Conjugative Plasmid pPPUT-Tik1-1 from a Permafrost Pseudomonas putida Strain and Its Present-Day Counterparts Inhabiting Environments and Clinics
Abstract
1. Introduction
2. Results
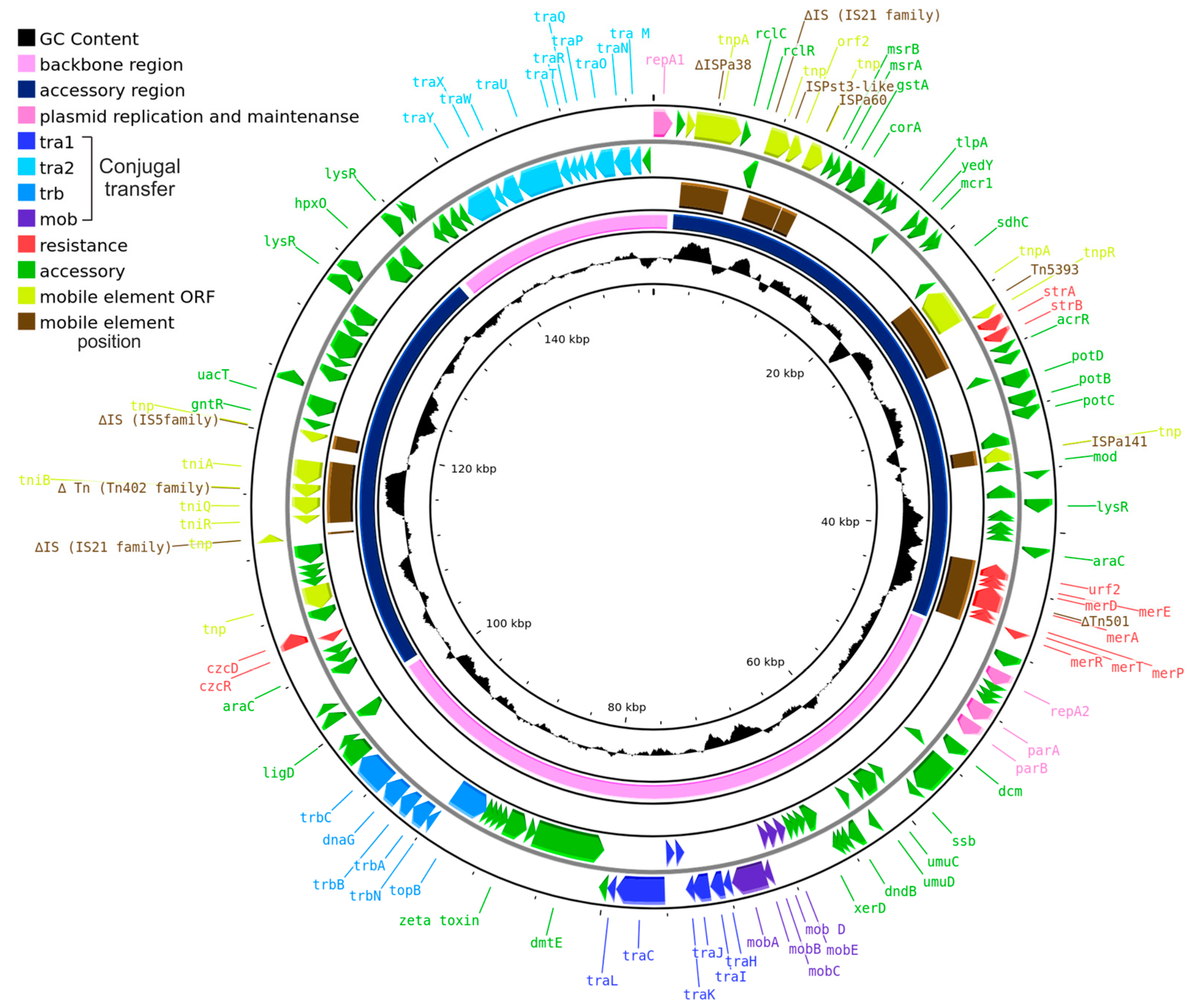
2.1. The Prototype Plasmid pPPUT-Tik1-1
2.2. Genome of pPPUT-Tik1-1 and Related Plasmids
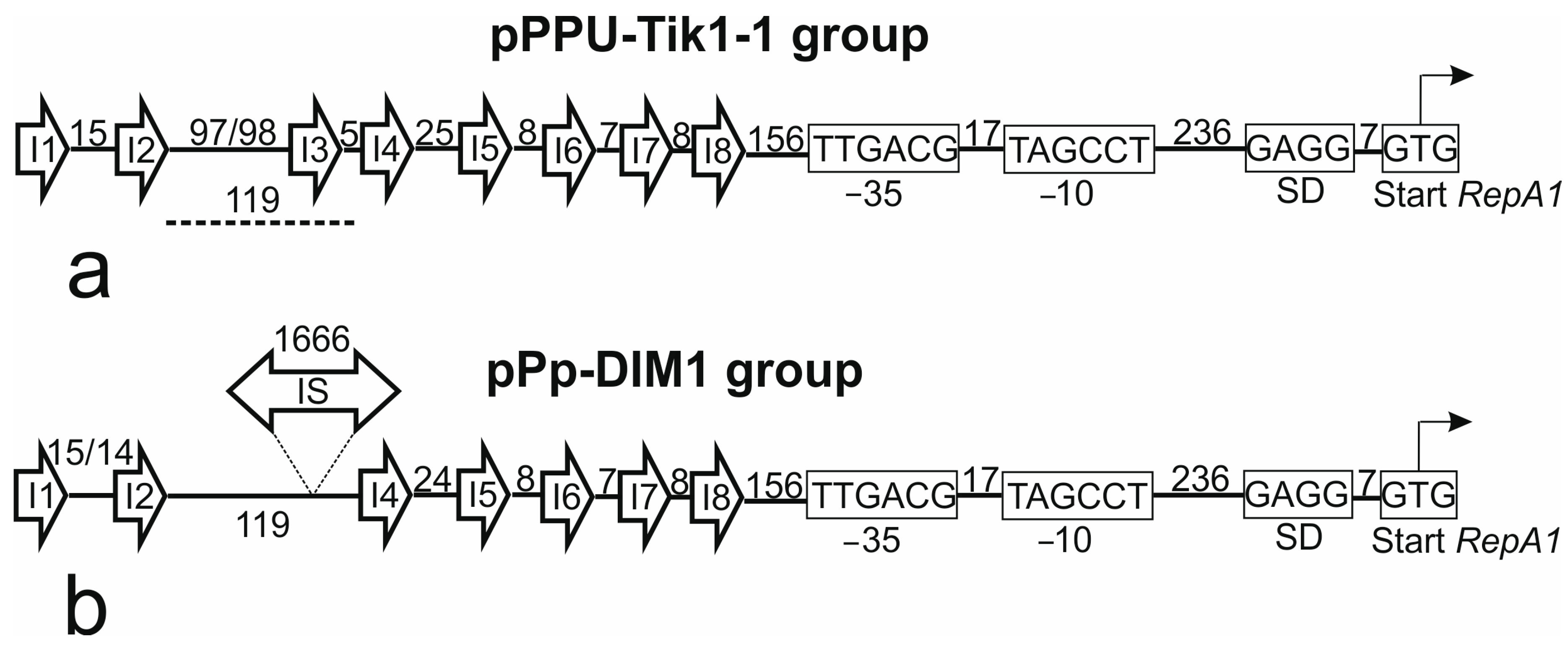
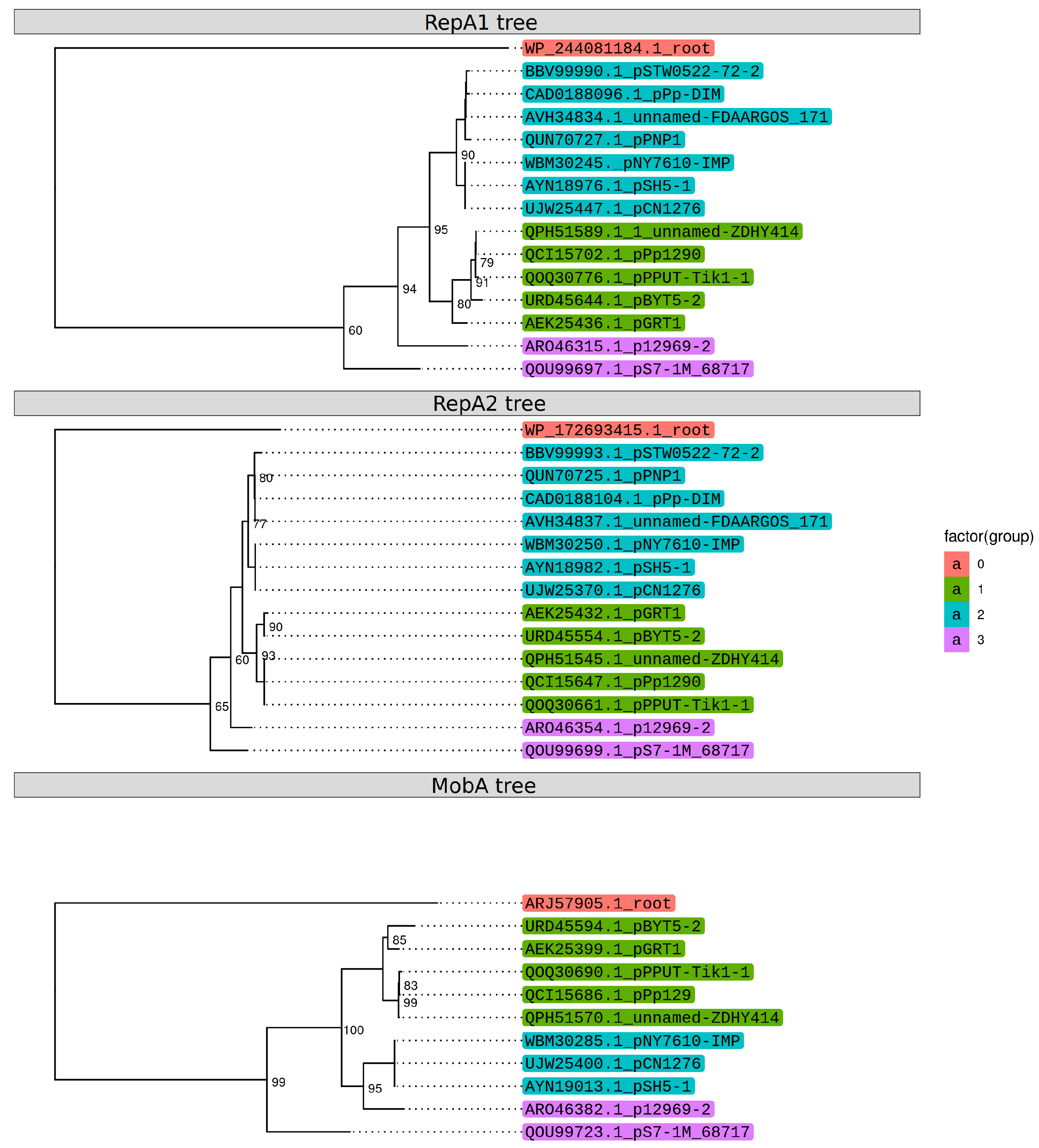
2.3. Identification of the Replicon and Relaxase Types of pPPUT-Tik1-1 and Related Plasmids
2.4. Coevolution of the Main Genes of the Backbone Region
2.5. Determination of the Conjugative Host Range of pPPUT-Tik1-1 and Its Stability in Different Hosts
2.6. The Accessory Region of pPPUT-Tik1-1 and Related Plasmids
2.6.1. Resistance to Heavy Metals
2.6.2. Biodegradation
2.6.3. Antibiotic Resistance
3. Discussion
4. Materials and Methods
4.1. Media and Growth Conditions
4.2. Bacterial Strains
4.3. Mating
4.4. Plasmid Stability Assays
4.5. Identification of the Backbone Region of pPPUT-Tik1-1
4.6. Search for Plasmids Related to pPPUT-Tik1-1 in GenBank
4.7. Whole-Genome Sequencing and Plasmid Assembly
4.8. Bioinformatic Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wackett, L.P. Pseudomonas putida—A versatile biocatalyst. Nat. Biotechnol. 2003, 21, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Barranquero, J.A.; Cazorla, F.M.; de Vicente, A.; Sund, G.W. Complete sequence and comparative genomic analysis of eight native Pseudomonas syringae plasmids belonging to the pPT23A family. BMC Genom. 2017, 18, 365. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cai, Z.; Cai, Z.; Zhang, Y.; Fu, T.; Jin, Y.; Cheng, Z.; Jin, S.; Wu, W.; Yang, L.; et al. Molecular genetic analysis of an XDR Pseudomonas aeruginosa ST664 clone carrying multiple conjugal plasmids. Antimicrob. Chemother. 2020, 75, 1443–1452. [Google Scholar] [CrossRef]
- Paul, D.; Chanda, D.D.; Chakravarty, A.; Bhattacharjee, A. An insight into analysis and elimination of plasmids encoding metallo-β-lactamases in Pseudomonas aeruginosa. J. Glob. Antimicrob. Resist. 2020, 21, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.; Hedges, R.W. Compatibility groups among fi—R factors. Nature 1971, 234, 222–223. [Google Scholar] [CrossRef] [PubMed]
- Novick, R.P. Plasmid incompatibility. Microbiol. Rev. 1987, 51, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Austin, S.; Nordström, K. Partition-mediated incompatibility of bacterial plasmids. Cell 1990, 60, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Shintani, M.; Sanchez, Z.K.; Kimbara, K. Genomics of microbial plasmids: Classification and identification based on replication and transfer systems and host taxonomy. Front. Microbiol. 2015, 6, 242. [Google Scholar] [CrossRef]
- Bardaji, L.; Añorga, M.; Ruiz-Masó, J.A.; Del Solar, G.; Murillo, J. Plasmid replicons from Pseudomonas are natural chimeras of functional, exchangeable modules. Front. Microbiol. 2017, 8, 190. [Google Scholar] [CrossRef]
- Sentchilo, V.S.; Perebituk, A.N.; Zehnder, A.J.; van der Meer, J.R. Molecular diversity of plasmids bearing genes that encode toluene and xylene metabolism in Pseudomonas strains isolated from different contaminated sites in Belarus. Appl. Environ. Microbiol. 2000, 66, 2842–2852. [Google Scholar] [CrossRef]
- Bonnin, R.A.; Bogaerts, P.; Girlich, D.; Huang, T.D.; Dortet, L.; Glupczynski, Y.; Naas, T. Molecular characterization of OXA-198 carbapenemase-producing Pseudomonas aeruginosa clinical isolates. Antimicrob. Agents Chemother. 2018, 62, 02496-17. [Google Scholar] [CrossRef]
- Jiang, X.; Yin, Z.; Yuan, M.; Cheng, Q.; Hu, L.; Xu, Y.; Yang, W.; Yang, H.; Zhao, Y.; Zhao, X.; et al. Plasmids of novel incompatibility group IncpRBL16 from Pseudomonas species. J. Antimicrob. Chemother. 2020, 75, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Garcillán-Barcia, M.P.; Francia, M.V.; de la Cruz, F. The diversity of conjugative relaxases and its application in plasmid classification. FEMS Microbiol. Rev. 2009, 33, 657–687. [Google Scholar] [CrossRef] [PubMed]
- Garcillán-Barcia, M.P.; de la Cruz, F. Ordering the bestiary of genetic elements transmissible by conjugation. Mob. Genet. Elem. 2013, 3, 24263. [Google Scholar] [CrossRef] [PubMed]
- Smillie, C.; Garcillán-Barcia, M.P.; Francia, M.V.; Rocha, E.P.; de la Cruz, F. Mobility of plasmids. Microbiol. Mol. Biol. Rev. 2010, 74, 434–452. [Google Scholar] [CrossRef]
- Orlek, A.; Phan, H.; Sheppard, A.E.; Doumith, M.; Ellington, M.; Peto, T.; Crook, D.; Walker, A.S.; Woodford, N.; Anjum, M.F.; et al. Ordering the mob: Insights into replicon and MOB typing schemes from analysis of a curated dataset of publicly available plasmids. Plasmid 2017, 91, 42–52. [Google Scholar] [CrossRef]
- Chen, F.; Wang, P.; Yin, Z.; Yang, H.; Hu, L.; Yu, T.; Jing, Y.; Guan, J.; Wu, J.; Zhou, D. VIM-encoding IncpSTY plasmids and chromosome-borne integrative and mobilizable elements (IMEs) and integrative and conjugative elements (ICEs) in Pseudomonas. Ann. Clin. Microbiol. Antimicrob. 2022, 21, 10. [Google Scholar] [CrossRef]
- Moura, A.; Oliveira, C.; Henriques, I.; Smalla, K.; Correia, A. Broad diversity of conjugative plasmids in integron-carrying bacteria from wastewater environments. FEMS Microbiol. Lett. 2012, 330, 157–164. [Google Scholar] [CrossRef]
- Mindlin, S.; Beletsky, A.; Rakitin, A.; Mardanov, A.; Petrova, M. Acinetobacter plasmids: Diversity and Development of classification strategies. Front. Microbiol. 2020, 11, 588410. [Google Scholar] [CrossRef]
- Mindlin, S.Z.; Petrova, M.; Gorlenko, Z.M.; Soina, V.S.; Khachikian, N.A.; Karaevskaya, E.A. Multidrug-resistant bacteria in permafrost: Isolation, biodiversity, phenotypic and genotypic analysis. In New Permafrost and Glacier Research, 1st ed.; Krugger, M.I., Stern, H.P., Eds.; Nova Science: Hauppauge, NY, USA, 2009; pp. 89–105. [Google Scholar]
- Volke, D.C.; Calero, P.; Nikel, P.I. Pseudomonas putida. Trends Microbiol. 2020, 28, 512–513. [Google Scholar] [CrossRef]
- Xu, Y.; Niu, Y.; Sun, F.; Yang, Y.; Luo, W.; Wang, Z. The novel Pseudomonas putida plasmid p12969-2 harbors an In127-carrying multidrug-resistant region. Future Microbiol. 2017, 12, 573–584. [Google Scholar] [CrossRef]
- Mutschler, H.; Meinhart, A.J. ε/ζ systems: Their role in resistance, virulence, and their potential for antibiotic development. Mol. Med. 2011, 89, 1183–1194. [Google Scholar] [CrossRef]
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Møller Aarestrup, F.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef] [PubMed]
- Virolle, C.; Goldlust, K.; Djermoun, S.; Bigot, S.; Lesterlin, C. Plasmid transfer by conjugation in gram-negative bacteria: From the cellular to the community level. Genes 2020, 11, 1239. [Google Scholar] [CrossRef] [PubMed]
- Hesse, C.; Schulz, F.; Bull, C.T.; Shaffer, B.T.; Yan, Q.; Shapiro, N.; Hassan, K.A.; Varghese, N.; Elbourne, L.D.H.; Paulsen, I.T.; et al. Genome-based evolutionary history of Pseudomonas spp. Environ. Microbiol. 2018, 20, 2142–2159. [Google Scholar] [CrossRef] [PubMed]
- Passarelli-Araujo, H.; Franco, G.R.; Venancio, T.M. Network analysis of ten thousand genomes shed light on Pseudomonas diversity and classification. Microbiol. Res. 2022, 254, 126919. [Google Scholar] [CrossRef] [PubMed]
- Gelder, L.D.; Ponciano, J.M.; Joyce, P.; Top, E.M. Stability of a promiscuous plasmid in different hosts: No guarantee for a long-term relationship. Microbiology 2007, 153, 452–463. [Google Scholar] [CrossRef]
- Yeo, C.C.; Tham, J.M.; Kwong, S.M.; Yiin, S.; Poh, C.L. Tn5563, a transposon encoding putative mercuric ion transport proteins located on plasmid pRA2 of Pseudomonas alcaligenes. FEMS Microbiol. Lett. 1998, 165, 253–260. [Google Scholar] [CrossRef][Green Version]
- Molina, L.; Duque, E.; Gomez, M.J.; Krell, T.; Lacal, J.; García-Puente, A.; García, V.; Matilla, M.A.; Ramos, J.-L.; Segura, A. The pGRT1 plasmid of Pseudomonas putida DOT-T1E encodes functions relevant for survival under harsh conditions in the environment. Environ. Microbiol. 2011, 13, 2315–2327. [Google Scholar] [CrossRef]
- Cabezón, E.; Ripoll-Rozada, J.; Peña, A.; de la Cruz, F.; Arechaga, I. Towards an integrated model of bacterial conjugation. FEMS Microbiol. Rev. 2015, 39, 81–95. [Google Scholar] [CrossRef]
- Gerdes, K.; Moller-Jensen, J.; Bugge Jensen, R. Plasmid and chromosome partitioning: Surprises from phylogeny. Mol. Microbiol. 2000, 37, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Lalucat, J.; Mulet, M.; Gomila, M.; García-Valdés, E. Genomics in Bacterial Taxonomy: Impact on the Genus Pseudomonas. Genes 2020, 11, 139. [Google Scholar] [CrossRef] [PubMed]
- Volkova, O.V.; Panov, A.V.; Kosheleva, I.A.; Boronin, A.M. IncP-7 plasmids classification based on structural diversity of their basic replicon. Mol. Biol. 2013, 47, 232–242. [Google Scholar] [CrossRef]
- Osborn, A.M.; Bruce, K.D.; Strike, P.; Ritchie, D.A. Distribution, diversity and evolution of the bacterial mercury resistance (mer) operon. FEMS Microbiol. Rev. 1997, 19, 239–262. [Google Scholar] [CrossRef] [PubMed]
- Liebert, C.A.; Hall, R.M.; Summers, A.O. Transposon Tn21, flagship of the floating genome. Microbiol. Mol. Biol. Rev. 1999, 63, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Schneiker, S.; Keller, M.; Dröge, M.; Lanka, E.; Pühler, A.; Selbitschka, W. The genetic organization and evolution of the broad host range mercury resistance plasmid pSB102 isolated from a microbial population residing in the rhizosphere of alfalfa. Nucleic Acids Res. 2001, 29, 5169–5181. [Google Scholar] [CrossRef]
- Mindlin, S.; Minakhin, L.; Petrova, M.; Kholodii, G.; Minakhina, S.; Gorlenko, Z.; Nikiforov, V. Present-day mercury resistance transposons are common in bacteria preserved in permafrost grounds since the Upper Pleistocene. Res. Microbiol. 2005, 156, 994–1004. [Google Scholar] [CrossRef]
- Barkay, T.; Miller, S.M.; Summers, A.O. Bacterial mercury resistance from atoms to ecosystems. FEMS Microbiol. Rev. 2003, 27, 355–384. [Google Scholar] [CrossRef]
- Driscoll, C.T.; Mason, R.P.; Chan, H.M.; Jacob, D.J.; Pirrone, N. Mercury as a global pollutant: Sources, pathways, and effects. Environ. Sci. Technol. 2013, 47, 4967–4983. [Google Scholar] [CrossRef]
- Obrist, D.; Kirk, J.L.; Zhang, L.; Sunderland, E.M.; Jiskra, M.; Selin, N.E. A review of global environmental mercury processes in response to human and natural perturbations: Changes of emissions, climate, and land use. Ambio 2018, 47, 116–140. [Google Scholar] [CrossRef]
- Raj, D.; Maiti, S.K. Sources, toxicity, and remediation of mercury: An essence review. Environ. Monit. Assess 2019, 191, 566. [Google Scholar] [CrossRef]
- Christakis, C.A.; Barkay, T.; Boyd, E.S. Expanded diversity and phylogeny of mer genes broadens mercury resistance paradigms and reveals an origin for MerA among thermophilic archaea. Front. Microbiol. 2021, 12, 682605. [Google Scholar] [CrossRef] [PubMed]
- Babich, K.; Engle, M.; Skinner, J.S.; Laddaga, R.A. Deletion mutant analysis of the Staphylococcus aureus plasmid pI258 mercury-resistance determinant. Can. J. Microbiol. 1991, 7, 624–631. [Google Scholar] [CrossRef]
- Hobman, J.L.; Brown, N.L. Overexpression of MerT, the mercuric ion transport protein of transposon Tn501, and genetic selection of mercury hypersensitivity mutations. Mol. Gen. Genet. 1996, 250, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.; Zamorano, L.; Mena, A.; Albertí, S.; Pérez, J.L.; Oliver, A. Metallo-beta-lactamase-producing Pseudomonas putida as a reservoir of multidrug resistance elements that can be transferred to successful Pseudomonas aeruginosa clones. J. Antimicrob. Chemother. 2010, 65, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.; Oberhettinger, P.; Schuele, L.; Dinkelacker, A.; Vogel, W. Genomic characterisation of clinical and environmental Pseudomonas putida group strains and determination of their role in the transfer of antimicrobial resistance genes to Pseudomonas aeruginosa. BMC Genom. 2017, 18, 859. [Google Scholar] [CrossRef]
- Mindlin, S.; Maslova, O.; Beletsky, A.; Nurmukanova, V.; Zong, Z.; Mardanov, A.; Petrova, M. Ubiquitous conjugative mega-plasmids of Acinetobacter species and their role in horizontal transfer of multi-drug resistance. Front. Microbiol. 2021, 12, 728644. [Google Scholar] [CrossRef]
- Aslam, B.; Khurshid, M.; Arshad, M.I.; Muzammil, S.; Rasool, M.; Yasmeen, N.; Shah, T.; Chaudhry, T.H.; Rasool, M.H.; Shahid, A.; et al. Antibiotic Resistance: One Health One World Outlook. Front. Cell Infect. Microbiol. 2021, 11, 771510. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory, 3rd ed.; Cold Spring: Harbor, NY, USA, 2001. [Google Scholar]
- Petrova, M.A.; Gorlenko, Z.M.; Mindlin, S.Z. Tn5045, a novel integron-containing antibiotic and chromate resistance transposon isolated from a permafrost bacterium. Res. Microbiol. 2011, 162, 337–345. [Google Scholar] [CrossRef]
- Vorobyova, E.; Soina, V.; Gorlenko, M.; Minkovskaya, N.; Zalinova, N.; Mamukelashvili, A.; Gilichinsky, D.; Rivkina, E.; Vishnivetskaya, T. The deep cold biosphere: Facts and hypothesis. FEMS Microbiol. Rev. 1997, 20, 277–290. [Google Scholar] [CrossRef]
- Deane, S.; Rawlings, D. Plasmid evolution and interaction between the plasmid addiction stability systems of two related broad-host-range IncQ-like plasmids. J. Bacteriol. 2004, 186, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Hamidian, M.; Ambrose, S.J.; Hall, R.M. A large conjugative Acinetobacter baumannii plasmid carrying the sul2 sulphonamide and strAB streptomycin resistance genes. Plasmid 2016, 87–88, 43–50. [Google Scholar] [CrossRef] [PubMed]

| Strain | Plasmid | Size (bp) | Source | Accessory Region 1 | Accession Number |
|---|---|---|---|---|---|
| Conjugative plasmids (Tra+) | |||||
| P. putida Tik1 | pPPUT-Tik1-1 | 153,663 | permafrost, Russia | Hg, Str | OQ920555 |
| P. putida 15420352 | p420352-strA | 153,678 | urine, China | Hg, Str | MT074087 |
| P. fulva ZDHY414 | Unnamed | 150,273 | clinic, China | Hg, Str | CP064947 |
| P. putida 12969 | p12969-2 | 109,708 | clinic, China | Hg, Str, Sul | KY270855 |
| P. putida 1290 | pPp1290 | 114,265 | pear pyllosphere | Aromatic compounds degradation | CP039372 |
| P. putida DOT-T1E | pGRT1 | 133,451 | unknown | Tolerance to toluene, UV-resistance | HM626202 |
| P. juntendi 18091276 | pCN1276 | 112,529 | urine, China | Hg | CP091312 |
| P. monteilii B5 | pSH5-1 | 130,536 | soil | Str | CP022562 |
| Pseudomonas sp. BYT-5 | pBYT5-2 | 136,400 | soil | Molybdate absorption | CP097489 |
| P. aeruginosa NY7610 | pNY7610-IMP | 152,682 | clinic, China | Str, Gm, Km, Cb, Cf | CP096914 |
| P. syringae pv. actinidiae S7-1M | pS7-1M | 68,717 | kiwifruit orchard, New Zealand | - | MK621014 |
| Non-conjugative plasmids (Tra−) | |||||
| P. putida | pPp-DIM | 69,823 | water, Spain | Sul, Km/Gm, Cb, Tc | LR822050 |
| P. monteilii | unnamed | 60,588 | sputum, USA | - | CP014061 |
| P. monteilii STW0522-72 | pSTW0522-72-2 | 59,057 | hospital sewage, Japan | - | AP022475 |
| Pseudomonas sp. | pPNP1 | 125,508 | activated sludge, USA | Sul, 4-Nitrophenol degradation | CP073662 |
| Group 1 | Strain | Species (Collection 2) | Transconjugant Frequency (Per Recipient) 3 |
|---|---|---|---|
| P. aeruginosa | PAO Type | P. aeruginosa (IMG) | <10−9 |
| B-6643 | P. aeruginosa (VKPM) | <10−9 | |
| B-5807 Type | P. aeruginosa (VKPM) | <10−9 | |
| TA37-4 | P. aeruginosa (IMG) | <10−9 | |
| FA8-1 | P. aeruginosa (IMG) | <10−9 | |
| TA40-1 | P. aeruginosa (IMG) | <10−9 | |
| P. resinovorans | B-14151 Type | P. resinovorans (VKPM) | 1.9 × 10−3 |
| P. stutzeri | B-975 Type | P. stutzeri (VKM) | 2.4 × 10−6 |
| B-903 | P. stutzeri (VKM) | 3.4 × 10−4 | |
| P. oleovorans | B-8623 | P. oleovorans (VKPM) | 9.8 × 10−5 |
| P. straminea | B-14152 Type | P. seleniipraecipitans (VKPM) | <10−9 |
| P. putida | B-4589 Type | P. putida (VKPM) | 3.7 × 10−3 |
| P. syringae | B-1546 | P. savastanoi (VKM) | 1.2 × 10−3 |
| P. fluorescens | B-6735 | P. fluorescens (VKPM) | 5.7 × 10−4 |
| P22-1-2 | P. fluorescens (IMG) | 2.6 × 10−4 | |
| B-14148 Type | P. fluorescens (VKPM) | 4.7 × 10−3 |
| Strain | Plasmid | mer Operon | Transposon |
|---|---|---|---|
| P. putida Tik1-1 | pPPUT-Tik1-1 | merRTPADE | ΔTn501 |
| P. putida 12969 | p12969-2 | merRTPABGD | ΔTn5041D |
| merRTPCADE | Tn5046 | ||
| P. juntendii 18091276 | pCN1276 | merRTPFADE | Tn512 |
| merRTPCADE | Tn5046 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maslova, O.; Beletsky, A.; Mindlin, S.; Petrova, N.; Mardanov, A.; Petrova, M. Conjugative Plasmid pPPUT-Tik1-1 from a Permafrost Pseudomonas putida Strain and Its Present-Day Counterparts Inhabiting Environments and Clinics. Int. J. Mol. Sci. 2023, 24, 13518. https://doi.org/10.3390/ijms241713518
Maslova O, Beletsky A, Mindlin S, Petrova N, Mardanov A, Petrova M. Conjugative Plasmid pPPUT-Tik1-1 from a Permafrost Pseudomonas putida Strain and Its Present-Day Counterparts Inhabiting Environments and Clinics. International Journal of Molecular Sciences. 2023; 24(17):13518. https://doi.org/10.3390/ijms241713518
Chicago/Turabian StyleMaslova, Olga, Alexey Beletsky, Sofia Mindlin, Nika Petrova, Andrey Mardanov, and Mayya Petrova. 2023. "Conjugative Plasmid pPPUT-Tik1-1 from a Permafrost Pseudomonas putida Strain and Its Present-Day Counterparts Inhabiting Environments and Clinics" International Journal of Molecular Sciences 24, no. 17: 13518. https://doi.org/10.3390/ijms241713518
APA StyleMaslova, O., Beletsky, A., Mindlin, S., Petrova, N., Mardanov, A., & Petrova, M. (2023). Conjugative Plasmid pPPUT-Tik1-1 from a Permafrost Pseudomonas putida Strain and Its Present-Day Counterparts Inhabiting Environments and Clinics. International Journal of Molecular Sciences, 24(17), 13518. https://doi.org/10.3390/ijms241713518






