed biogenetic pathway of 1 and 2.
2. Results and Discussion
2.1. Compound Identification
Dried leaves of I. rubescens were subjected to MeOH extraction, yielding a corresponding MeOH extract, which was chromatographed with RP-8 and silica gel chromatography to yield guidongnin I (1) and guidongnin J (2).
Compound 1 was isolated and crystalized as block crystals in MeOH (m.p. 247–248 °C), −127.27 (c 0.22, MeOH). It was identified to have the molecular formula of C23H32O6 elucidated from its HRESIMS analyses (found m/z 427.2091 [M + Na]+, calcd. for C23H32O6Na, 427.2086), indicating eight degrees of unsaturation. The IR absorption of 1 displayed the presence of hydroxyl (νmax 3380 cm−1) and carbonyl (1697, 1749 cm−1) groups. The 13C NMR (Table 1) data of 1 displayed 23 carbon signals, which were identified to belong to three non-oxy quaternary carbons [δC 41.2 (C-4), 56.1 (C-8), 39.2 (C-10)], a ketone group [δC 215.9 (C-15)], two lactones [δC 177.0 (C-6), 174.9 (C-7)], five non-oxy methines [δC 47.7 (C-5), 43.3 (C-9), 32.0 (C-13), 52.8 (C-16), 27.3 (C-21)], an oxy methine [δC 65.2 (C-11)], six non-oxy methylenes [δC 28.5 (C-1), 19.3 (C-2), 33.6 (C-3), 32.3 (C-12), 36.3 (C-14), 37.3 (C-17)], two oxy methylenes [δC 76.9 (C-19), 72.9 (C-20)], and three methyl groups [δC 22.1 (C-18), 21.8 (C-22), 23.7 (C-23)]. On the basis of the literature data [14] and the chemotaxonomic considerations, 1 was determined to have a 6,7-seco-7,20-olide-ent-kaurane skeleton structure, which was confirmed by the analysis of the 2D NMR correlation data (Figure 2A). However, unlike the common ent-kaurane diterpenoids that contain 20 carbons in the skeletons, 1 was shown to have 23 carbons in its skeletal structure. The extra three carbons were found to belong to an isopropyl group, which formed a carbon-carbon bond directly connected to C-17, as determined by the analysis of the 1D and 2D NMR data. In addition, the COSY spectrum of 1 showed the chained cross-peak correlations between H2-1, H2-2, and H2-3, between H-9, H-11, H2-12, H-13, and H2-14, and between H-13, H-16, H2-17, H-21, H2-22, and H2-23, and the HMBC correlations from H-11 to C-8 and C-13, H-19 to C-3, C-5, C-6, C-18, H-20 to C-5, C-7 and C-18, H-21 to C-17, and C-22 and C-23. The stereochemistry of 1 was further elucidated by observing the spatially closed cross-peak signals of the protons in the NOESY spectrum. Because of the NOE correlations of H-11 with H-2β, H-9β and H-12β, and H-16 with H-13α and H-14β, the H-11 was determined to be β-oriented, whereas H-16 was α-oriented.

Table 1.
1H (600 MHz) and 13C (150 MHz) NMR data for 1 in CD3OD, 2 in CDCl3.
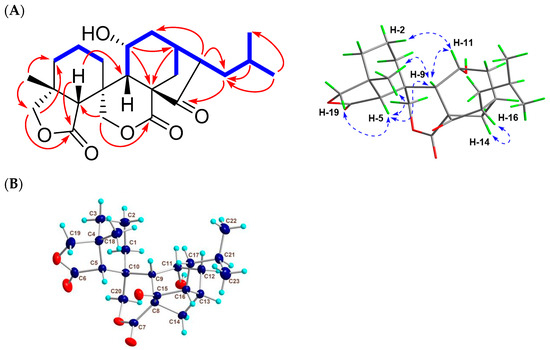
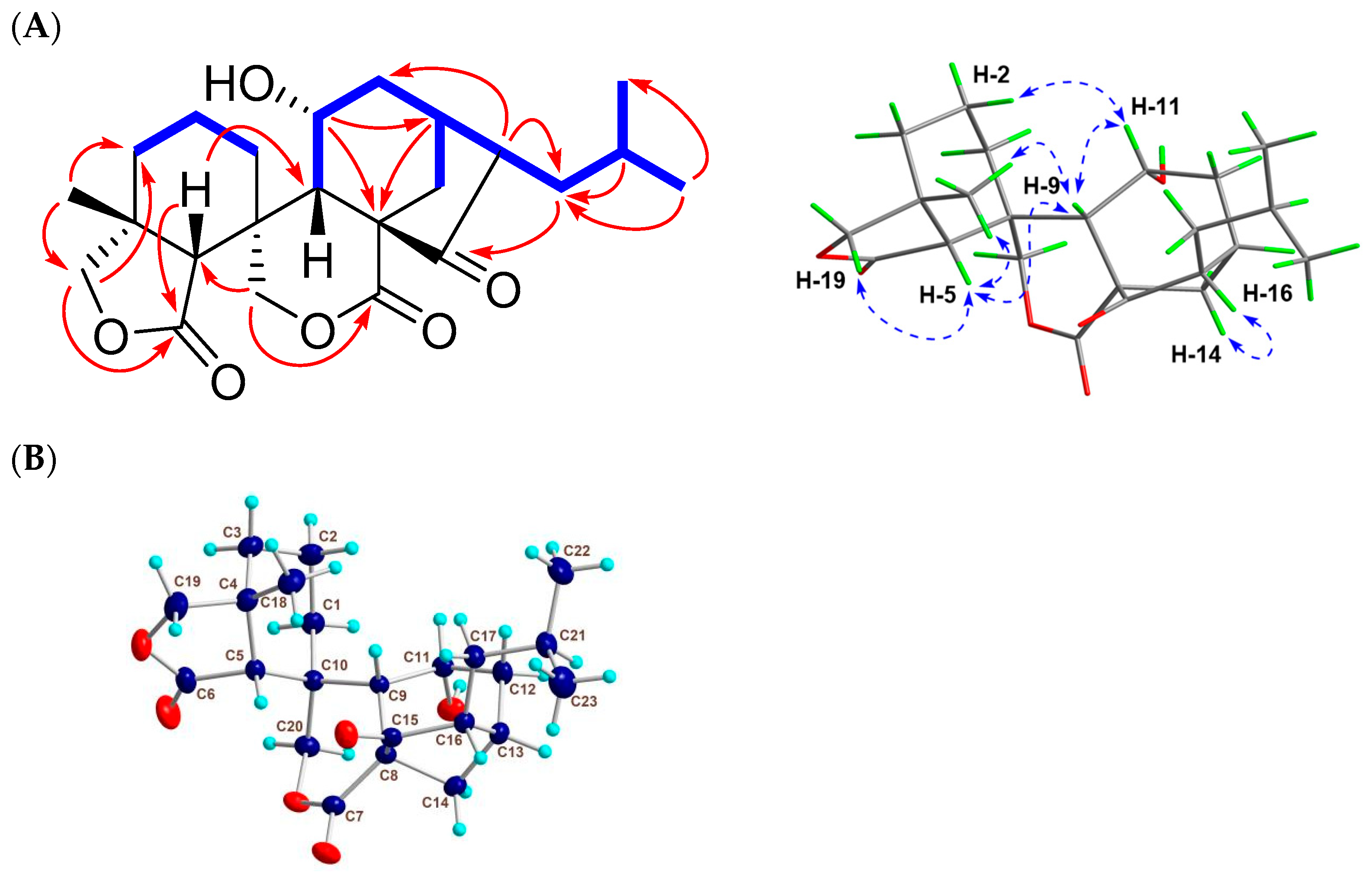
Figure 2.
2D NMR correlations and X-ray data of 1. (A) Key 1H-1H COSY ( ), HMBC (
), HMBC ( ), and NOESY (
), and NOESY ( ) correlations of 1. (B) Single-crystal X-ray structure of 1.
) correlations of 1. (B) Single-crystal X-ray structure of 1.
 ), HMBC (
), HMBC ( ), and NOESY (
), and NOESY ( ) correlations of 1. (B) Single-crystal X-ray structure of 1.
) correlations of 1. (B) Single-crystal X-ray structure of 1.
To confirm the absolute stereochemistry, 1 was dissolved in MeOH, followed by crystallization to afford a crystal suitable for an X-ray diffraction analysis. As a result of the final refinement, the Flack parameter of 0.00 (6) suggested its (4R, 5R, 8S, 9S, 10R, 11R, 13S, and 16R) absolute configuration (Figure 2B). Thus, compound 1 (guidongnin I) was defined as 16β-isopropyl-11α-hydroxy-6,7-seco-6,19:7,20-diolide-ent-kaur-15-one.
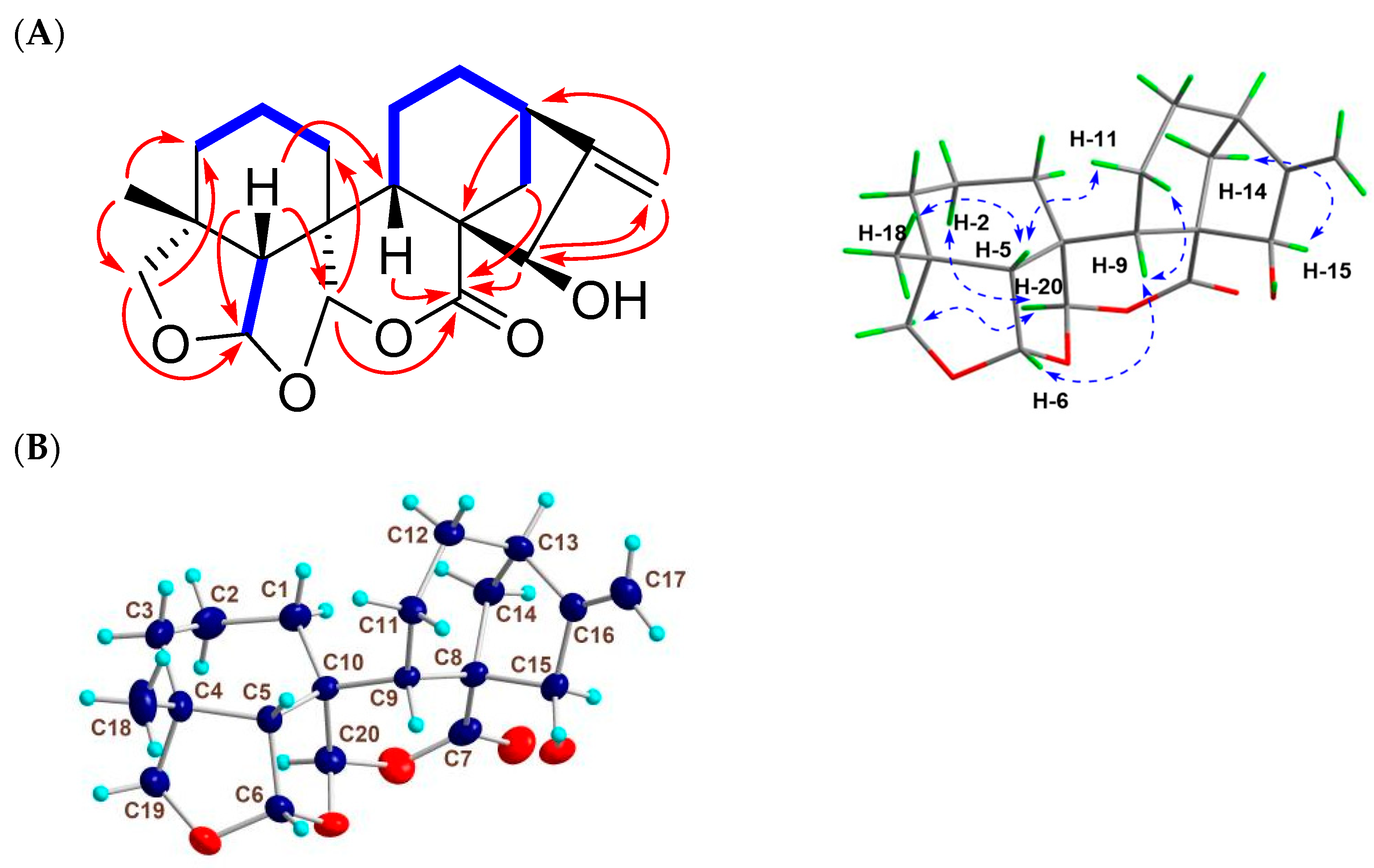
Compound 2 was isolated and finally obtained as colorless flaky crystals (MeOH, melting point: 223.9–226.1 °C), and the HRESIMS sodium adduct at m/z 369.1673 [M + Na]+ (calcd for C20H26O5Na, 369.1666) determined its molecular formula as C20H26O5. Its IR absorptions at νmax 1722 and 3419 cm−1 implied the existence of carbonyl and hydroxyl groups in 2. Based on the analysis of the 13C- and DEPT-NMR spectral data (Table 1), the 20 carbons in 2 were identified to be two olefinic carbons [δC 153.3 (C-16), 107.7 (C-17)], the carbonyl carbon of a lactone [δC 173.5 (C-7)], three non-oxygenated quaternary carbons [δC 40.2 (C-4), 51.1 (C-8), 44.5 (C-10)], three non-oxy methine carbons [δC 56.0 (C-5), 33.3 (C-9), 39.6 (C-13)], an oxy methine [δC 79.2 (C-15)], two acetal carbons [δC 106.7 (C-6), 104.1 (C-20)], six non-oxy methylene carbons [δC 26.4 (C-1), 17.1 (C-2), 30.6 (C-3), 18.0 (C-11), 32.7 (C-12), 37.9 (C-14)], one oxy methylene carbon [δC 76.3 (C-19)], and one methyl carbon [δC 31.0 (C-18)]. By searching for the literature compounds of ent-kaurenes, 2 was found to show similar 1H and 13C NMR data to the known compound macrocalyxoformin E [15]. The main structural difference between the two compounds was that 2 formed an extra tetrahydrofuran ring between C-6 and C-19, as evidenced by the presence of the HMBC correlations of H-6 to C-19 (δC 76.3) and of Ha-19 (δH 3.46, d, J = 9.0) to C-6 (Figure 3A). The presence of the NOE correlations of H-6 with H-5β, H-9β and H3-18, H-20 with H-2α and H-19α, and H-15 with H-14β suggested that H-6 was β-oriented, while H-15 and H-20 were α-oriented (Figure 3A). Furthermore, the X-ray diffraction analysis of 2 confirmed the relative configurations of 2 (Figure 3B) as 4R*, 5R*, 6S*, 8S*, 9S*, 10R*, 13R*, 15R*, and 20R*. Compound 2 was thus identified as 15β-hydroxy-6,19: 6,20-diepoxy-6,7-seco-7,20-olide-ent-kaur-16-en, and given the trivial name guidongnin J.
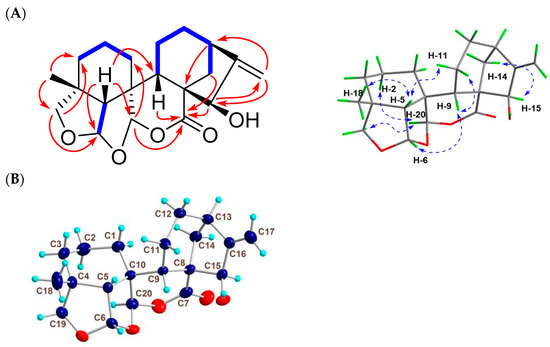
Figure 3.
2D NMR correlations and X-ray data of 2. (A) Key 1H-1H COSY ( ), HMBC (
), HMBC ( ), and NOESY (
), and NOESY ( ) correlations of 2. (B) Single-crystal X-ray structure of 2.
) correlations of 2. (B) Single-crystal X-ray structure of 2.
 ), HMBC (
), HMBC ( ), and NOESY (
), and NOESY ( ) correlations of 2. (B) Single-crystal X-ray structure of 2.
) correlations of 2. (B) Single-crystal X-ray structure of 2.
Plausible biogenetic pathways for compounds 1 and 2 are postulated in Scheme 1. Guidongnins I (1) and J (2) could be formed through a series of reactions starting from kau-16-en-15-one (A), the normal ent-kaurene that commonly exists in Isodon plants. The oxidations at C-7, -19, and -20 of A produce compound B, which undergoes dehydration to form a double bond between C-6 and C-7 by losing an H2O molecule. The cleavage of the 6,7 double bond in C by dioxygenase leads to the formation of compound D with two carbaldehyde groups. D then undergoes two separate biogenetic pathways to produce 1 and 2. When the carbaldehyde at C-5 in D reacts with 19-OH to form the first tetrahydrofuran group through a hemiacetal linkage, followed by the 2nd and 3rd hemiacetal formations between C-6 and C-20 (J) and between C-20 and C-7 (K), respectively, compound L with a three-fused cyclic ether ring system is yielded. Further oxidation of the C-7 acetal group, followed by the reduction of the carbonyl carbon at C-15, L is transformed to compound 2 through M. On the other hand, when hemiacetal conversions take place for both carbaldehyde groups in D at the same time, compound E is produced. With the subsequent oxidations at C-7 and C-11, E is converted to ludongnin A (G), a major component (0.63%) in Isodon rubescens Hara var lushiensis [16]. Ludongnin A (G) further reacts with isobutyryl-CoA via a Michael-like addition reaction to afford H. Upon losing CO2, compound 1 is eventually produced from H.
2.2. Biological Activity
It is reported that the I. rubescens extract was found to inhibit a variety of cancer cells [17]. Previously, the diterpenoids isolated from this species, such as oridonin, rosthorin, lushanrubescensin H, and rabdosin A, showed significant inhibitory activity against the growth of tumor cell lines [18]. Therefore, guidongnins I (1) and J (2) were evaluated for their cytotoxicities against various human cancer cell lines [A-549 (lung), HL-60 (leukemia), MCF-7 (breast), HepG2 (liver), and COLO-205 (colon)] using the CCK-8 method as previously reported [19,20,21]. The two compounds were inactive at a concentration of 100 µM, except for 2 on HepG2 cells. Compound 2 was found to show antiproliferative activity against HepG2 cells at an IC50 of 27.14 ± 3.43 µM. Since the carbonyl group is reduced to a hydroxy group and 2 contains no α, β-conjugate system, which is normally required for the biological activity of an ent-kaurane diterpenoid, the antiproliferative activity of 2 against HepG2 cells could arise from the three-fused ring system. Further study of the fused ring system with an activity relationship is thus needed to explore this unique structural unit of ent-kaurene compounds.
3. Materials and Methods
3.1. General Experimental Procedures
An XRC-1 micro-melting point apparatus (Sichuan Weitai, Chengdu, China) was used for the measurement of the compounds’ melting points. Optical rotations were determined using a Jasco P-1010 polarimeter (Perkin-Elmer, Waltham, MA, USA). The IR spectra were recorded on a VECTOR22 infrared spectrometer using the KBr pellets method. A Bruker Avance spectrometer was used for recording the NMR spectra. The HR-ESI-MS data were obtained using a Bruker Q-TOF mass spectrometer (Bruker, Karlsruhe, Germany). X-ray data were collected on a Bruker APEX-II CCD instrument (Bruker, Rheinstetten, Germany) equipped with Cu Kα radiation for 1 and a Bruker APEX-II instrument equipped with Mo Kα radiation for 2. Silica gel (200–300 mesh) was used for column chromatography, while silica gel GF254 (0.2 mm) was used for analytical TLC. Prior to use, all solvents were distilled. Detailed information is displayed in the Supplementary Materials.
3.2. Plant Material
I. rubesens twigs and leaves were collected from Guiyang, Guizhou Province, China, in July 2018. The plant material was identified by Professor Junhua Zhao, and a voucher specimen (No. 20180713) was deposited at Guizhou University of Traditional Chinese Medicine.
3.3. Extraction and Isolation
I. rubescens twigs and leaves (8.8 kg) were dried, powdered, and extracted with MeOH (3 × 10 L) at room temperature to give a crude extract (1.3 kg) after concentration. The resultant residue was suspended in H2O, which was then extracted with petroleum ether (1 L × 2) and EtOAc (1 L × 3). Subsequently, the EtOAc-soluble fraction (230.2 g) was divided into 6 fractions (A-F) using silica gel column chromatography, eluting with gradients of petroleum ether/EtOAc (100:1–0:1). Fraction C (37.4 g) was applied on a RP-8 (octylsilane bonded silica gel) column (MeOH/H2O, 5:95–50:50) to produce subfractions C1-C4. Fraction C2 (6.8 g) was separated by silica gel column chromatography (CH2Cl2/EtOAc, 100:1–1:1) to obtain compounds 1 (20 mg) and 2 (14 mg), followed by recrystallization in MeOH to yield suitable crystals for X-ray crystallographic analysis.
Guidongnin I (1): colorless flaky crystals; m.p. 247~248 °C; −127.27 (c 0.2200, CH2Cl2); IR (KBr) νmax 3380, 2941, 2864, 2357, 1749, 1697, 1468, 1106, 1059, 1033, 868 cm−1; 1D NMR data, see Table 1; HR-ESI-MS m/z 427.2091 [M + Na]+ (calcd. for C23H32O6Na, 427.2086).
Guidongnin J (2): colorless transparent sheet-shaped crystals; m.p. 223.9~226.1 °C; −32.92 (c 2.4300, MeOH); IR (KBr) νmax 3419, 2938, 1723, 1458, 1417, 1297, 1252, 1125, 1086, 1057, 980 cm−1; 1D NMR data, see Table 1; HR-ESI-MS m/z 369.1673 [M + Na]+ (calcd for C20H26O5 Na, 369.1666).
3.4. X-ray Data of Compounds 1–2
Colorless block crystals of guidongnins I–J (1–2) were obtained from the MeOH solution. Crystallographic data for 1 were collected on a Bruker APEX-II CCD instrument using Cu Kα radiation, and for 2 were collected on a Bruker APEX II CCD instrument using Mo Kα radiation (Table 2). With the use of Bruker SAINT, cell refinement and data reduction were accomplished, where the structure was solved by means of SHELXTL [22,23]. The crystallographic data for 1 (CCDC 2288451), 2, and (CCDC 2261016) can be obtained on request at www.ccdc.cam.ac.uk/data_request/cif (accessed on 10 May 2023).

Table 2.
Sample and crystal data for compounds 1 and 2.
3.5. Cytotoxicity Assay of Compounds 1–2
The anti-proliferative activities of compounds 1–2 against human cancer cell lines were evaluated using the CCK-8 method, as previously described [18,19]. In the CCK-8 assay, the A549 cells were grown in Roswell Park Memorial Institute (RPMI) 1640 medium. HL-60, MCF-7, HepG2, and COLO-205 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM). All used medium was supplemented with 10% fetal bovine serum (FBS) in a 37 °C incubator with a humidified atmosphere containing 5% CO2. A density of 2 × 105 cell/mL cancer cells (200 μL) were seeded in 96-well microtiter plates and exposed to 200 μL of various concentrations of compounds in triplicate for 72 h. The initial concentrations of the compounds were 10.0 mM in DMSO and then diluted into 20, 10, 5, 2.5, 1.25, and 0.625 μM in RPMI-1640/DMEM medium. Then, 100 μL of the test solutions were added to each well and further incubated for 72 h. The CCK-8 solution (10 μL) was then added to each well and incubated for 2–3 h. Absorbance was then measured at 450 nm.
4. Conclusions
In conclusion, the present phytochemical study of I. rubescens resulted in the isolation of two novel 6,7-seco-7,20-olide-ent-kaurene diterpenes (1 and 2). Their chemical structures were elucidated by means of comprehensive spectroscopic data analysis (Figures S1–S18) and further confirmed by single-crystal X-ray crystallographic data. The isolates were evaluated for their bioactivities on a panel of human cancer cell lines. Compound 2 demonstrated antiproliferative activity against HepG2 cells, which may warrant 2 as a lead molecule for further chemical and biological exploration since it contains a three-fused ring system instead of α,β-conjugate system that is normally required for the biological activity of an ent-kaurane diterpenoid.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241713451/s1.
Author Contributions
Methodology, J.Z. (Juan Zou), and J.Y.; investigation, Y.L., and J.Z. (Jingjie Zhang); writing—original draft preparation, J.Z. (Juan Zou); writing—review and editing, C.Z., and H.Z.; visualization, J.Z. (Juan Zou); supervision, H.Z.; project administration, K.H.; funding acquisition, L.P. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported financially by the Natural-Scientific Research Program of Department of Education of Guizhou Province, Qianjiaoji [No (2023)070], National Natural Science Foundation of China (No 82204605), the Research Grants Council of the Hong Kong Special Administrative Region, China (Project No. 12102219), Hong Kong Baptist University, Research Committee, and University Grants Committee of the Hong Kong Special Administrative Region, China (UGC Research Matching Grant Scheme RMGS2019-1-19).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yan, B.C.; Hu, K.; Sun, H.D.; Puno, P. Recent Advances in the Synthesis of Isodon Diterpenoids and Schinortriterpenoids. Chin. J. Org. Chem. 2018, 38, 2259–2280. [Google Scholar] [CrossRef]
- Tan, Q.; Hu, K.; Li, X.N.; Yang, X.Z.; Sun, H.D.; Puno, P.T. Cytotoxic C-20 non-oxygenated ent-kaurane diterpenoids from Isodon wardii. Bioorg. Chem. 2023, 135, 106512. [Google Scholar] [CrossRef]
- Li, X.R.; Hu, K.; Yan, B.C.; Li, X.N.; Sun, H.D.; Liu, Y.; Puno, P.T. Scopariusicides D–M, ent-clerodane-based isomeric meroditerpenoids with a cyclobutane-fused γ/δ-lactone core from Isodon scoparius. Bioorg. Chem. 2022, 127, 105973. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.Y.; Li, X.N.; Sun, H.D.; Zhang, H.B.; Puno, P.T. Scopariusols L-T, nine new ent-kaurane diterpenoids isolated from Isodon scoparius. Chin. J. Nat. Med. 2018, 16, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, W.G.; Sun, H.D.; Pu, J.X. Diterpenoids from Isodon species: An update. Nat. Prod. Rep. 2017, 34, 1090–1140. [Google Scholar] [CrossRef] [PubMed]
- National Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Zou, J.; Pan, L.T.; Li, Q.J.; Zhao, J.H.; Pu, J.X.; Yao, P.; Gong, N.B.; Lu, Y.; Kondratyuk, T.P.; Pezzuto, J.M.; et al. Rubesanolides A and B: Diterpenoids from Isodon Rubescens. Org. Lett. 2011, 13, 1406–1409. [Google Scholar] [CrossRef]
- Zou, J.; Pan, L.T.; Li, Q.J.; Pu, J.X.; Yao, P.; Zhu, M.; Banas, J.A.; Zhang, H.J.; Sun, H.D. Rubesanolides C–E: Abietane diterpenoids isolated from Isodon rubescens and evaluation of their anti-biofilm activity. Org. Biomol. Chem. 2012, 10, 5039–5044. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zou, J.; Wang, Y.X.; Zhao, C.L.; Ye, J.H.; Zhang, J.J.; Pan, L.T.; Zhang, H.J. Rubesanolides F and G: Two Novel Lactone-Type Norditerpenoids from Isodon Rubescens. Molecules 2021, 26, 3865. [Google Scholar] [CrossRef] [PubMed]
- Belaabed, S.; De Leo, M.; Velotto, S.; Malafronte, N.; D’Ambola, M. A new glucosidic iridoid from Isodon rubescens. Rev. Bras. Farmacogn. 2018, 28, 294–297. [Google Scholar] [CrossRef]
- Wen, C.M.; Chen, S.; Yuan, F.; Liu, X.M.; Song, F.J.; Mei, Z.N.; Yang, X.F.; Yang, G.Z. Diterpenoids from Isodon rubescens and their nitric oxide production inhibitory activity. RSC Adv. 2019, 9, 40628–40635. [Google Scholar] [CrossRef]
- Gao, X.M.; Luo, X.; Pu, J.X.; Wu, Y.L.; Zhao, Y.; Yang, L.B.; He, F.; Li, X.N.; Xiao, W.L.; Chen, G.Q.; et al. Antiproliferative Diterpenoids from the Leaves of Isodon rubescens. Planta Med. 2011, 77, 169–174. [Google Scholar] [CrossRef]
- Han, Q.B.; Li, M.L.; Li, S.H.; Mou, Y.K.; Lin, Z.W.; Sun, H.D. Ent-kaurane Diterpenoids from Isodon rubescens var. Lushanensis. Chem. Pharm. Bull. 2003, 51, 790–793. [Google Scholar] [CrossRef]
- Han, Q.B.; Zhao, Q.S.; Li, S.H.; Peng, L.Y.; Sun, H.D. Ent-Kaurane Diterpenoids from Isodon rubescens Collected in Guizhou Province. Acta Chim. Sin. 2003, 61, 1077–1082. [Google Scholar]
- Wang, Z.Q.; Wang, X.R.; Dong, J.G. Chemical Structures of Macrocalyxoformin B, C and E. Acta Bot. Sin. 1986, 28, 79–85. [Google Scholar]
- Zheng, X.R.; Gao, Z.Y.; Sun, H.D.; Lin, Z.W. The Structure of Ludongnin. Acta Bot. Yunnan 1984, 6, 316–320. [Google Scholar]
- Ding, Y.; Ding, C.Y.; Ye, N.; Liu, Z.Q.; Wold, E.A.; Chen, H.Y.; Wild, C.; Shen, Q.; Zhou, J. Discovery and development of natural product oridonin-inspired anticancer agents. Eur. J. Med. Chem. 2016, 122, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.F.; Dai, X.F.; Liu, Y.H.; He, X.R.; Gong, G. Isodon rubescens (Hemls.) Hara.: A comprehensive review on traditional uses, phytochemistry, and pharmacological activities. Front. Pharmacol. 2022, 13, 766581. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.L.; Liu, Y.Y.; Pei, Y.Z. Synthesis and biological evaluation of 3-(piperidin-4-yl)isoxazolo[4,5-d]pyrimidine derivatives as novel PI3Kδ inhibitors. Chin. Chem. Lett. 2015, 26, 1283–1288. [Google Scholar] [CrossRef]
- Guo, T.; Liu, X.L.; Zhang, Z.K.; Luo, Y.Q.; Li, T.; Li, L.; Wang, H.X.; Huang, Y.; He, J.; Chen, Q.Y.; et al. A recombinant Newcastle disease virus expressing MMP8 promotes oncolytic efficacy. Chin. Chem. Lett. 2021, 32, 3962–3966. [Google Scholar] [CrossRef]
- Wu, Z.Q.; Liu, Z.; Jiang, X.L.; Mi, Z.Y.; Meng, M.B.; Wang, H.; Zhao, J.L.; Zheng, B.Y.; Yuan, Z.Y. Depleting PTOV1 Sensitizes Non-Small Cell Lung Cancer Cells to Chemotherapy through Attenuating Cancer Stem Cell Traits. J. Exp. Clin. Cancer Res. 2019, 38, 341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXTL-PC, Version 5.1; Siemens Analytical Instruments. Inc.: Madison, WI, USA, 1997.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).