The Antitumoral Effect In Ovo of a New Inclusion Complex from Dimethoxycurcumin with Magnesium and Beta-Cyclodextrin
Abstract
1. Introduction
| Compound | Breast Cancer (Cell Line) | Reference |
|---|---|---|
| Curcumin | T47D, MDA-MB-415, SK-BR-3, BT-20, MDA-MB-231, MDA-MB-468, MDA-MB-453, MCF-7, MCF-10A | Latifah et al. (2006) [31] Hu et al. (2018) [32] Farghadani et al. (2021) [1] Abdelkader et at. (2022) [33] Ismail-Alhasawi (2022) [34] Fawzy et al. (2024) [35] |
| DAC | MCF-7 | Meza-Morales et al. (2023) [24] |
| DiMeOC | MCF-10A, MDA-MB-231, MDA-MB 435S, MCF-7 | Fuchs et al. (2009) [36] Yoon et al. (2014) [37] Kunwar et al. (2011) [38] Muhammad et al. (2021) [18] |
| DAC–Magnesium | MCF-7 | Meza-Morales et al. (2019) [22] |
| DAC–Zinc | MCF-7 | Meza-Morales et al. (2019) [22] |
| DAC–Manganese | MCF-7 | Meza-Morales et al. (2019) [22] |
| DiMeOC–Zinc | MCF-7 | Meza-Morales et al. (2023) [21] |
| DiMeOC–Copper | MCF-7 | Meza-Morales et al. (2019) [21] |
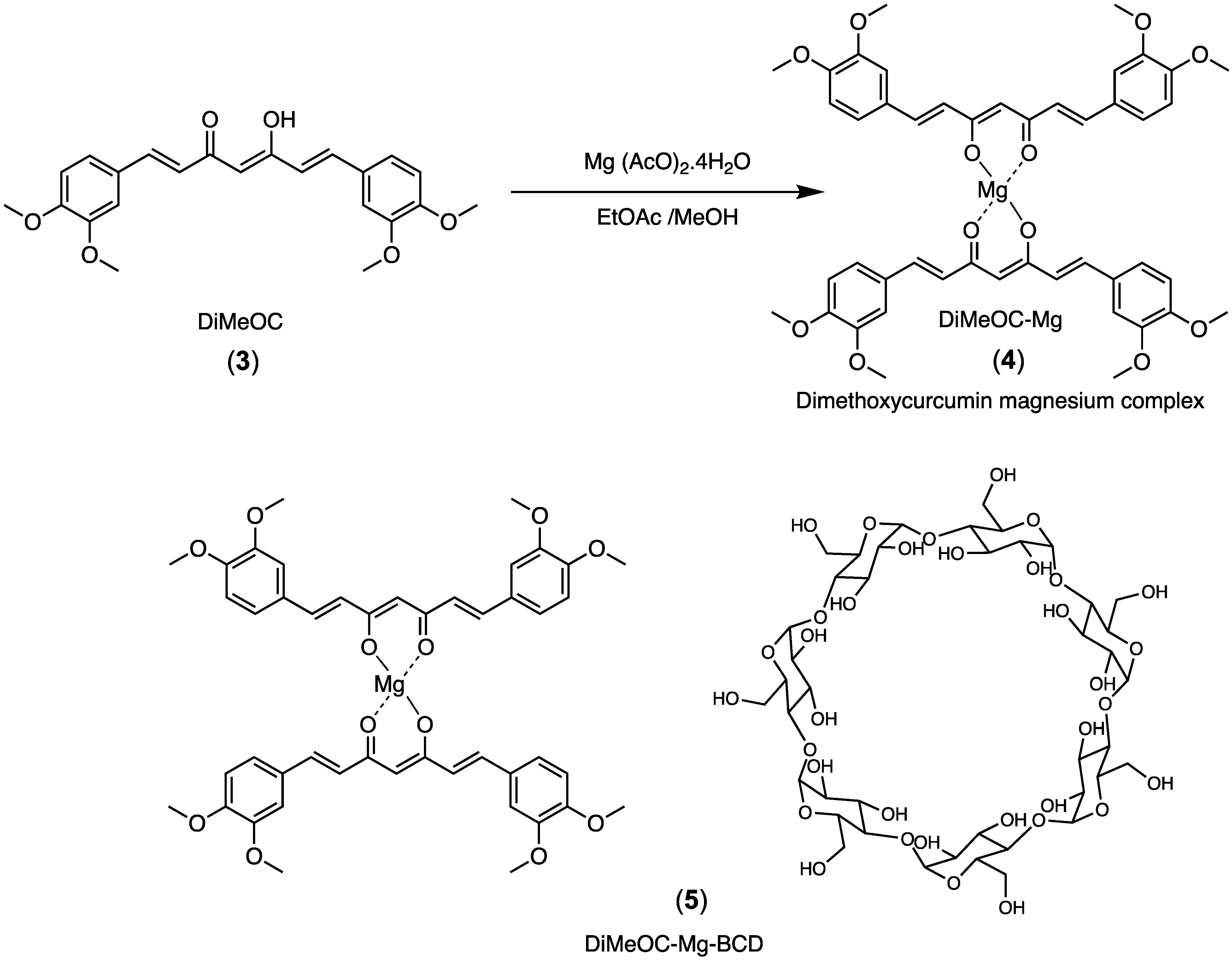
2. Results
3. Discussion
3.1. Infrarred
3.2. Mass Spectrometry
3.3. 1H NMR Liquid State
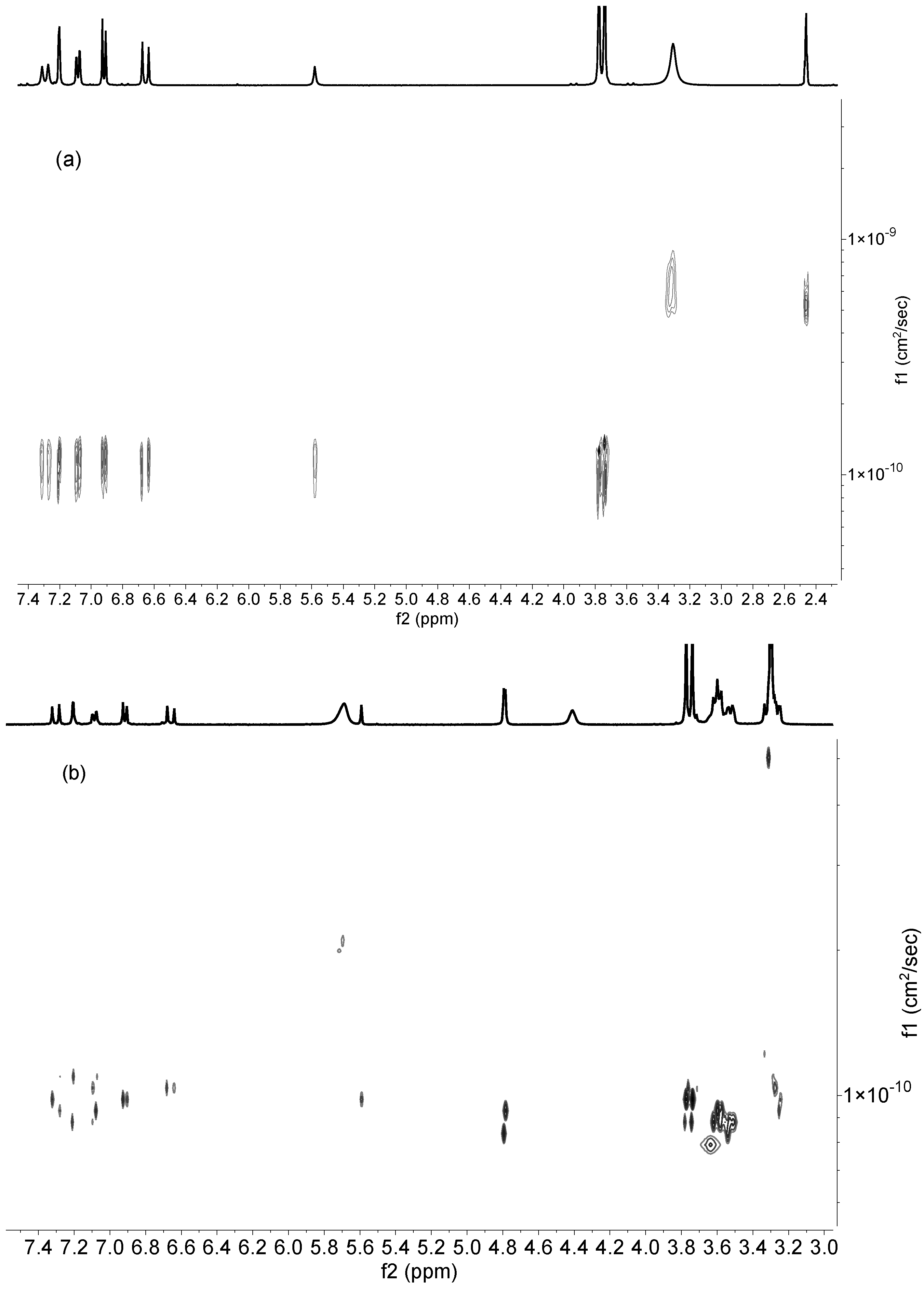
3.4. DOSY Spectra
3.5. Solubility, Ratio of Guest–Host, and HPLC
3.6. 13C CPMAS Solid-State NMR
- The guest (DiMeOC-Mg) was adequately complexed with BCD using the selected coprecipitation method.
- The relaxation phenomena for the guest molecule becomes very fast (low intensity of signals) when located at the cavity of BCD.
- The inclusion complex reveals an amorphization (broadening of signals) in agreement with the data obtained by scanning electron microscopy (SEM).
3.7. SEM Analysis
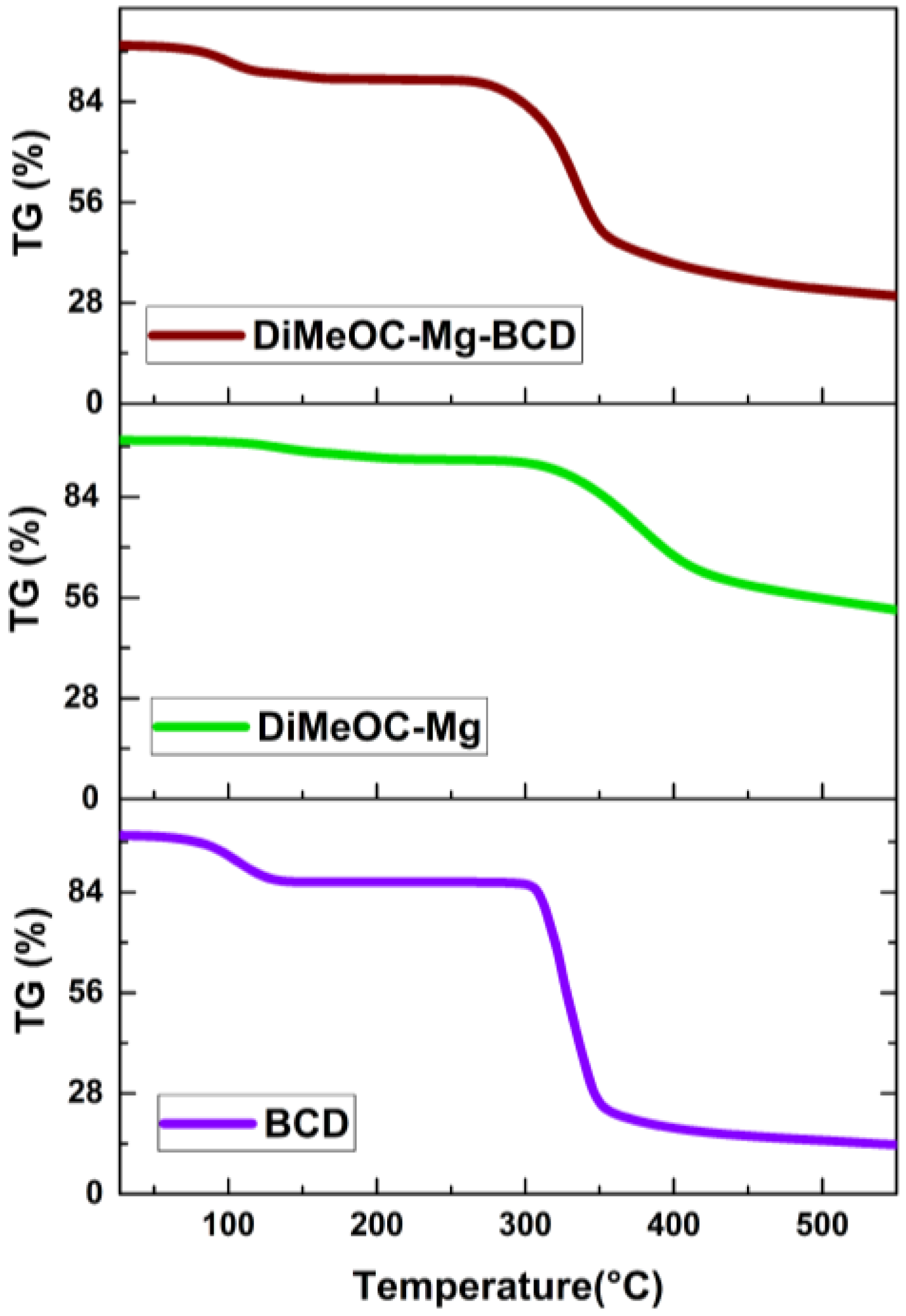
3.8. TGA and DSC Analysis
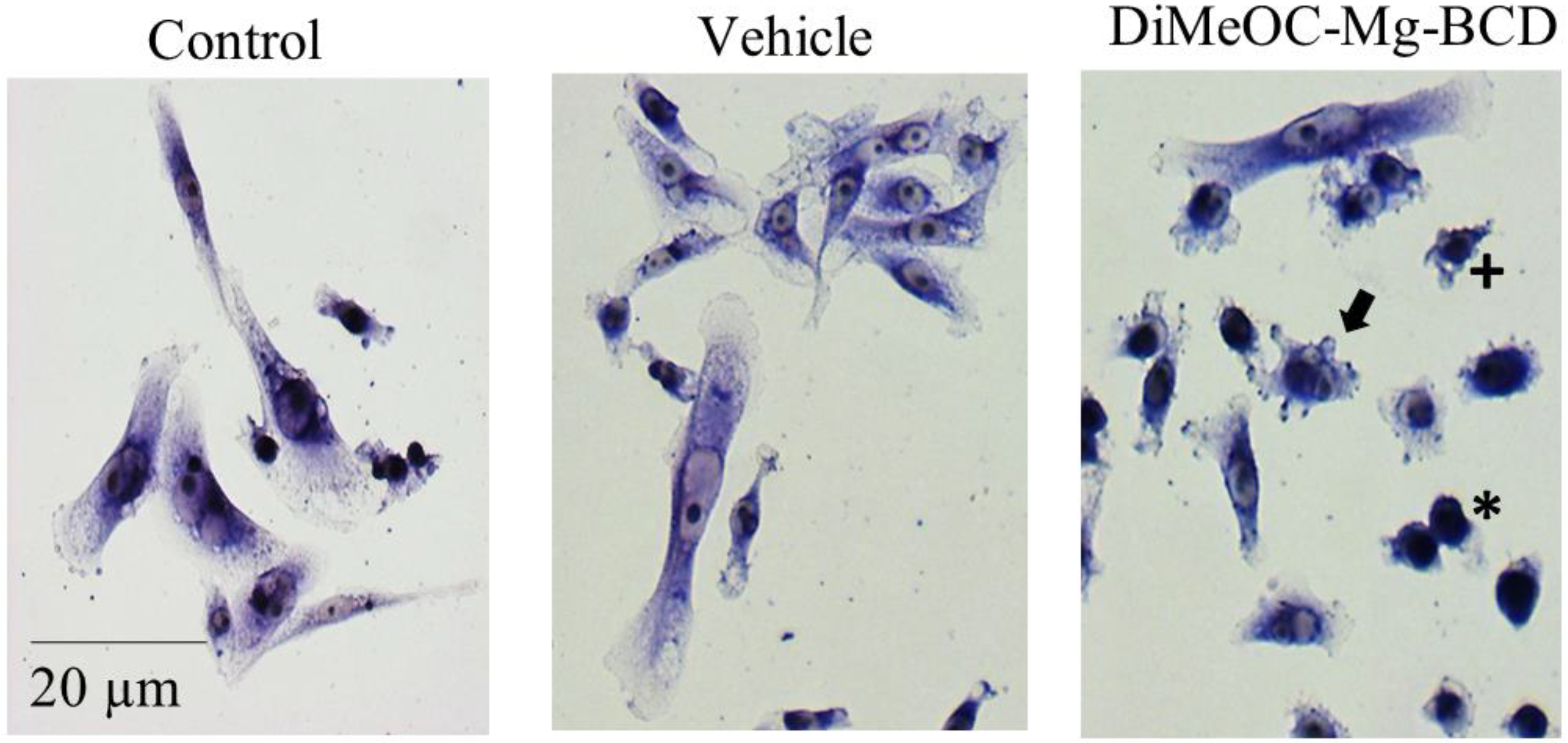
3.9. Cytotoxic Activity in Human Tumour Cells and Evaluation of Morphological Changes
3.10. Esterase Activity and Membrane Damage Induced by DiMeOC-Mg-BCD on MDA-MB-231
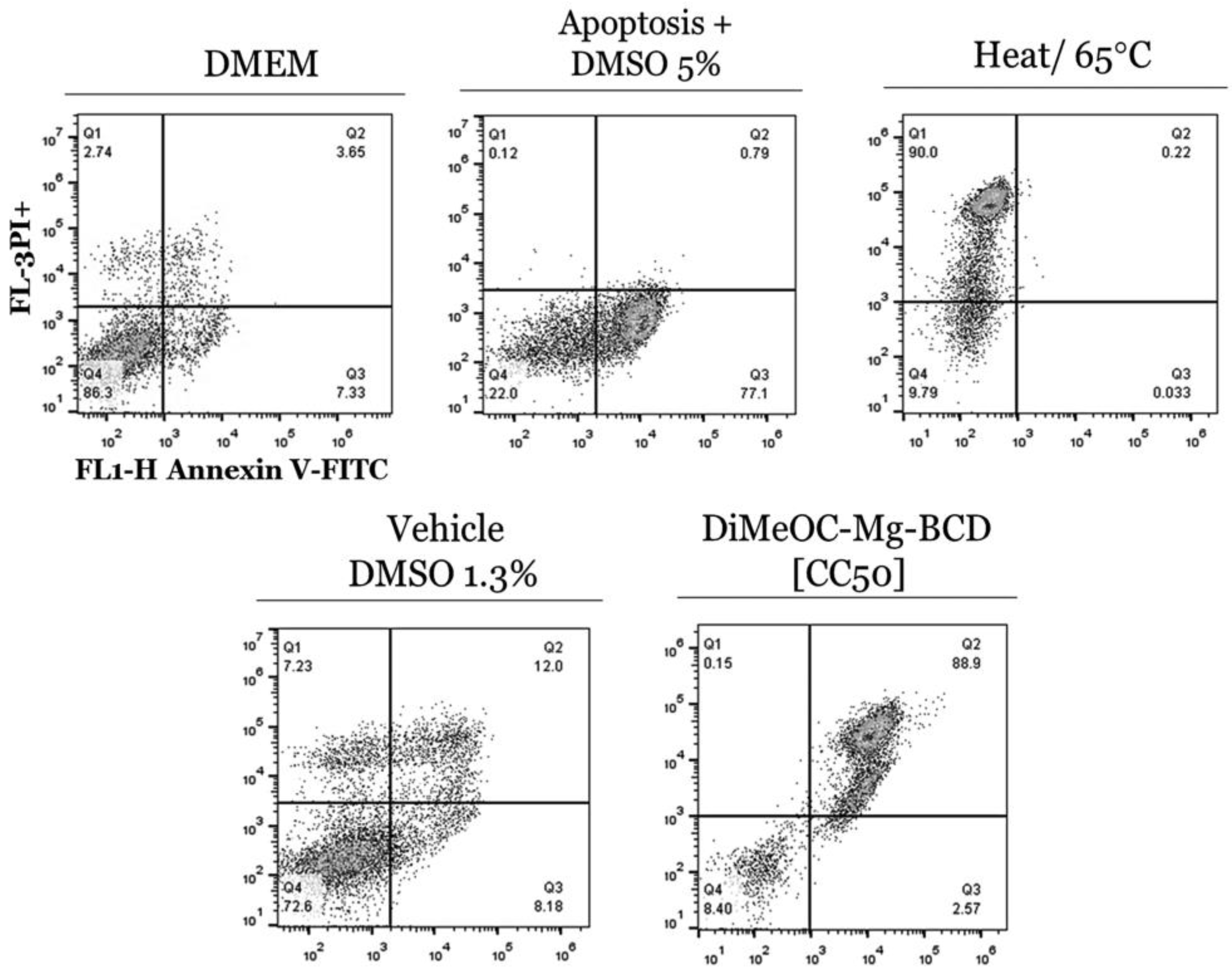
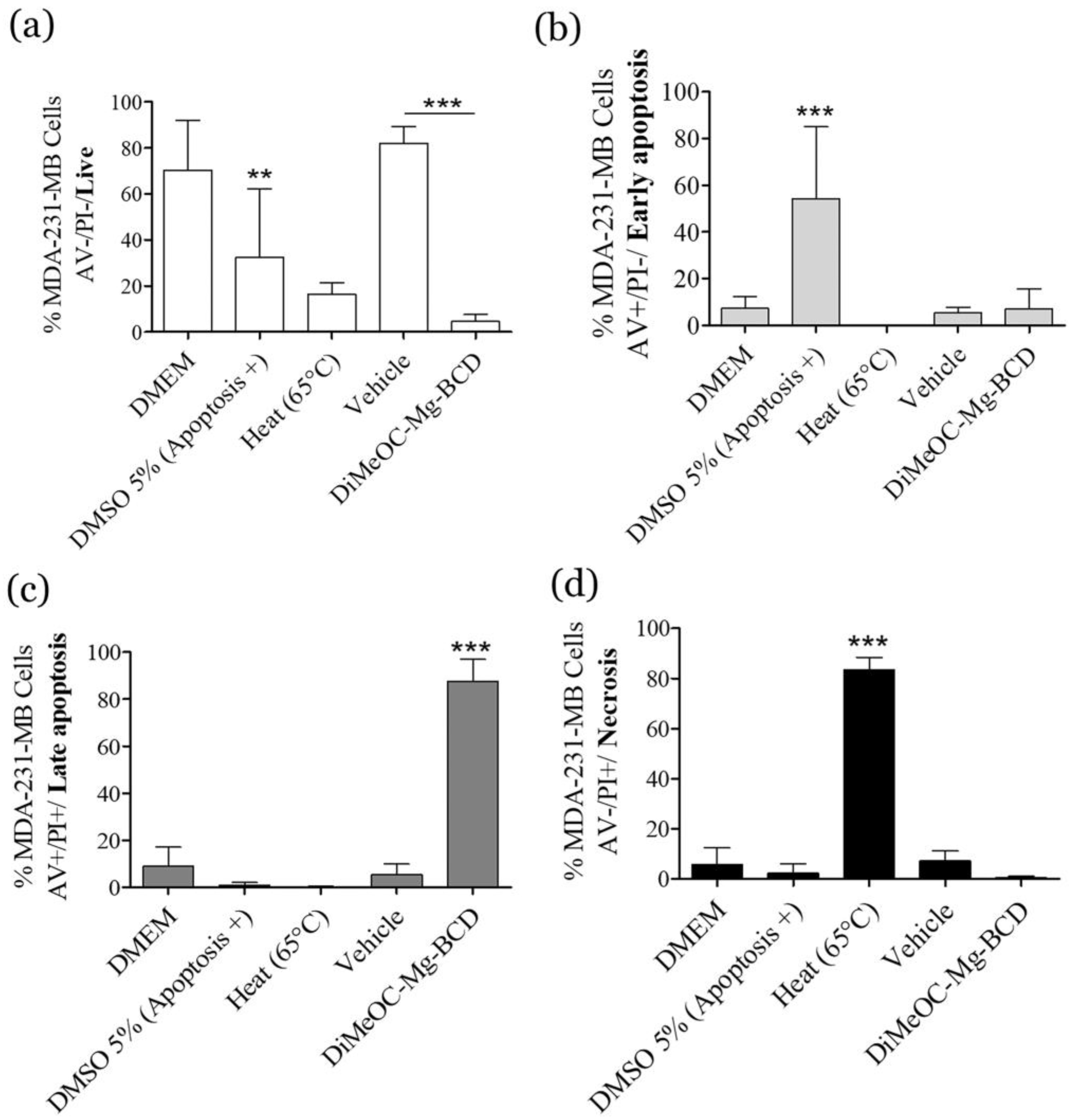
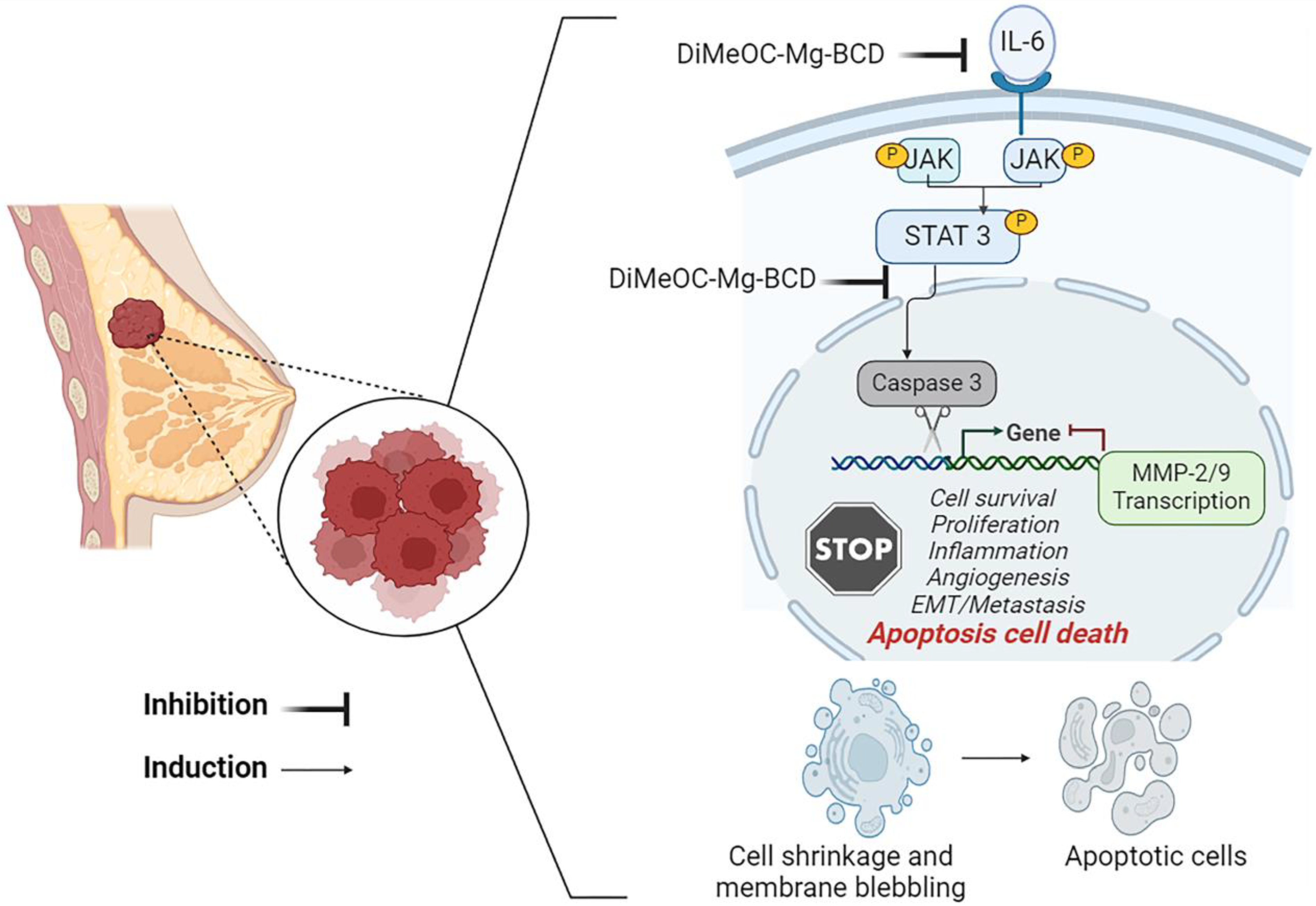
3.11. Apoptosis Induced by DiMeOC-Mg-BCD on MDA-231-MB Breast Cancer
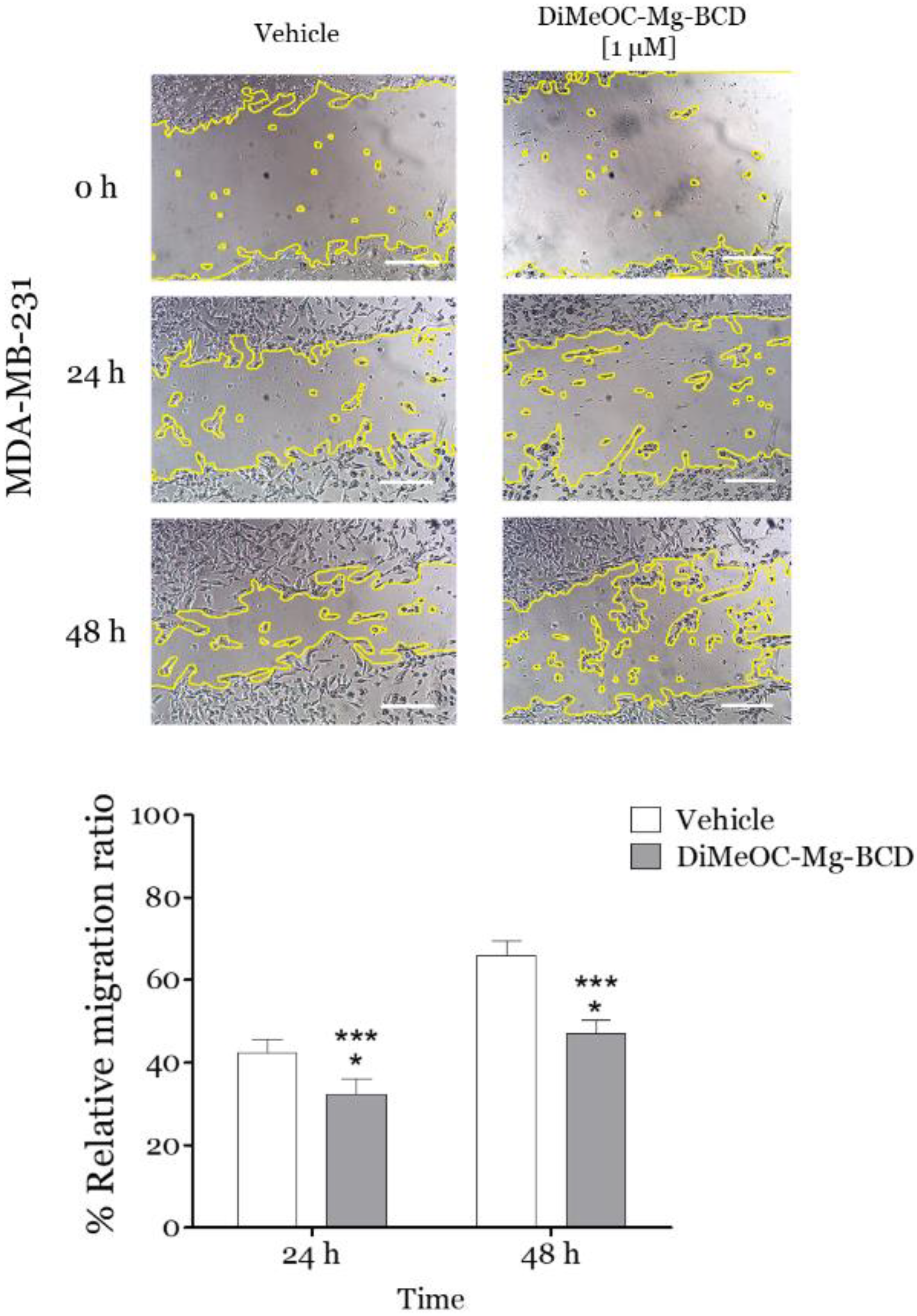
3.12. Effect of DiMeOC-Mg-BCD on MDA-MB-231 Cell Migration (Wound-Healing Assay)
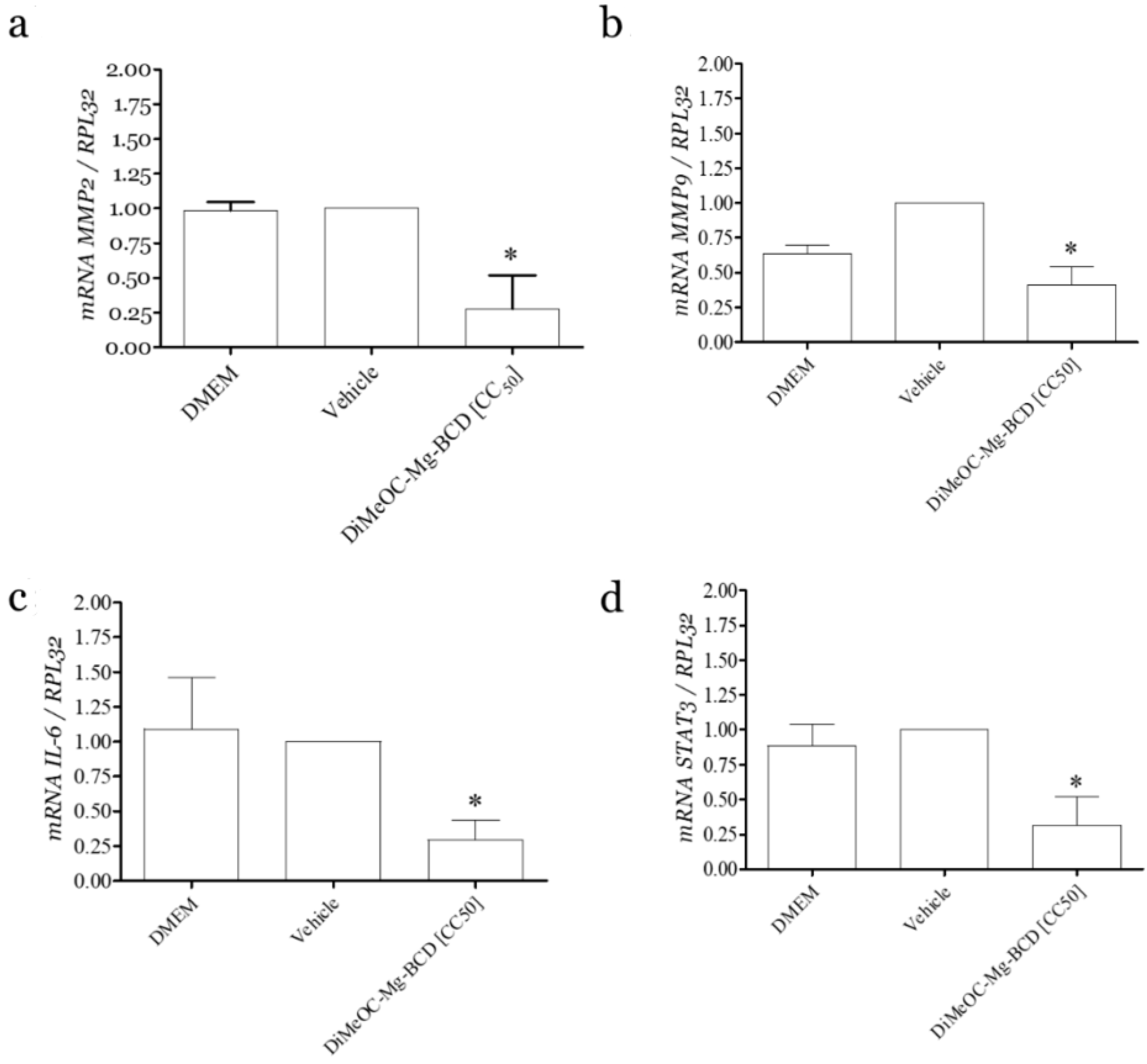
3.13. Effect of DiMeOC-Mg-BCD on MMP-2, MMP-9 and IL-6, and Total STAT3 Gene Expression on MDA-MB-231
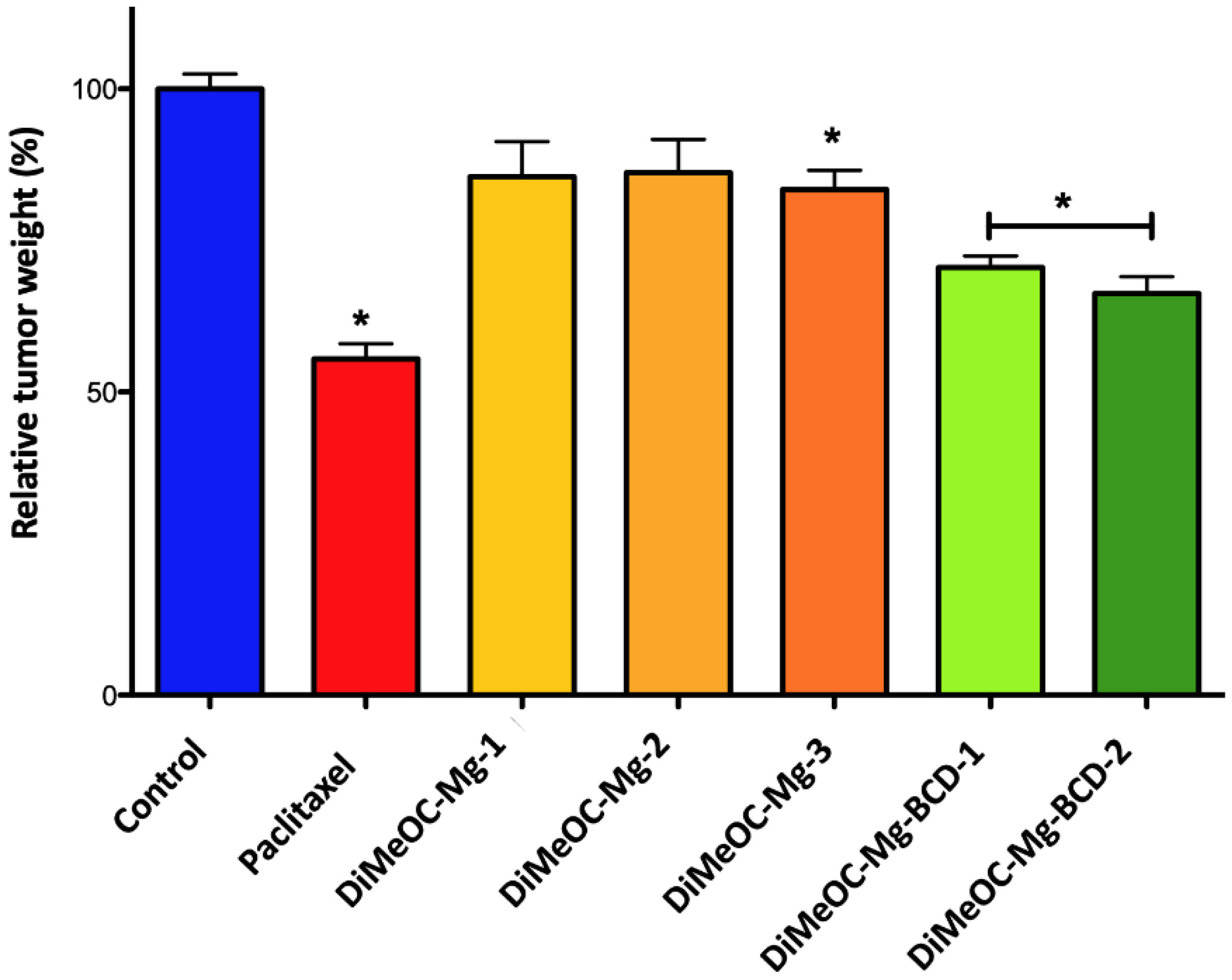
3.14. Antitumoral Activity and Embryotoxicity
4. Materials and Methods
Synthetic Procedures
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farghadani, R.; Naidu, R. Curcumin: Modulator of Key Molecular Signaling Pathways in Hormone-Independent Breast Cancer. Cancers 2021, 13, 3427. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Xiang, L.; Li, T.; Bai, Z. Cancer Hallmarks, Biomarkers and Breast Cancer Molecular Subtypes. J. Cancer 2016, 7, 1281–1294. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Li, J.; Wang, H.Q.; Zheng, H.B.; Ma, S.W.; Zhou, G.Z. Activation of Autophagy Promotes the Inhibitory Effect of Curcumin Analog EF-24 against MDA-MB-231 Cancer Cells. J. Biochem. Mol. Toxicol. 2024, 38, e23642. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ali, A.; Tahir, A.; Bakht, M.A.; Salahuddin; Ahsan, M.J. Molecular Engineering of Curcumin, an Active Constituent of Curcuma longa L. (Turmeric) of the Family Zingiberaceae with Improved Antiproliferative Activity. Plants 2021, 10, 1559. [Google Scholar] [CrossRef] [PubMed]
- Orona-Ortiz, A.; Medina-Torres, L.; Velázquez-Moyado, J.A.; Pineda-Peña, E.A.; Balderas-López, J.L.; Bernad-Bernad, M.J.; Carlos, J.; Carvalho, T.; Navarrete, A. Mucoadhesive Effect of Curcuma Longa Extract and Curcumin Decreases the Ranitidine Effect, but Not Bismuth Subsalicylate on Ethanol-Induced Ulcer Model. Sci. Rep. 2019, 9, 16622–16633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Fu, Y.; Wang, H.W.; Gong, T.; Qin, Y.; Zhang, Z.R. Synthesis and Cytotoxic Activity of Novel Curcumin Analogues. Chin. Chem. Lett. 2008, 19, 281–285. [Google Scholar] [CrossRef]
- Kunwar, A.; Barik, A.; Mishra, B.; Rathinasamy, K.; Pandey, R.; Priyadarsini, K.I. Quantitative Cellular Uptake, Localization and Cytotoxicity of Curcumin in Normal and Tumor Cells. Biochim. Biophys. Acta Gen. Subj. 2008, 1780, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Lien, J.C.; Chen, C.Y.; Hung, C.C.; Lin, H.C. Design, Synthesis and Evaluation of Novel Derivatives of Curcuminoids with Cytotoxicity. Int. J. Mol. Sci. 2021, 22, 2171. [Google Scholar] [CrossRef]
- Kong, Y.; Ma, W.; Liu, X.; Zu, Y.; Fu, Y.; Wu, N.; Liang, L.; Yao, L.; Efferth, T. Cytotoxic Activity of Curcumin towards CCRF-CEM Leukemia Cells and Its Effect on DNA Damage. Molecules 2009, 14, 5328–5338. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Nguyen, N.N.T.; Tran, N.T.N.; Le, P.N.; Nguyen, T.B.T.; Nguyen, N.H.; Bach, L.G.; Doan, V.N.; Tran, H.L.B.; Le, V.T.; et al. Synergic Activity against MCF-7 Breast Cancer Cell Growth of Nanocurcumin-Encapsulated and Cisplatin-Complexed Nanogels. Molecules 2018, 23, 3347. [Google Scholar] [CrossRef]
- Zamrus, S.N.H.; Akhtar, M.N.; Yeap, S.K.; Quah, C.K.; Loh, W.S.; Alitheen, N.B.; Zareen, S.; Tajuddin, S.N.; Hussin, Y.; Shah, S.A.A. Design, Synthesis and Cytotoxic Effects of Curcuminoids on HeLa, K562, MCF-7 and MDA-MB-231 Cancer Cell Lines. Chem. Cent. J. 2018, 12, 31. [Google Scholar] [CrossRef]
- Strojny, B.; Grodzik, M.; Sawosz, E.; Winnicka, A.; Kurantowicz, N.; Jaworski, S.; Kutwin, M.; Urbańska, K.; Hotowy, A.; Wierzbicki, M.; et al. Diamond Nanoparticles Modify Curcumin Activity: In Vitro Studies on Cancer and Normal Cells and in Ovo Studies on Chicken Embryo Model. PLoS ONE 2016, 11, e0164637. [Google Scholar] [CrossRef] [PubMed]
- Lateh, L.; Kaewnopparat, N.; Yuenyongsawad, S.; Panichayupakaranant, P. Enhancing the Water-Solubility of Curcuminoids-Rich Extract Using a Ternary Inclusion Complex System: Preparation, Characterization, and Anti-Cancer Activity. Food Chem. 2022, 368, 130827. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Siviero, A.; Gallo, E.; Maggini, V.; Gori, L.; Mugelli, A.; Firenzuoli, F.; Vannacci, A. Curcumin, a Golden Spice with a Low Bioavailability. J. Herb. Med. 2015, 5, 57–70. [Google Scholar] [CrossRef]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological Activities of Curcuminoids, Other Biomolecules from Turmeric and Their Derivatives—A Review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef]
- Ajavakom, V.; Yutthaseri, T.; Chantanatrakul, R.; Suksamrarm, A.; Ajavakom, A. Curcuminoids in Multi-Component Synthesis. J. Heterocycl. Chem. 2017, 46, 1259–1265. [Google Scholar] [CrossRef]
- Sohail, M.; Guo, W.; Yang, X.; Li, Z.; Li, Y.; Xu, H.; Zhao, F. A Promising Anticancer Agent Dimethoxycurcumin: Aspects of Pharmacokinetics, Efficacy, Mechanism, and Nanoformulation for Drug Delivery. Front. Pharmacol. 2021, 12, 665387. [Google Scholar] [CrossRef]
- Teymouri, M.; Barati, N.; Pirro, M.; Sahebkar, A. Biological and Pharmacological Evaluation of Dimethoxycurcumin: A Metabolically Stable Curcumin Analogue with a Promising Therapeutic Potential. J. Cell Physiol. 2018, 233, 124–140. [Google Scholar] [CrossRef]
- Khan, N.; Afghah, Z.; Baral, A.; Geiger, J.D.; Chen, X. Dimethoxycurcumin Acidifies Endolysosomes and Inhibits SARS-CoV-2 Entry. Front. Virol. 2022, 2, 923018. [Google Scholar] [CrossRef]
- Meza-Morales, W.; Machado-Rodriguez, J.C.; Alvarez-Ricardo, Y.; Obregón-Mendoza, M.A.; Nieto-Camacho, A.; Toscano, R.A.; Soriano-García, M.; Cassani, J.; Enríquez, R.G. A New Family of Homoleptic Copper Complexes of Curcuminoids: Synthesis, Characterization and Biological Properties. Molecules 2019, 24, 910. [Google Scholar] [CrossRef] [PubMed]
- Meza-Morales, W.; Mirian Estévez-Carmona, M.; Alvarez-Ricardo, Y.; Obregón-Mendoza, M.A.; Cassani, J.; Ramírez-Apan, M.T.; Escobedo-Martínez, C.; Soriano-García, M.; Reynolds, W.F.; Enríquez, R.G. Full Structural Characterization of Homoleptic Complexes of Diacetylcurcumin with Mg, Zn, Cu, and Mn: Cisplatin-Level Cytotoxicity in Vitro with Minimal Acute Toxicity in Vivo. Molecules 2019, 24, 1598. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, K.; Thompson, K.H.; Patrick, B.O.; Storr, T.; Martins, C.; Polishchuk, E.; Yuen, V.G.; McNeill, J.H.; Orvig, C. Synthesis and Characterization of Dual Function Vanadyl, Gallium and Indium Curcumin Complexes for Medicinal Applications. J. Inorg. Biochem. 2005, 99, 2217–2225. [Google Scholar] [CrossRef] [PubMed]
- Meza-Morales, W.; Alvarez-Ricardo, Y.; Obregón-Mendoza, M.A.; Arenaza-Corona, A.; Ramírez-Apan, M.T.; Toscano, R.A.; Poveda-Jaramillo, J.C.; Enríquez, R.G. Three New Coordination Geometries of Homoleptic Zn Complexes of Curcuminoids and Their High Antiproliferative Potential. RSC Adv. 2023, 13, 8577–8585. [Google Scholar] [CrossRef]
- Kim, J.Y.; Song, M.; Kim, M.S.; Natarajan, P.; Do, R.; Myung, W.; Won, H.H. An Atlas of Associations between 14 Micronutrients and 22 Cancer Outcomes: Mendelian Randomization Analyses. BMC Med. 2023, 21, 316. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yu, Y.; Shi, H.; Cao, Y.; Liu, X.; Hao, Z.; Ren, Y.; Qin, G.; Huang, Y.; Wang, B. Magnesium in Combinatorial With Valproic Acid Suppressed the Proliferation and Migration of Human Bladder Cancer Cells. Front. Oncol. 2020, 10, 589112. [Google Scholar] [CrossRef] [PubMed]
- Mendes, P.M.V.; Bezerra, D.L.C.; dos Santos, L.R.; de Oliveira Santos, R.; de Sousa Melo, S.R.; Morais, J.B.S.; Severo, J.S.; Vieira, S.C.; do Nascimento Marreiro, D. Magnesium in Breast Cancer: What Is Its Influence on the Progression of This Disease? Biol. Trace Elem. Res. 2018, 184, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.A.; Shekatkar, M.; Raj, A.T.; Kheur, S. Potential Role of Magnesium in Cancer Initiation and Progression. Pathol. Oncol. Res. 2020, 26, 2001–2002. [Google Scholar] [CrossRef] [PubMed]
- Uçmak, Z.G.; Koenhemsi, L.; Uçmak, M.; Yalçın, E.; Ateş, A.; Gönül, R. Evaluation of Serum and Tissue Magnesium, Vascular Endothelial Growth Factor, and Osteopontin Levels in Dogs with Mammary Tumors with/without Pulmonary Metastases and in Healthy Dogs. J. Elem. 2021, 26, 125–135. [Google Scholar] [CrossRef]
- Bezerra, D.L.C.; Mendes, P.M.V.; Melo, S.R.d.S.; dos Santos, L.R.; Santos, R.d.O.; Vieira, S.C.; Henriques, G.S.; Freitas, B.d.J.e.S.d.A.; Marreiro, D.d.N. Hypomagnesemia and Its Relationship with Oxidative Stress Markers in Women with Breast Cancer. Biol. Trace Elem. Res. 2021, 199, 4466–4474. [Google Scholar] [CrossRef]
- Latifah, S.Y.; Faujan, H.A.; Sze, L.P.; Raha, A.R.; Hisyam, A.H.; Li, O.C. Curcumin from Turmeric (Curcuma Longa) Induced Apoptosis in Human Mammary Carcinoma Cells (MDA-MB-231). Malays. J. Med. Health Sci. 2006, 2, 71–79. [Google Scholar]
- Hu, S.; Xu, Y.; Meng, L.; Huang, L.; Sun, H. Curcumin Inhibits Proliferation and Promotes Apoptosis of Breast Cancer Cells. Exp. Ther. Med. 2018, 16, 1266–1272. [Google Scholar] [CrossRef]
- Abdelkader, H.; Fatease, A.A.; Fathalla, Z.; Shoman, M.E.; Abou-Taleb, H.A.; Abourehab, M.A.S. Design, Preparation and Evaluation of Supramolecular Complexes with Curcumin for Enhanced Cytotoxicity in Breast Cancer Cell Lines. Pharmaceutics 2022, 14, 2283. [Google Scholar] [CrossRef] [PubMed]
- Alhasawi, M.A.I.; Aatif, M.; Muteeb, G.; Alam, M.W.; El Oirdi, M.; Farhan, M. Curcumin and Its Derivatives Induce Apoptosis in Human Cancer Cells by Mobilizing and Redox Cycling Genomic Copper Ions. Molecules 2022, 27, 7410. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, R.M.; Abdel-Aziz, A.A.; Bassiouny, K.; Fayed, A.M. Phytocompounds-Based Therapeutic Approach: Investigating Curcumin and Green Tea Extracts on MCF-7 Breast Cancer Cell Line. J. Genet. Eng. Biotechnol. 2024, 22, 100339. [Google Scholar] [CrossRef]
- Fuchs, J.R.; Pandit, B.; Bhasin, D.; Etter, J.P.; Regan, N.; Abdelhamid, D.; Li, C.; Lin, J.; Li, P.K. Structure-Activity Relationship Studies of Curcumin Analogues. Bioorg. Med. Chem. Lett. 2009, 19, 2065–2069. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.J.; Kang, Y.J.; Lee, J.A.; Kim, I.Y.; Kim, M.A.; Lee, Y.S.; Park, J.H.; Lee, B.Y.; Kim, I.A.; Kim, H.S.; et al. Stronger Proteasomal Inhibition and Higher CHOP Induction Are Responsible for More Effective Induction of Paraptosis by Dimethoxycurcumin than Curcumin. Cell Death Dis. 2014, 5, e1112. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, A.; Simon, E.; Singh, U.; Chittela, R.K.; Sharma, D.; Sandur, S.K.; Priyadarsini, I.K. Interaction of a Curcumin Analogue Dimethoxycurcumin with DNA. Chem. Biol. Drug Des. 2011, 77, 281–287. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Q.; Zhang, Z.; Yuan, L.; Liu, X.; Zhou, L. Preparation of Curcumin-Loaded Liposomes and Evaluation of Their Skin Permeation and Pharmacodynamics. Molecules 2012, 17, 5972–5987. [Google Scholar] [CrossRef]
- Woźniak, M.; Nowak, M.; Lazebna, A.; Więcek, K.; Jabłońska, I.; Szpadel, K.; Grzeszczak, A.; Gubernator, J.; Ziółkowski, P. The Comparison of in Vitro Photosensitizing Efficacy of Curcumin-Loaded Liposomes Following Photodynamic Therapy on Melanoma Mug-Mel2, Squamous Cell Carcinoma Scc-25, and Normal Keratinocyte Hacat Cells. Pharmaceuticals 2021, 14, 374. [Google Scholar] [CrossRef]
- Araya-Sibaja, A.M.; Wilhelm-Romero, K.; Quirós-Fallas, M.I.; Huertas, L.F.V.; Vega-Baudrit, J.R.; Navarro-Hoyos, M. Bovine Serum Albumin-Based Nanoparticles: Preparation, Characterization, and Antioxidant Activity Enhancement of Three Main Curcuminoids from Curcuma Longa. Molecules 2022, 27, 2758. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchi, S.S.; Dunuweera, S.P.; Dunuweera, A.N.; Rajapakse, R.M.G. Synthesis of Curcumin Nanoparticles from Raw Turmeric Rhizome. ACS Omega 2021, 6, 8246–8252. [Google Scholar] [CrossRef] [PubMed]
- Romero, K.W.; Quirós, M.I.; Huertas, F.V.; Vega-Baudrit, J.R.; Navarro-Hoyos, M.; Araya-Sibaja, A.M. Design of Hybrid Polymeric-Lipid Nanoparticles Using Curcumin as a Model: Preparation, Characterization, and in Vitro Evaluation of Demethoxycurcumin and Bisdemethoxycurcumin-Loaded Nanoparticles. Polymers 2021, 13, 4207. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Shameli, K.; Sazili, A.Q.; Selamat, J.; Kumari, S. Rapid Green Synthesis and Characterization of Silver Nanoparticles Arbitrated by Curcumin in an Alkaline Medium. Molecules 2019, 24, 719. [Google Scholar] [CrossRef] [PubMed]
- Boarescu, P.M.; Boarescu, I.; Bocșan, I.C.; Gheban, D.; Bulboacă, A.E.; Nicula, C.; Pop, R.M.; Râjnoveanu, R.M.; Bolboacă, S.D. Antioxidant and Anti-Inflammatory Effects of Curcumin Nanoparticles on Drug-Induced Acute Myocardial Infarction in Diabetic Rats. Antioxidants 2019, 8, 504. [Google Scholar] [CrossRef] [PubMed]
- Patro, N.M.; Sultana, A.; Terao, K.; Nakata, D.; Jo, A.; Urano, A.; Ishida, Y.; Gorantla, R.N.; Pandit, V.; Devi, K.; et al. Comparison and Correlation of in Vitro, in Vivo and in Silico Evaluations of Alpha, Beta and Gamma Cyclodextrin Complexes of Curcumin. J. Incl. Phenom. Macrocycl. Chem. 2014, 78, 471–483. [Google Scholar] [CrossRef]
- Moussa, Z.; Hmadeh, M.; Abiad, M.G.; Dib, O.H.; Patra, D. Encapsulation of Curcumin in Cyclodextrin-Metal Organic Frameworks: Dissociation of Loaded CD-MOFs Enhances Stability of Curcumin. Food Chem. 2016, 212, 485–494. [Google Scholar] [CrossRef]
- Low, Z.X.; Teo, M.Y.M.; Nordin, F.J.; Dewi, F.R.P.; Palanirajan, V.K.; In, L.L.A. Biophysical Evaluation of Water-Soluble Curcumin Encapsulated in β-Cyclodextrins on Colorectal Cancer Cells. Int. J. Mol. Sci. 2022, 23, 2866. [Google Scholar] [CrossRef]
- Benucci, I.; Mazzocchi, C.; Lombardelli, C.; Del Franco, F.; Cerreti, M.; Esti, M. Inclusion of Curcumin in B-Cyclodextrin: A Promising Prospective as Food Ingredient. Food Addit. Contam. Part A 2022, 39, 1942–1952. [Google Scholar] [CrossRef]
- Alizadeh, N.; Malakzadeh, S. Changes in Chemical Stability and Bioactivities of Curcumin by Forming Inclusion Complexes of Beta- and Gama-Cyclodextrins. J. Polym. Res. 2020, 27, 42. [Google Scholar] [CrossRef]
- Ntuli, S.; Leuschner, M.; Bester, M.J.; Serem, J.C. Stability, Morphology, and Effects of In Vitro Digestion on the Antioxidant Properties of Polyphenol Inclusion Complexes with β-Cyclodextrin. Molecules 2022, 27, 3808. [Google Scholar] [CrossRef] [PubMed]
- Marcolino, V.A.; Zanin, G.M.; Durrant, L.R.; Benassi, M.D.T.; Matioli, G. Interaction of Curcumin and Bixin with β-Cyclodextrin: Complexation Methods, Stability, and Applications in Food. J. Agric. Food Chem. 2011, 59, 3348–3357. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Nagpal, M.; Vashistha, V.K.; Arora, R.; Issar, U. Recent Advances in the Antioxidant Activity of Metal-Curcumin Complexes: A Combined Computational and Experimental Review. Free Radic. Res. 2024, 58, 11–26. [Google Scholar] [CrossRef]
- Miebach, L.; Berner, J.; Bekeschus, S. In Ovo Model in Cancer Research and Tumor Immunology. Front. Immunol. 2022, 13, 1006064. [Google Scholar] [CrossRef]
- Obregón-Mendoza, M.A.; Meza-Morales, W.; Alvarez-Ricardo, Y.; Estévez-Carmona, M.M.; Enríquez, R.G. High Yield Synthesis of Curcumin and Symmetric Curcuminoids: A “Click” and “Unclick” Chemistry Approach. Molecules 2023, 28, 289. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qiu, Z.; Li, F.; Wang, C. The Relationship between MMP-2 and MMP-9 Expression Levels with Breast Cancer Incidence and Prognosis. Oncol. Lett. 2017, 14, 5865–5870. [Google Scholar] [CrossRef]
- Arya, P.; Raghav, N. In-Vitro Studies of Curcumin-β-Cyclodextrin Inclusion Complex as Sustained Release System. J. Mol. Struct. 2021, 1228, 129774. [Google Scholar] [CrossRef]
- Pessine, F.B.T.; Calderini, A.; Alexandrino, G.L. Review: Cyclodextrin Inclusion Complexes Probed by NMR Techniques. Magn. Reson. Spectrosc. 2012, 237–262. [Google Scholar] [CrossRef]
- Jahed, V.; Zarrabi, A.; Bordbar, A.K.; Hafezi, M.S. NMR (1H, ROESY) Spectroscopic and Molecular Modelling Investigations of Supramolecular Complex of β-Cyclodextrin and Curcumin. Food Chem. 2014, 165, 241–246. [Google Scholar] [CrossRef]
- Kida, T.; Iwamoto, T.; Fujino, Y.; Tohnai, N.; Miyata, M.; Akashi, M. Strong Guest Binding by Cyclodextrin Hosts in Competing Nonpolar Solvents and the Unique Crystalline Structure. Org. Lett. 2011, 13, 4570–4573. [Google Scholar] [CrossRef]
- dos Santos, C.; Buera, P.; Mazzobre, F. Novel Trends in Cyclodextrins Encapsulation. Applications in Food Science. Curr. Opin. Food Sci. 2017, 16, 106–113. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. β-Cyclodextrin-Curcumin Self-Assembly Enhances Curcumin Delivery in Prostate Cancer Cells. Colloids Surf. B Biointerfaces 2010, 79, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Bie, C.; Ji, Q.; Ling, H.; Li, C.; Fu, Y.; Zhao, L.; Ye, F. Preparation and Characterization of Cyanazine-Hydroxypropyl-Beta-Cyclodextrin Inclusion Complex. RSC Adv. 2019, 9, 26109–26115. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, A.H.; Szeleszczuk, Ł.; Bethanis, K.; Christoforides, E.; Dudek, M.K.; Zielińska-Pisklak, M.; Pisklak, D.M. 17-β-Estradiol—β-Cyclodextrin Complex as Solid: Synthesis, Structural and Physicochemical Characterization. Molecules 2023, 28, 3747. [Google Scholar] [CrossRef] [PubMed]
- Al Hagbani, T.; Nazzal, S. Curcumin Complexation with Cyclodextrins by the Autoclave Process: Method Development and Characterization of Complex Formation. Int. J. Pharm. 2017, 520, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.E. Animal Models for Studying Prevention and Treatment of Breast Cancer. In Animal Models for the Study of Human Disease; Elsevier: Amsterdam, The Netherlands, 2013; pp. 997–1018. ISBN 9780124158948. [Google Scholar]
- Stoica, L.; Stoica, B.A.; Olinici, D.; Onofrei, P.; Botez, E.A.; Cotrutz, C.E. Correlations between Morphological Changes Induced by Curcumin and Its Biological Activities. Rom. J. Morphol. Embryol. 2018, 59, 65–69. [Google Scholar] [PubMed]
- Ahmad, I.; Ahmad, S.; Ahmad, A.; Zughaibi, T.A.; Alhosin, M.; Tabrez, S. Curcumin, Its Derivatives, and Their Nanoformulations: Revolutionizing Cancer Treatment. Cell Biochem. Funct. 2024, 42, e3911. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, K.D.; Martínez, I.; Reyes-Chilpa, R.; Espinoza, B. Mammea Type Coumarins Isolated from Calophyllum Brasiliense Induced Apoptotic Cell Death of Trypanosoma Cruzi through Mitochondrial Dysfunction, ROS Production and Cell Cycle Alterations. Bioorg Chem. 2020, 100, 103894. [Google Scholar] [CrossRef]
- Tian, M.; Ma, Y.; Lin, W. Fluorescent Probes for the Visualization of Cell Viability. Acc. Chem. Res. 2019, 52, 2147–2157. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Kornblatt, A.P.; Nicoletti, V.G.; Travaglia, A. The Neglected Role of Copper Ions in Wound Healing. J. Inorg. Biochem. 2016, 161, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Mai, Z.; Liu, C.; Yin, S.; Cai, Y.; Xia, C. Natural Products in Preventing Tumor Drug Resistance and Related Signaling Pathways. Molecules 2022, 27, 3513. [Google Scholar] [CrossRef] [PubMed]
- Manore, S.G.; Doheny, D.L.; Wong, G.L.; Lo, H.W. IL-6/JAK/STAT3 Signaling in Breast Cancer Metastasis: Biology and Treatment. Front. Oncol. 2022, 12, 866014. [Google Scholar] [CrossRef] [PubMed]
- Alaaeldin, R.; Ali, F.E.M.; Bekhit, A.A.; Zhao, Q.L.; Fathy, M. Inhibition of NF-KB/IL-6/JAK2/STAT3 Pathway and Epithelial-Mesenchymal Transition in Breast Cancer Cells by Azilsartan. Molecules 2022, 27, 7825. [Google Scholar] [CrossRef]
- Obregón-Mendoza, M.A.; Estévez-Carmona, M.M.; Hernández-Ortega, S.; Soriano-García, M.; Ramírez-Apan, M.T.; Orea, L.; Pilotzi, H.; Gnecco, D.; Cassani, J.; Enríquez, R.G. Retro-Curcuminoids as Mimics of Dehydrozingerone and Curcumin: Synthesis, NMR, X-Ray, and Cytotoxic Activity. Molecules 2017, 22, 33. [Google Scholar] [CrossRef]
- Obregón-Mendoza, M.A.; Arias-Olguín, I.I.; Estévez-Carmona, M.M.; Meza-Morales, W.; Alvarez-Ricardo, Y.; Toscano, R.A.; Arenas-Huertero, F.; Cassani, J.; Enríquez, R.G. Non-Cytotoxic Dibenzyl and Difluoroborate Curcuminoid Fluorophores Allow Visualization of Nucleus or Cytoplasm in Bioimaging. Molecules 2020, 25, 3205. [Google Scholar] [CrossRef]
- Mestrelab Research. MNova Software. Available online: https://mestrelab.com/download/mnova/ (accessed on 10 March 2024).
- Higuchi, T.; Shih, F.M.; Kimura, T.; Rytting, J.H. Solubility Determination of Barely Aqueous-Soluble Organic Solids. J. Pharm. Sci. 1979, 68, 1267–1272. [Google Scholar] [CrossRef]
- Jadhav, B.K.; Mahadik, K.R.; Paradkar, A.R. Development and Validation of Improved Reversed Phase-HPLC Method for Simultaneous Determination of Curcumin, Demethoxycurcumin and Bis-Demethoxycurcumin. Chromatographia 2007, 65, 483–488. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B Colorimetric Assay for Cytotoxicity Screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next Generation of Scientific Image Data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Ashland, O.R.; Becton-Dickinson and Company. FlowJoTM v10.0.7r2.8 Software for Mac Software Application Version 7.3.2. Available online: https://www.flowjo.com (accessed on 10 March 2024).
- Chomczynski, P.; Sacchi, N. Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate-Phenol-Chloroform Extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Noyola-Martínez, N.; Halhali, A.; Zaga-Clavellina, V.; Olmos-Ortiz, A.; Larrea, F.; Barrera, D. A Time-Course Regulatory and Kinetic Expression Study of Steroid Metabolizing Enzymes by Calcitriol in Primary Cultured Human Placental Cells. J. Steroid Biochem. Mol. Biol. 2017, 167, 98–105. [Google Scholar] [CrossRef] [PubMed]
- GraphPad Software Inc. Available online: http://www.graphpad.com/faq/viewfaq.cfm?faq=1362 (accessed on 10 March 2024).
- Sigma Plot Statistical Software. Jandel Corporation: Las Vegas, NV, USA. Available online: https://sigmaplot.software.informer.com/11.0 (accessed on 10 March 2024).
- Weiss, H.; Reichel, J.; Görls, H.; Schneider, K.R.A.; Micheel, M.; Pröhl, M.; Gottschaldt, M.; Dietzek, B.; Weigand, W. Curcuminoid-BF2 Complexes: Synthesis, Fluorescence and Optimization of BF2 Group Cleavage. Beilstein J. Org. Chem. 2017, 13, 2264–2272. [Google Scholar] [CrossRef]
- Liu, K.; Chen, J.; Chojnacki, J.; Zhang, S. BF3·OEt2-Promoted Concise Synthesis of Difluoroboron-Derivatized Curcumins from Aldehydes and 2,4-Pentanedione. Tetrahedron Lett. 2013, 54, 2070–2073. [Google Scholar] [CrossRef] [PubMed]


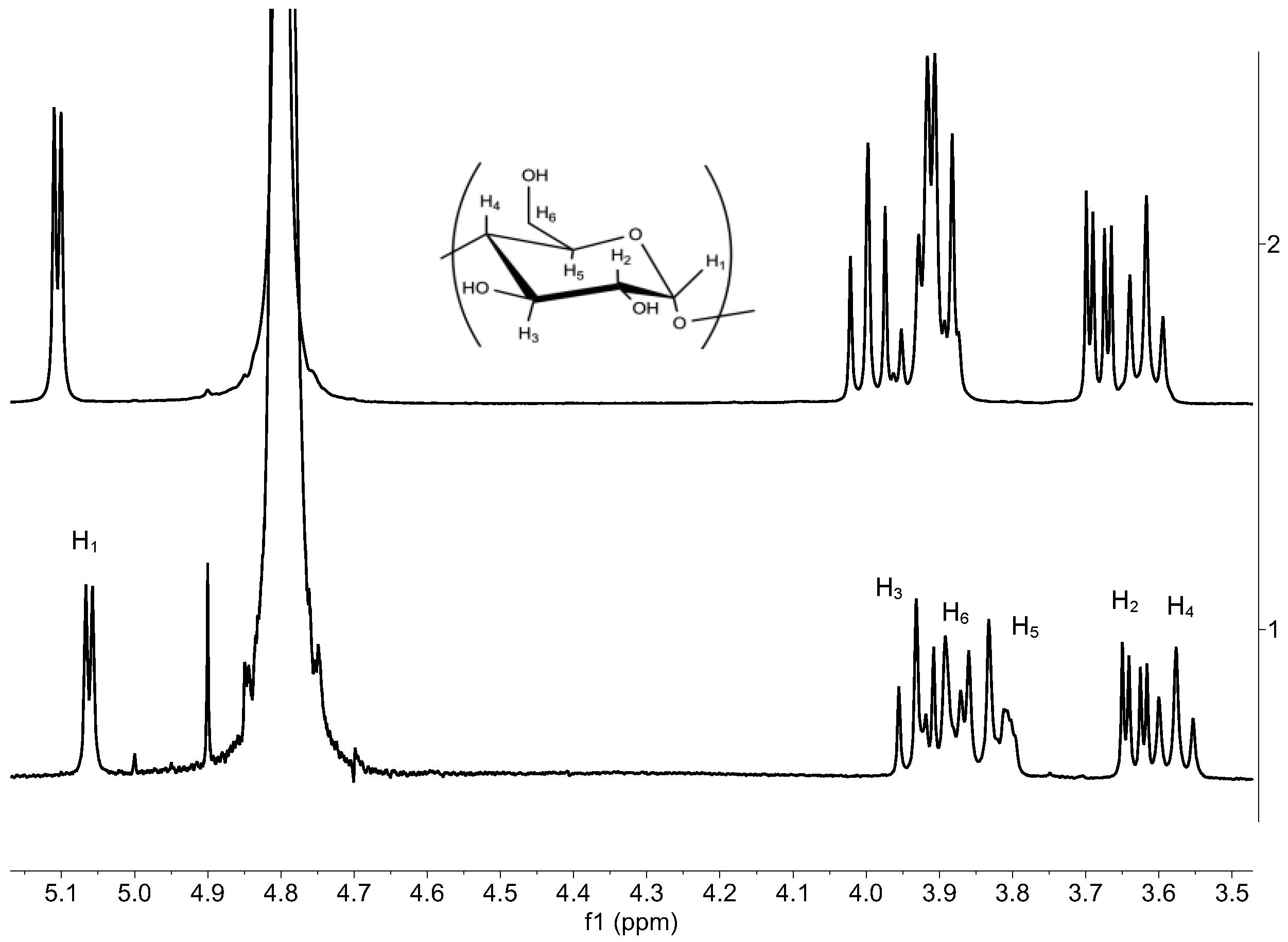





| Proton | BCD Free (ppm) | BCD Complex (ppm) | Δδ (ppm) |
|---|---|---|---|
| H1 | 5.11 | 5.06 | −0.05 |
| H2 | 3.68 | 3.63 | −0.05 |
| H3 | 3.99 | 3.93 | −0.06 |
| H4 | 3.62 | 3.58 | −0.04 |
| H5 | 3.88 | 3.80 | −0.08 |
| H6 | 3.91 | 3.89 | −0.02 |
| Compound | Dfree | Dinclusion Complex | HOD |
|---|---|---|---|
| DiMeOC-Mg | 1.20 × 10−10 cm2/s | 9.82 × 10−11 cm2/s | 6.61 × 10−10 cm2/s |
| BCD | 1.26 × 10−10 cm2/s | 8.34 × 10−11 cm2/s | 7.5 × 10−10 cm2/s |
| Compounds | Solubility μg/mL |
|---|---|
| DiMeOC-Mg | 15.6 ± 0.048 |
| DiMeOC-Mg-BCD | 98.2 ± 0.038 |
| Compounds | MDA-MB-231 |
|---|---|
| DiMeOC | >100 |
| DiMeOC-Mg | 22.04 ± 0.06 |
| DiMeOC-Mg-BCD | 10.73 ± 0.1 |
| Group | Injected Concentration (μM) | In Ovo Final Dose (mg/kg) |
|---|---|---|
| Control (DMSO 1%) | - | - |
| Paclitaxel | 10.0 | 0.014 |
| DiMeOC-Mg-1 | 276.1 | 0.375 |
| DiMeOC-Mg-2 | 552.3 | 0.750 |
| DiMeOC-Mg-3 | 1104.5 | 1.5 |
| DiMeOC-Mg-BCD-1 | 10 | 0.065 |
| DiMeOC-Mg-BCD-2 | 100 | 0.650 |
| Gene | Forward | Reverse | * Probe | Accession Number |
|---|---|---|---|---|
| RPL32 | GAAGTTCCTGGTCCACAACG | GAGCGATCTCGGCACAGTA | 17 | NM_000994.3 |
| MMP-2 | ATAACCTGGATG CCGTCGT | AGGCACCCTTGAAGAAGTAGC | 70 | NM_001302510.1 |
| MMP-9 | GCCACCCGAGTGTAACCATA | GAACCAATCTCACCGACAGG | 6 | NM_004994.2 |
| IL-6 | GATGAGTACAAAAGTCCTGATCCA | CTGCAGCCACTGGTTCTGT | 40 | NM 000600.1 |
| STAT3 | CCTCTGCCGGAGAAACAGT | CATTGGGAAGCTGTCACTGTAG | 1 | NM_139276.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obregón-Mendoza, M.A.; Meza-Morales, W.; Rodríguez-Hernández, K.D.; Estévez-Carmona, M.M.; Pérez-González, L.L.; Tavera-Hernández, R.; Ramírez-Apan, M.T.; Barrera-Hernández, D.; García-Olivares, M.; Monroy-Torres, B.; et al. The Antitumoral Effect In Ovo of a New Inclusion Complex from Dimethoxycurcumin with Magnesium and Beta-Cyclodextrin. Int. J. Mol. Sci. 2024, 25, 4380. https://doi.org/10.3390/ijms25084380
Obregón-Mendoza MA, Meza-Morales W, Rodríguez-Hernández KD, Estévez-Carmona MM, Pérez-González LL, Tavera-Hernández R, Ramírez-Apan MT, Barrera-Hernández D, García-Olivares M, Monroy-Torres B, et al. The Antitumoral Effect In Ovo of a New Inclusion Complex from Dimethoxycurcumin with Magnesium and Beta-Cyclodextrin. International Journal of Molecular Sciences. 2024; 25(8):4380. https://doi.org/10.3390/ijms25084380
Chicago/Turabian StyleObregón-Mendoza, Marco A., William Meza-Morales, Karla Daniela Rodríguez-Hernández, M. Mirian Estévez-Carmona, Leidys L. Pérez-González, Rosario Tavera-Hernández, María Teresa Ramírez-Apan, David Barrera-Hernández, Mitzi García-Olivares, Brian Monroy-Torres, and et al. 2024. "The Antitumoral Effect In Ovo of a New Inclusion Complex from Dimethoxycurcumin with Magnesium and Beta-Cyclodextrin" International Journal of Molecular Sciences 25, no. 8: 4380. https://doi.org/10.3390/ijms25084380
APA StyleObregón-Mendoza, M. A., Meza-Morales, W., Rodríguez-Hernández, K. D., Estévez-Carmona, M. M., Pérez-González, L. L., Tavera-Hernández, R., Ramírez-Apan, M. T., Barrera-Hernández, D., García-Olivares, M., Monroy-Torres, B., Nieto-Camacho, A., Chávez, M. I., Sánchez-Obregón, R., & Enríquez, R. G. (2024). The Antitumoral Effect In Ovo of a New Inclusion Complex from Dimethoxycurcumin with Magnesium and Beta-Cyclodextrin. International Journal of Molecular Sciences, 25(8), 4380. https://doi.org/10.3390/ijms25084380






