Impact of Developmental Changes of GABAA Receptors on Interneuron-NG2 Glia Transmission in the Hippocampus
Abstract
1. Introduction
2. Results
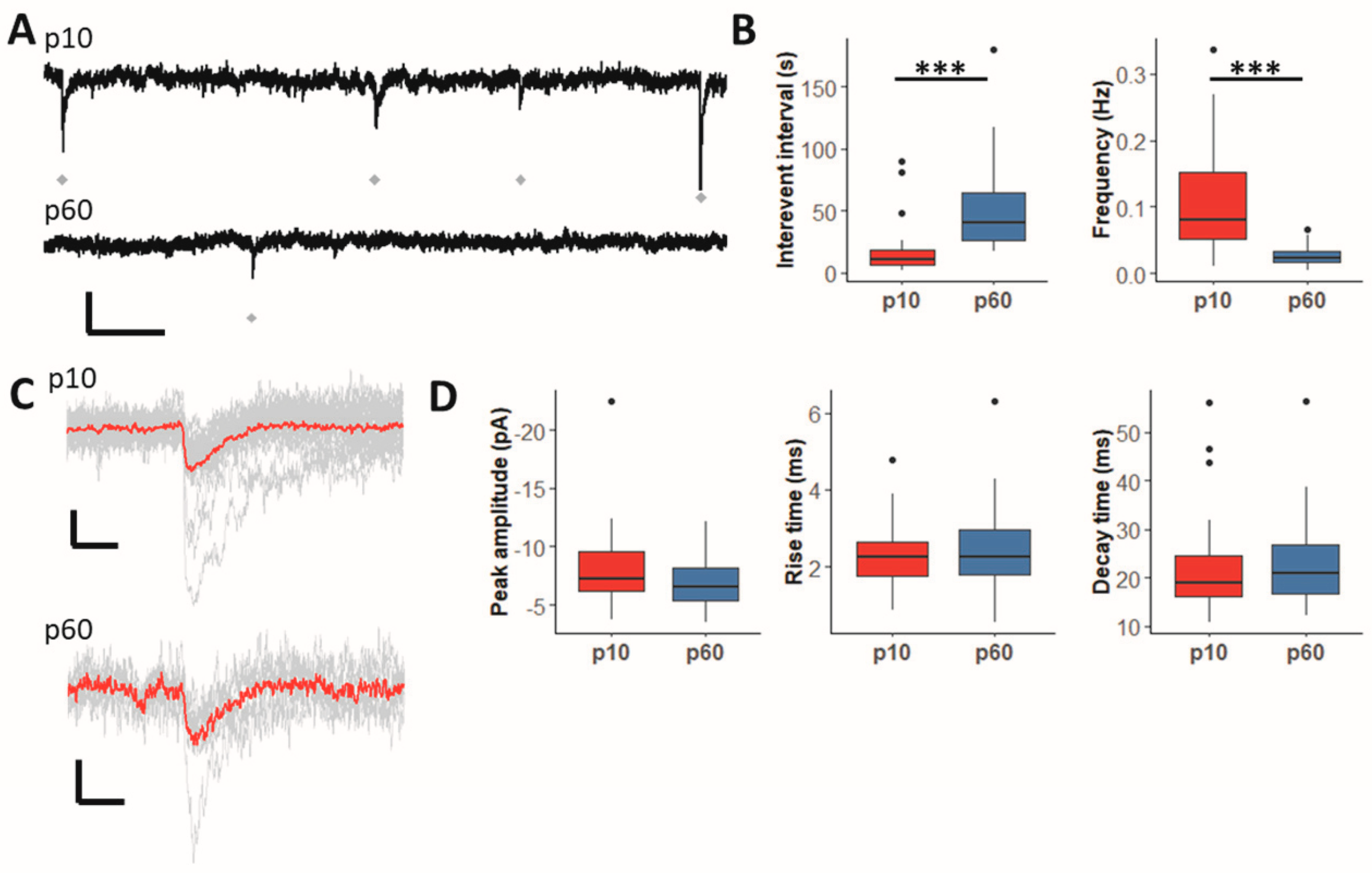
2.1. GABAergic Synaptic Input to Hippocampal NG2 Glia Declines during Maturation
2.2. Pharmacological Analysis of Evoked Postsynaptic Currents (ePSCs)
2.3. Enhanced GABA Spillover and Extrasynaptic Receptor Activation in Adult NG2 Glia
2.4. GABAergic Input onto Adult Hippocampal NG2 Glia Is Mediated by α5-Containing Receptors
2.5. RT-PCR Substantiates Developmental Rearrangements of Glial GABAA Receptors
2.6. GABAA Receptor Clustering in Hippocampal NG2 Glia
3. Discussion
3.1. GABAergic Innervation of NG2 Glia Declines during Development
3.2. Molecular Changes of Glial GABA Receptors
3.3. Tonic GABA Receptor Currents
3.4. Auxiliary Subunits Und Anchoring Proteins
3.5. Impact of GABA Receptors in NG2 Glia
4. Materials and Methods
4.1. Animals
4.2. Preparation of Acute Hippocampal Slices for Patch-Clamp Recordings and Cell Harvesting
4.3. Electrophysiology
4.3.1. Whole Cell Recordings
4.3.2. Spontaneous and Miniature PSC
4.3.3. Evoked PSCs
4.3.4. Tonic Currents
4.4. Cell Harvesting and Single-Cell RT-PCR
4.5. FACsorting of NG2 Glial Cells and Semiquantitative Real-Time RT-PCR
4.6. Expansion Microscopy
4.7. Data Analysis and Statistics
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergles, D.E.; Jabs, R.; Steinhäuser, C. Neuron-glia synapses in the brain. Brain Res. Rev. 2010, 63, 130–137. [Google Scholar] [CrossRef]
- Dimou, L.; Simon, C.; Kirchhoff, F.; Takebayashi, H.; Götz, M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J. Neurosci. 2008, 28, 10434–10442. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Fukaya, M.; Yang, J.K.; Rothstein, J.D.; Bergles, D.E. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 2010, 68, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Karram, K.; Goebbels, S.; Schwab, M.; Jennissen, K.; Seifert, G.; Steinhäuser, C.; Nave, K.A.; Trotter, J. NG2-expressing cells in the nervous system revealed by the NG2-EYFP-knockin mouse. Genesis 2008, 46, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Degen, J.; Dublin, P.; Zhang, J.; Dobrowolski, R.; Jokwitz, M.; Karram, K.; Trotter, J.; Jabs, R.; Willecke, K.; Steinhäuser, C.; et al. Dual reporter approaches for identification of Cre efficacy and astrocyte heterogeneity. FASEB J. 2012, 26, 4576–4583. [Google Scholar] [CrossRef]
- Seifert, G.; Steinhäuser, C. Heterogeneity and function of hippocampal macroglia. Cell Tissue Res. 2018, 373, 653–670. [Google Scholar] [CrossRef]
- Bergles, D.E.; Roberts, J.D.; Somogyi, P.; Jahr, C.E. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 2000, 405, 187–191. [Google Scholar] [CrossRef]
- Lin, S.C.; Bergles, D.E. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat. Neurosci. 2004, 7, 24–32. [Google Scholar] [CrossRef]
- Jabs, R.; Pivneva, T.; Hüttmann, K.; Wyczynski, A.; Nolte, C.; Kettenmann, H.; Steinhäuser, C. Synaptic transmission onto hippocampal glial cells with hGFAP promoter activity. J. Cell Sci. 2005, 118, 3791–3803. [Google Scholar] [CrossRef]
- Mehta, A.K.; Ticku, M.K. An update on GABAA receptors. Brain Res. Brain Res. Rev. 1999, 29, 196–217. [Google Scholar] [CrossRef]
- Hevers, W.; Luddens, H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol. Neurobiol. 1998, 18, 35–86. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.W.; Sieghart, W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: Classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol. Rev. 2008, 60, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Garaschuk, O.; Hanse, E.; Konnerth, A. Developmental profile and synaptic origin of early network oscillations in the CA1 region of rat neonatal hippocampus. J. Physiol. Lond. 1998, 507, 219–236. [Google Scholar] [CrossRef]
- Rivera, C.; Voipio, J.; Payne, J.A.; Ruusuvuori, E.; Lahtinen, H.; Lamsa, K.; Pirvola, U.; Saarma, M.; Kaila, K. The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 1999, 397, 251–255. [Google Scholar] [CrossRef]
- Stein, V.; Hermans-Borgmeyer, I.; Jentsch, T.J.; Hubner, C.A. Expression of the KCl cotransporter KCC2 parallels neuronal maturation and the emergence of low intracellular chloride. J. Comp. Neurol. 2004, 468, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Tozuka, Y.; Takata, T.; Shimazu, N.; Matsumura, N.; Ohta, A.; Hisatsune, T. Excitatory GABAergic activation of cortical dividing glial cells. Cereb. Cortex 2009, 19, 2181–2195. [Google Scholar] [CrossRef]
- Passlick, S.; Grauer, M.; Schafer, C.; Jabs, R.; Seifert, G.; Steinhäuser, C. Expression of the gamma2-subunit distinguishes synaptic and extrasynaptic GABA(A) receptors in NG2 cells of the hippocampus. J. Neurosci. 2013, 33, 12030–12040. [Google Scholar] [CrossRef]
- Arellano, R.O.; Sanchez-Gomez, M.V.; Alberdi, E.; Canedo-Antelo, M.; Chara, J.C.; Palomino, A.; Perez-Samartin, A.; Matute, C. Axon-to-Glia Interaction Regulates GABAA Receptor Expression in Oligodendrocytes. Mol. Pharmacol. 2016, 89, 63–74. [Google Scholar] [CrossRef]
- Balia, M.; Velez-Fort, M.; Passlick, S.; Schafer, C.; Audinat, E.; Steinhäuser, C.; Seifert, G.; Angulo, M.C. Postnatal down-regulation of the GABAA receptor gamma2 subunit in neocortical NG2 cells accompanies synaptic-to-extrasynaptic switch in the GABAergic transmission mode. Cereb. Cortex 2015, 25, 1114–1123. [Google Scholar] [CrossRef]
- Velez-Fort, M.; Maldonado, P.P.; Butt, A.M.; Audinat, E.; Angulo, M.C. Postnatal switch from synaptic to extrasynaptic transmission between interneurons and NG2 cells. J. Neurosci. 2010, 30, 6921–6929. [Google Scholar] [CrossRef]
- Yamasaki, T.; Hoyos-Ramirez, E.; Martenson, J.S.; Morimoto-Tomita, M.; Tomita, S. GARLH Family Proteins Stabilize GABA(A) Receptors at Synapses. Neuron 2017, 93, 1138–1152. [Google Scholar] [CrossRef] [PubMed]
- Davenport, E.C.; Pendolino, V.; Kontou, G.; McGee, T.P.; Sheehan, D.F.; Lopez-Domenech, G.; Farrant, M.; Kittler, J.T. An Essential Role for the Tetraspanin LHFPL4 in the Cell-Type-Specific Targeting and Clustering of Synaptic GABA(A) Receptors. Cell Rep. 2017, 21, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Poulopoulos, A.; Aramuni, G.; Meyer, G.; Soykan, T.; Hoon, M.; Papadopoulos, T.; Zhang, M.; Paarmann, I.; Fuchs, C.; Harvey, K.; et al. Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin. Neuron 2009, 63, 628–642. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, T.; Soykan, T. The role of collybistin in gephyrin clustering at inhibitory synapses: Facts and open questions. Front. Cell Neurosci. 2011, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Hausrat, T.J.; Muhia, M.; Gerrow, K.; Thomas, P.; Hirdes, W.; Tsukita, S.; Heisler, F.F.; Herich, L.; Dubroqua, S.; Breiden, P.; et al. Radixin regulates synaptic GABAA receptor density and is essential for reversal learning and short-term memory. Nat. Commun. 2015, 6, 6872. [Google Scholar] [CrossRef]
- Loebrich, S.; Bahring, R.; Katsuno, T.; Tsukita, S.; Kneussel, M. Activated radixin is essential for GABAA receptor alpha5 subunit anchoring at the actin cytoskeleton. EMBO J. 2006, 25, 987–999. [Google Scholar] [CrossRef]
- Olsen, R.W.; Sieghart, W. GABAA receptors: Subtypes provide diversity of function and pharmacology. Neuropharmacology 2009, 56, 141–148. [Google Scholar] [CrossRef]
- Frahm, C.; Draguhn, A. GAD and GABA transporter (GAT-1) mRNA expression in the developing rat hippocampus. Brain Res. Dev. Brain Res. 2001, 132, 1–13. [Google Scholar] [CrossRef]
- Brunskine, C.; Passlick, S.; Henneberger, C. Structural Heterogeneity of the GABAergic Tripartite Synapse. Cells 2022, 11, 3150. [Google Scholar] [CrossRef]
- Kersante, F.; Rowley, S.C.; Pavlov, I.; Gutierrez-Mecinas, M.; Semyanov, A.; Reul, J.M.; Walker, M.C.; Linthorst, A.C. A functional role for both -aminobutyric acid (GABA) transporter-1 and GABA transporter-3 in the modulation of extracellular GABA and GABAergic tonic conductances in the rat hippocampus. J. Physiol. 2013, 591, 2429–2441. [Google Scholar] [CrossRef]
- Olsen, R.W. GABA(A) receptor: Positive and negative allosteric modulators. Neuropharmacology 2018, 136, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Haberlandt, C.; Derouiche, A.; Wyczynski, A.; Haseleu, J.; Pohle, J.; Karram, K.; Trotter, J.; Seifert, G.; Frotscher, M.; Steinhäuser, C.; et al. Gray Matter NG2 Cells Display Multiple Ca-Signaling Pathways and Highly Motile Processes. PLoS ONE 2011, 6, e17575. [Google Scholar] [CrossRef]
- Passlick, S.; Trotter, J.; Seifert, G.; Steinhäuser, C.; Jabs, R. The NG2 Protein Is Not Required for Glutamatergic Neuron-NG2 Cell Synaptic Signaling. Cereb. Cortex 2016, 26, 51–57. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Orduz, D.; Benamer, N.; Ortolani, D.; Coppola, E.; Vigier, L.; Pierani, A.; Angulo, M.C. Developmental cell death regulates lineage-related interneuron-oligodendroglia functional clusters and oligodendrocyte homeostasis. Nat. Commun. 2019, 10, 4249. [Google Scholar] [CrossRef] [PubMed]
- Kessaris, N.; Fogarty, M.; Iannarelli, P.; Grist, M.; Wegner, M.; Richardson, W.D. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci. 2006, 9, 173–179. [Google Scholar] [CrossRef]
- Essrich, C.; Lorez, M.; Benson, J.A.; Fritschy, J.M.; Luscher, B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat. Neurosci. 1998, 1, 563–571. [Google Scholar] [CrossRef]
- Kneussel, M.; Betz, H. Clustering of inhibitory neurotransmitter receptors at developing postsynaptic sites: The membrane activation model. Trends Neurosci. 2000, 23, 429–435. [Google Scholar] [CrossRef]
- Alldred, M.J.; Mulder-Rosi, J.; Lingenfelter, S.E.; Chen, G.; Luscher, B. Distinct gamma2 subunit domains mediate clustering and synaptic function of postsynaptic GABAA receptors and gephyrin. J. Neurosci. 2005, 25, 594–603. [Google Scholar] [CrossRef]
- Burgard, E.C.; Tietz, E.I.; Neelands, T.R.; Macdonald, R.L. Properties of recombinant gamma-aminobutyric acid A receptor isoforms containing the alpha 5 subunit subtype. Mol. Pharmacol. 1996, 50, 119–127. [Google Scholar]
- Capogna, M.; Pearce, R.A. GABA A, slow: Causes and consequences. Trends Neurosci. 2011, 34, 101–112. [Google Scholar] [CrossRef]
- Balia, M.; Benamer, N.; Angulo, M.C. A specific GABAergic synapse onto oligodendrocyte precursors does not regulate cortical oligodendrogenesis. Glia 2017, 65, 1821–1832. [Google Scholar] [CrossRef] [PubMed]
- Moshrefi-Ravasdjani, B.; Dublin, P.; Seifert, G.; Jennissen, K.; Steinhäuser, C.; Kafitz, K.W.; Rose, C.R. Changes in the proliferative capacity of NG2 cell subpopulations during postnatal development of the mouse hippocampus. Brain Struct. Funct. 2017, 222, 831–847. [Google Scholar] [CrossRef] [PubMed]
- Timmermann, A.; Tascio, D.; Jabs, R.; Boehlen, A.; Domingos, C.; Skubal, M.; Huang, W.; Kirchhoff, F.; Henneberger, C.; Bilkei-Gorzo, A.; et al. Dysfunction of NG2 glial cells affects neuronal plasticity and behavior. Glia 2023, 71, 1481–1501. [Google Scholar] [CrossRef]
- Hamilton, N.B.; Clarke, L.E.; Arancibia-Carcamo, I.L.; Kougioumtzidou, E.; Matthey, M.; Karadottir, R.; Whiteley, L.; Bergersen, L.H.; Richardson, W.D.; Attwell, D. Endogenous GABA controls oligodendrocyte lineage cell number, myelination, and CNS internode length. Glia 2017, 65, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Mangin, J.M.; Kunze, A.; Chittajallu, R.; Gallo, V. Satellite NG2 progenitor cells share common glutamatergic inputs with associated interneurons in the mouse dentate gyrus. J. Neurosci. 2008, 28, 7610–7623. [Google Scholar] [CrossRef]
- Orduz, D.; Maldonado, P.P.; Balia, M.; Velez-Fort, M.; de Sars, V.; Yanagawa, Y.; Emiliani, V.; Angulo, M.C. Interneurons and oligodendrocyte progenitors form a structured synaptic network in the developing neocortex. Elife 2015, 4, e06953. [Google Scholar] [CrossRef]
- Pelkey, K.A.; Chittajallu, R.; Craig, M.T.; Tricoire, L.; Wester, J.C.; McBain, C.J. Hippocampal GABAergic Inhibitory Interneurons. Physiol. Rev. 2017, 97, 1619–1747. [Google Scholar] [CrossRef]
- Booker, S.A.; Vida, I. Morphological diversity and connectivity of hippocampal interneurons. Cell Tissue Res. 2018, 373, 619–641. [Google Scholar] [CrossRef]
- Caraiscos, V.B.; Elliott, E.M.; You-Ten, K.E.; Cheng, V.Y.; Belelli, D.; Newell, J.G.; Jackson, M.F.; Lambert, J.J.; Rosahl, T.W.; Wafford, K.A.; et al. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors. Proc. Natl. Acad. Sci. USA 2004, 101, 3662–3667. [Google Scholar] [CrossRef]
- Glykys, J.; Mody, I. Hippocampal network hyperactivity after selective reduction of tonic inhibition in GABA A receptor alpha5 subunit-deficient mice. J. Neurophysiol. 2006, 95, 2796–2807. [Google Scholar] [CrossRef]
- Glykys, J.; Mody, I. The main source of ambient GABA responsible for tonic inhibition in the mouse hippocampus. J. Physiol. 2007, 582, 1163–1178. [Google Scholar] [CrossRef] [PubMed]
- Scimemi, A.; Semyanov, A.; Sperk, G.; Kullmann, D.M.; Walker, M.C. Multiple and plastic receptors mediate tonic GABAA receptor currents in the hippocampus. J. Neurosci. 2005, 25, 10016–10024. [Google Scholar] [CrossRef] [PubMed]
- Glykys, J.; Mann, E.O.; Mody, I. Which GABA(A) receptor subunits are necessary for tonic inhibition in the hippocampus? J. Neurosci. 2008, 28, 1421–1426. [Google Scholar] [CrossRef] [PubMed]
- Dembitskaya, Y.; Wu, Y.W.; Semyanov, A. Tonic GABA(A) Conductance Favors Spike-Timing-Dependent over Theta-Burst-Induced Long-Term Potentiation in the Hippocampus. J. Neurosci. 2020, 40, 4266–4276. [Google Scholar] [CrossRef]
- Martin, L.J.; Zurek, A.A.; MacDonald, J.F.; Roder, J.C.; Jackson, M.F.; Orser, B.A. Alpha5GABAA receptor activity sets the threshold for long-term potentiation and constrains hippocampus-dependent memory. J. Neurosci. 2010, 30, 5269–5282. [Google Scholar] [CrossRef]
- Kneussel, M.; Brandstätter, J.H.; Laube, B.; Stahl, S.; Müller, U.; Betz, H. Loss of postsynaptic GABAA receptor clustering in gephyrin-deficient mice. J. Neurosci. 1999, 19, 9289–9297. [Google Scholar] [CrossRef]
- Kneussel, M.; Loebrich, S. Trafficking and synaptic anchoring of ionotropic inhibitory neurotransmitter receptors. Biol. Cell 2007, 99, 297–309. [Google Scholar] [CrossRef]
- Zonouzi, M.; Scafidi, J.; Li, P.; McEllin, B.; Edwards, J.; Dupree, J.L.; Harvey, L.; Sun, D.; Hubner, C.A.; Cull-Candy, S.G.; et al. GABAergic regulation of cerebellar NG2 cell development is altered in perinatal white matter injury. Nat. Neurosci. 2015, 18, 674–682. [Google Scholar] [CrossRef]
- Benamer, N.; Vidal, M.; Balia, M.; Angulo, M.C. Myelination of parvalbumin interneurons shapes the function of cortical sensory inhibitory circuits. Nat. Commun. 2020, 11, 5151. [Google Scholar] [CrossRef]
- Oh, W.C.; Lutzu, S.; Castillo, P.E.; Kwon, H.B. De novo synaptogenesis induced by GABA in the developing mouse cortex. Science 2016, 353, 1037–1040. [Google Scholar] [CrossRef]
- Gibson, E.M.; Purger, D.; Mount, C.W.; Goldstein, A.K.; Lin, G.L.; Wood, L.S.; Inema, I.; Miller, S.E.; Bieri, G.; Zuchero, J.B.; et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 2014, 344, 1252304. [Google Scholar] [CrossRef]
- Seifert, G.; Rehn, L.; Weber, M.; Steinhäuser, C. AMPA receptor subunits expressed by single astrocytes in the juvenile mouse hippocampus. Mol. Brain Res. 1997, 47, 286–294. [Google Scholar] [CrossRef]
- Matthias, K.; Kirchhoff, F.; Seifert, G.; Hüttmann, K.; Matyash, M.; Kettenmann, H.; Steinhäuser, C. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J. Neurosci. 2003, 23, 1750–1758. [Google Scholar] [CrossRef]
- Deshpande, T.; Li, T.; Herde, M.K.; Becker, A.; Vatter, H.; Schwarz, M.K.; Henneberger, C.; Steinhäuser, C.; Bedner, P. Subcellular reorganization and altered phosphorylation of the astrocytic gap junction protein connexin43 in human and experimental temporal lobe epilepsy. Glia 2017, 65, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Asano, S.M.; Gao, R.; Wassie, A.T.; Tillberg, P.W.; Chen, F.; Boyden, E.S. Expansion Microscopy: Protocols for Imaging Proteins and RNA in Cells and Tissues. Curr. Protoc. Cell Biol. 2018, 80, e56. [Google Scholar] [CrossRef] [PubMed]
- Herde, M.K.; Bohmbach, K.; Domingos, C.; Vana, N.; Komorowska-Müller, J.A.; Passlick, S.; Schwarz, I.; Jackson, C.J.; Dietrich, D.; Schwarz, M.K.; et al. Local efficacy of glutamate uptake decreases with synapse size. Cell Rep. 2020, 32, 108182. [Google Scholar] [CrossRef] [PubMed]








| Gene | Primer Sequence | Product Length | Position | Genbank Accession Number |
|---|---|---|---|---|
| Collybistin (Arhgef9) | se 5′-CACGGAGCGCCATTACATCAA as 5′-GGCGGCAGGCCTCAAAGA | 338 bp | 339 659 | NM_001033329 |
| Collybistin (Arhgef9) (nested) | se 5′-GTGCCGAAAGAGAAGGGACAT as 5′-CAGGCATCCAGGTGGTTGTTA | 219 bp | 396 594 | |
| Gephyrin | se 5′-AGGTGCAGCAGCAAGGAGAACATT as 5′-GTTGTAACCCGCATCACTTGTCCT | 365 bp | 982 1323 | NM_145965 |
| Gephyrin (nested) | se 5′-TCTCCTTTTCCCCTGACG as 5′-TCCCCAATGATGAAACGAT | 221 bp | 1063 1265 | |
| Radixin | se 5′-CAGCGAGCAAAAGAGGAGGCAGAG as 5′-TGCTTCTTCACGCGTTCATTCTTC | 443 bp | 1138 1557 | NM_009041 |
| Radixin (nested) | se 5′-GCAAGCTGCTGACCAGATGAAG as 5′-TCCGTGGGAGGGATGACTG | 234 bp | 1215 1430 | |
| LHFPL4 | se 5′-TCTACAAGATCTGCGCCTGGATGC as 5′-TGGCCGCAGCACGGAACTTACTGT | 311 bp | 371 658 | NM_177763 |
| LHFPL4 (nested) | se 5′-ATGGCTGGGATGCTGAGACC as 5′-ACTGTAGTGCCCACGAAATCTTTG | 220 bp | 443 639 | |
| GABAA-R α3 (Gabra3) | se 5′-TAACCGGCTTCGACCTGGACTTG as 5′-CAGCGTGTATTGTTAACCTCATTG | 332 bp | 237 545 | NM_008067 |
| GABAA-R α3 (Gabra3) (nested) | se 5′-AGTTTTGGCCCTGTGTCAGAC as 5′-AGAGGAGGGTCCCATTATCTACC | 241 bp | 301 519 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patt, L.; Tascio, D.; Domingos, C.; Timmermann, A.; Jabs, R.; Henneberger, C.; Steinhäuser, C.; Seifert, G. Impact of Developmental Changes of GABAA Receptors on Interneuron-NG2 Glia Transmission in the Hippocampus. Int. J. Mol. Sci. 2023, 24, 13490. https://doi.org/10.3390/ijms241713490
Patt L, Tascio D, Domingos C, Timmermann A, Jabs R, Henneberger C, Steinhäuser C, Seifert G. Impact of Developmental Changes of GABAA Receptors on Interneuron-NG2 Glia Transmission in the Hippocampus. International Journal of Molecular Sciences. 2023; 24(17):13490. https://doi.org/10.3390/ijms241713490
Chicago/Turabian StylePatt, Linda, Dario Tascio, Catia Domingos, Aline Timmermann, Ronald Jabs, Christian Henneberger, Christian Steinhäuser, and Gerald Seifert. 2023. "Impact of Developmental Changes of GABAA Receptors on Interneuron-NG2 Glia Transmission in the Hippocampus" International Journal of Molecular Sciences 24, no. 17: 13490. https://doi.org/10.3390/ijms241713490
APA StylePatt, L., Tascio, D., Domingos, C., Timmermann, A., Jabs, R., Henneberger, C., Steinhäuser, C., & Seifert, G. (2023). Impact of Developmental Changes of GABAA Receptors on Interneuron-NG2 Glia Transmission in the Hippocampus. International Journal of Molecular Sciences, 24(17), 13490. https://doi.org/10.3390/ijms241713490






