Biomarkers of Oxidative Stress in Diabetes Mellitus with Diabetic Nephropathy Complications
Abstract
:1. Introduction
2. Results
2.1. Plasma Levels of NO, eNOS and iNOS
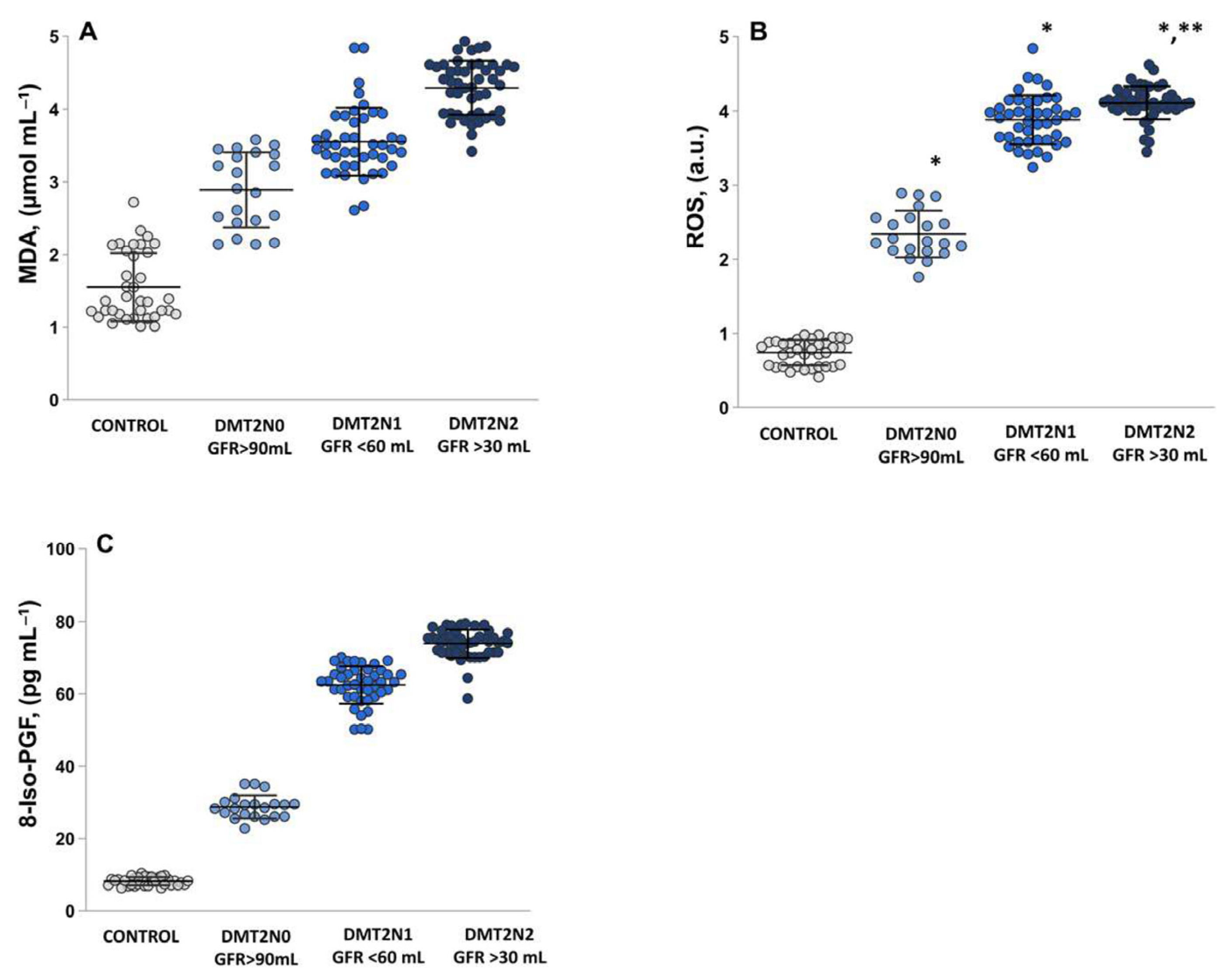
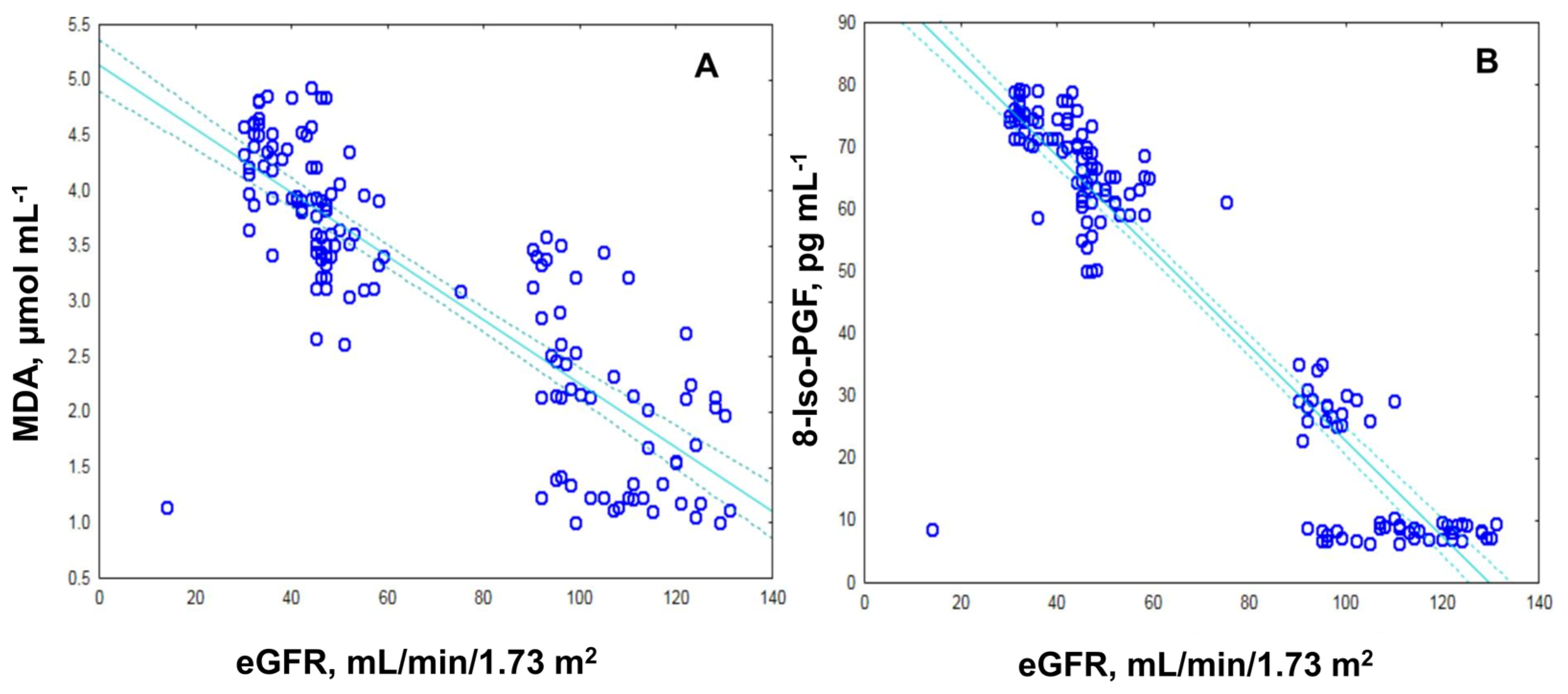
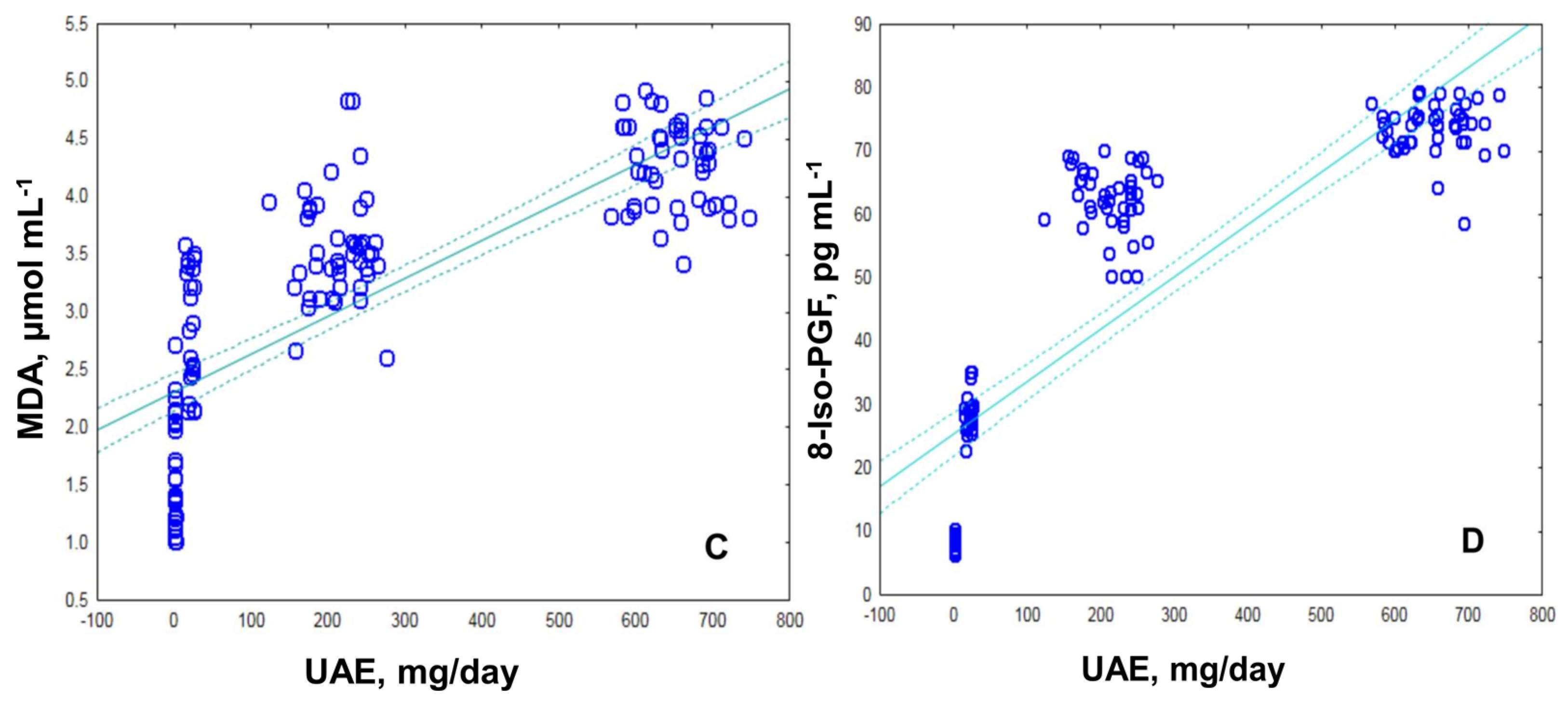
2.2. Assessment of Oxidative Stress by Measuring the MDA, ROS and 8-Iso-prostaglandin Levels
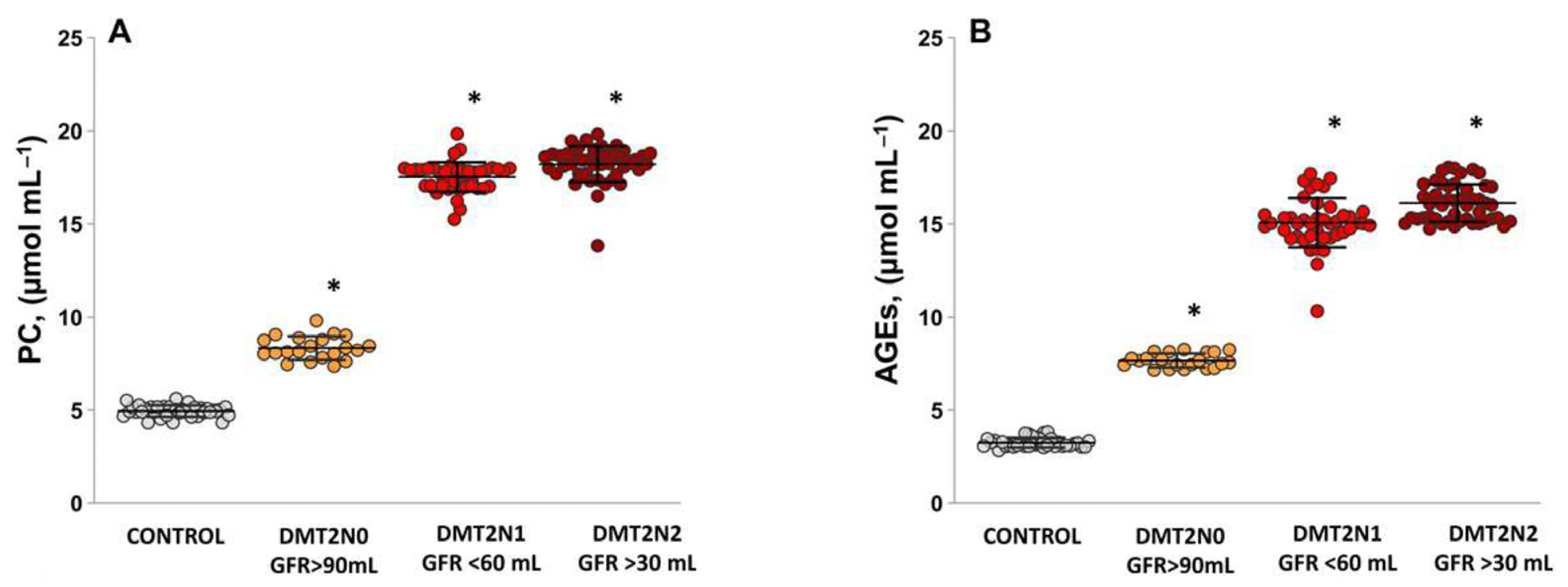
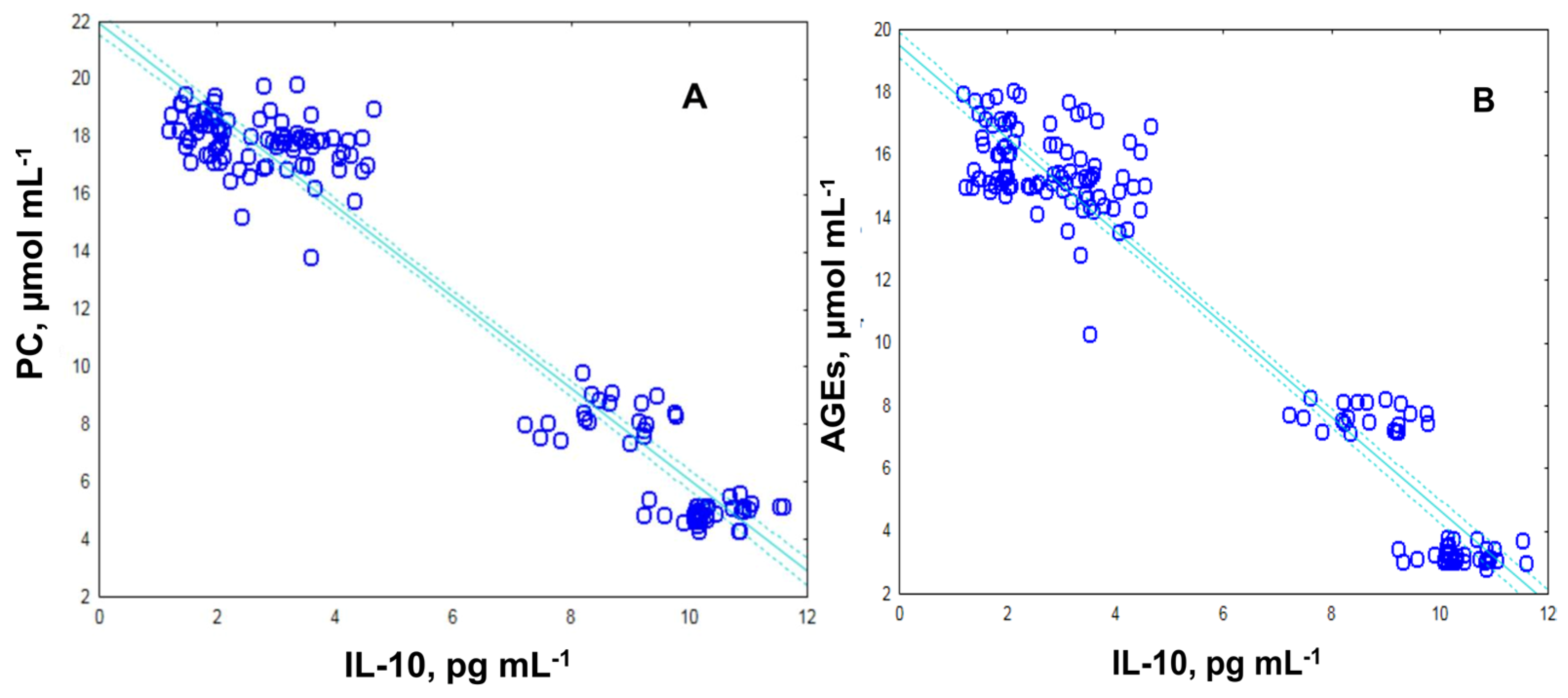
2.3. Levels of Protein Carbonyl and Advanced Glycation End Products, as Suitable Surrogate Biomarkers of Oxidative Damage in Plasma Samples Diabetic Patients
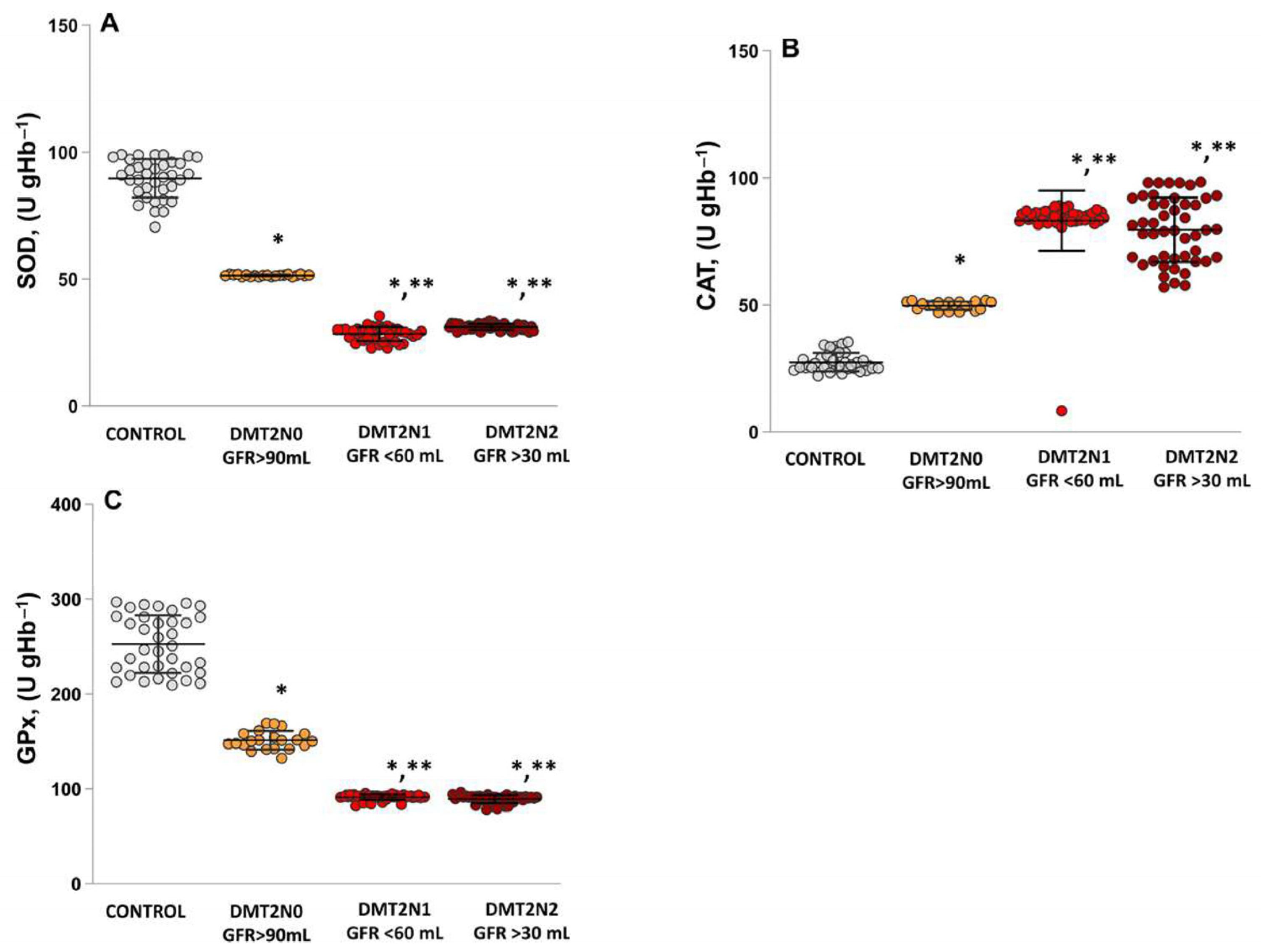
2.4. Antioxidant Enzymes’ Activities (SOD, CAT and GPx) in Patients with DN
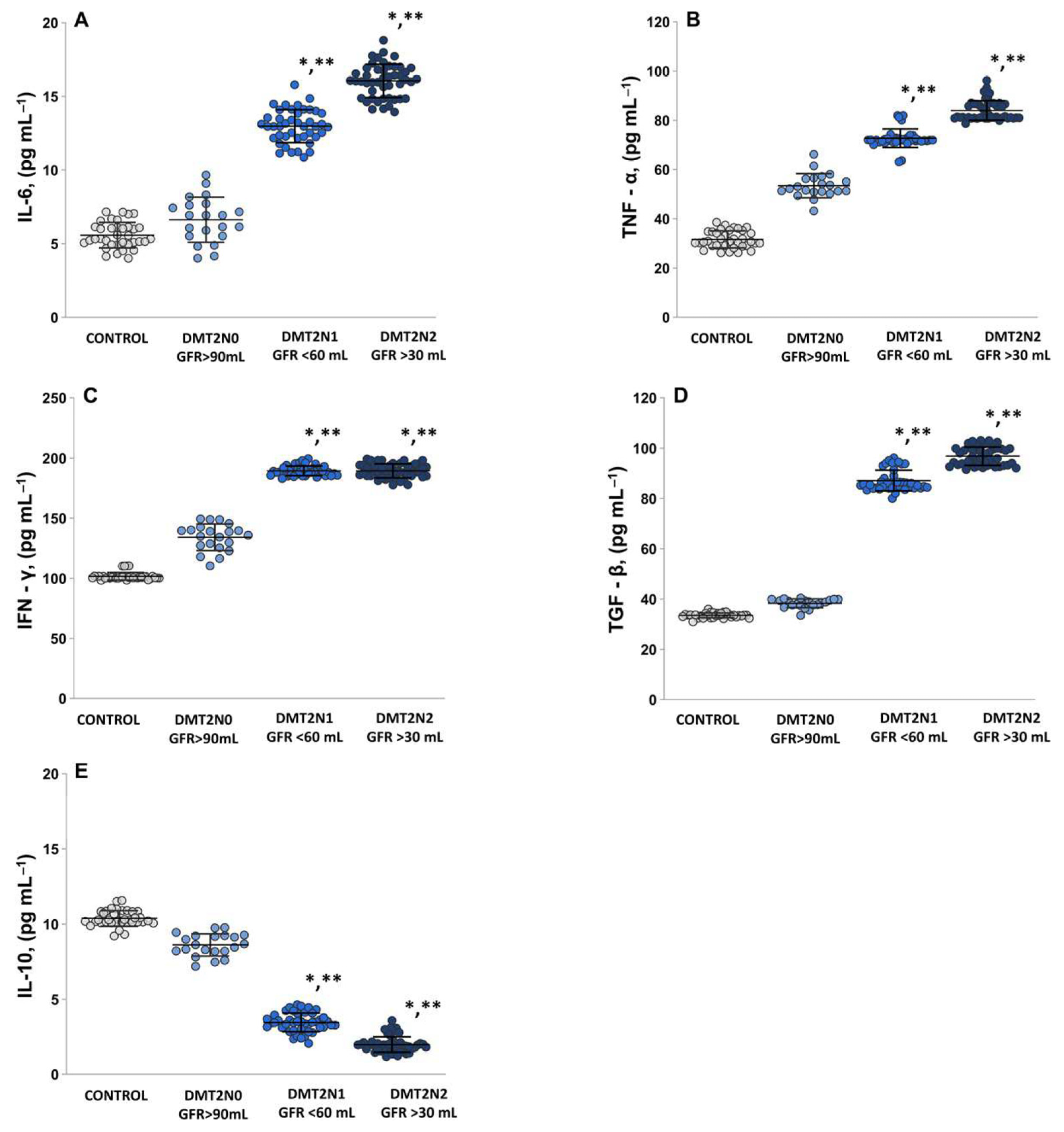
2.5. The Levels of Pro-Inflammatory Cytokines IL-6, TNF-α, IFN-γ
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Subjects
4.3. Electron Paramagnetic Resonance (EPR) Study
4.3.1. Evaluation of the ROS Product Levels
4.3.2. Evaluation of the •NO Radical Levels
4.4. Enzyme-Linked Immunosorbent Assay
4.5. Spectrophotometric Determination of Lipid Peroxidation Products—Malondialdehyde (MDA)
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Gabbay, R.A. 2. Classification and diagnosis of diabetes: Standards of care in diabetes—2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef] [PubMed]
- Giaccari, A.; Sorice, G.; Muscogiuri, G. Glucose toxicity: The leading actor in the pathogenesis and clinical history of type 2 diabetes—Mechanisms and potentials for treatment. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 365–377. [Google Scholar] [CrossRef] [PubMed]
- John, S. Complication in diabetic nephropathy. Diabetes Metab. Syndr. Clin. Res. Rev. 2016, 10, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative stress and diabetes: Antioxidative strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Sol, M.; Kamps, J.A.A.M.; van den Born, J.; van den Heuvel, M.C.; van der Vlag, J.; Krenning, G.; Hillebrands, J.L. Glomerular Endothelial Cells as Instigators of Glomerular Sclerotic Diseases. Front. Pharmacol. 2020, 11, 573557. [Google Scholar] [CrossRef]
- Higashi, Y. Roles of Oxidative Stress and Inflammation in Vascular Endothelial Dysfunction-Related Disease. Antioxidants 2022, 11, 1958. [Google Scholar] [CrossRef]
- Babel, R.A.; Dandekar, M.P. A Review on Cellular and Molecular Mechanisms Linked to the Development of Diabetes Complications. Curr. Diabetes Rev. 2021, 17, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: Eustress and distress in redox homeostasis. In Stress: Physiology, Biochemistry, and Pathology; Academic Press: Cambridge, MA, USA, 2019; pp. 153–163. [Google Scholar] [CrossRef]
- Niki, E. Oxidative stress and antioxidants: Distress or eustress? Arch. Biochem. Biophys. 2016, 595, 19–24. [Google Scholar] [CrossRef]
- Ziegler, D.; Porta, M.; Papanas, N.; Mota, M.; Jermendy, G.; Beltramo, E.; Kempler, P. The Role of Biofactors in Diabetic Microvascular Complications. Curr. Diabetes Rev. 2021, 18, e2508211958302021. [Google Scholar] [CrossRef] [PubMed]
- Leyane, T.S.; Jere, S.W.; Houreld, N.N. Oxidative stress in ageing and chronic degenerative pathologies: Molecular mechanisms involved in counteracting oxidative stress and chronic inflammation. Int. J. Mol. Sci. 2022, 23, 7273. [Google Scholar] [CrossRef] [PubMed]
- Mormone, E.; Iorio, E.L. Regenerative medicine in neurodegenerative diseases and aging: Challenging the redox homeostasis. Front. Neurosci. 2023, 17, 1238781. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Role of metabolic H2O2 generation: Redox signaling and oxidative stress. J. Biol. Chem 2014, 289, 8735–8741. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Lennicke, C.; Cochemé, H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef]
- Tessari, P.; Cecchet, D.; Cosma, A.; Vettore, M.; Coracina, A.; Millioni, R.; Iori, E.; Puricelli, L.; Avogaro, A.; Vedovato, M. Nitric oxide synthesis is reduced in subjects with type 2 diabetes and nephropathy. Diabetes 2010, 59, 2152–2159. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Bir, S.C.; Kevil, C.G.; Calvert, J.W. Endothelial Dysfunction and Diabetes: Effects on Angiogenesis, Vascular Remodeling, and Wound Healing. Int. J. Vasc. Med. 2012, 2012, 918267. [Google Scholar] [CrossRef]
- Sokolovska, J.; Dekante, A.; Baumane, L.; Pahirko, L.; Valeinis, J.; Dislere, K.; Rovite, V.; Pirags, V.; Sjakste, N. Nitric oxide metabolism is impaired by type 1 diabetes and diabetic nephropathy. Biomed. Rep. 2020, 12, 251–258. [Google Scholar] [CrossRef]
- Juin, S.K.; Ouseph, R.; Gondim, D.D.; Jala, V.R.; Sen, U. Diabetic Nephropathy and Gaseous Modulators. Antioxidants 2023, 12, 1088. [Google Scholar] [CrossRef]
- Pautz, A.; Li, H.; Kleinert, H. Regulation of NOS expression in vascular diseases. Front. Biosci. 2021, 26, 85–101. [Google Scholar] [CrossRef]
- Duni, A.; Liakopoulos, V.; Roumeliotis, S.; Peschos, D.; Dounousi, E. Oxidative Stress in the Pathogenesis and Evolution of Chronic Kidney Disease: Untangling Ariadne’s Thread. Int. J. Mol. Sci. 2019, 20, 3711. [Google Scholar] [CrossRef] [PubMed]
- Dellamea, B.S.; Leitão, C.B.; Friedman, R.; Canani, L.H. Nitric oxide system and diabetic nephropathy. Diabetol. Metab. Syndr. 2014, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Rayego-Mateos, S.; Morgado-Pascual, J.L.; Opazo-Ríos, L.; Guerrero-Hue, M.; García-Caballero, C.; Vázquez-Carballo, C.; Mas, S.; Sanz, A.B.; Herencia, C.; Mezzano, S.; et al. Pathogenic Pathways and Therapeutic Approaches Targeting Inflammation in Diabetic Nephropathy. Int. J. Mol. Sci. 2020, 21, 3798. [Google Scholar] [CrossRef] [PubMed]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Valle, E.; Prola, L.; Vergnano, D.; Borghi, R.; Monacelli, F.; Traverso, N.; Bruni, N.; Bovero, A.; Schiavone, A.; Nery, J.; et al. Investigation of hallmarks of carbonyl stress and formation of end products in feline chronic kidney disease as markers of uraemic toxins. J. Feline Med. Surg. 2018, 21, 465–474. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef]
- Pușcașu, C.; Ungurianu, A.; Șeremet, O.C.; Andrei, C.; Mihai, D.P.; Negreș, S. The Influence of Sildenafil–Metformin Combination on Hyperalgesia and Biochemical Markers in Diabetic Neuropathy in Mice. Medicina 2023, 59, 1375. [Google Scholar] [CrossRef]
- Coco, C.; Sgarra, L.; Potenza, A.; Nacci, C.; Pasculli, B.; Barbano, R.; Montagnani, M. Can epigenetics of endothelial dysfunction represent the key to precision medicine in type 2 diabetes mellitus? Int. J. Mol. Sci. 2019, 20, 2949. [Google Scholar] [CrossRef]
- Alhayaza, R.; Haque, E.; Karbasiafshar, C.; Sellke, F.W.; Abid, M.R. The Relationship Between Reactive Oxygen Species and Endothelial Cell Metabolism. Front. Chem. 2020, 8, 592688. [Google Scholar] [CrossRef]
- Ray, A.; Maharana, K.C.; Meenakshi, S.; Singh, S. Endothelial dysfunction and its relation in different disorders: Recent update. Health Sci. Rev. 2023, 7, 100084. [Google Scholar] [CrossRef]
- Hojs, N.V.; Bevc, S.; Ekart, R.; Hojs, R. Oxidative Stress Markers in Chronic Kidney Disease with Emphasis on Diabetic Nephropathy. Antioxidants 2020, 9, 925. [Google Scholar] [CrossRef] [PubMed]
- Rajlic, S.; Treede, H.; Münzel, T.; Daiber, A.; Duerr, G.D. Early Detection Is the Best Prevention—Characterization of Oxidative Stress in Diabetes Mellitus and Its Consequences on the Cardiovascular System. Cells 2023, 12, 583. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Scioli, M.G.; Storti, G.; D’amico, F.; Guzmán, R.R.; Centofanti, F.; Doldo, E.; Miranda, E.M.C.; Orlandi, A. Oxidative Stress and New Pathogenetic Mechanisms in Endothelial Dysfunction: Potential Diagnostic Biomarkers and Therapeutic Targets. J. Clin. Med. 2020, 9, 1995. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Mechanistic Insight into Oxidative Stress-Triggered Signaling Pathways and Type 2 Diabetes. Molecules 2022, 27, 950. [Google Scholar] [CrossRef]
- Shabalala, S.C.; Johnson, R.; Basson, A.K.; Ziqubu, K.; Hlengwa, N.; Mthembu, S.X.H.; Mabhida, S.E.; Mazibuko-Mbeje, S.E.; Hanser, S.; Cirilli, I.; et al. Detrimental Effects of Lipid Peroxidation in Type 2 Diabetes: Exploring the Neutralizing Influence of Antioxidants. Antioxidants 2022, 11, 2071. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Ma, E.; Ingram, K.H.; Milne, G.L.; Garvey, W.T. F2-isoprostanes reflect oxidative stress correlated with lean mass and bone density but not insulin resistance. J. Endocr. Soc. 2017, 1, 436–448. [Google Scholar] [CrossRef]
- Kaviarasan, S.; Muniandy, S.; Qvist, R.; Ismail, I.S. F(2)-isoprostanes as novel biomarkers for type 2 diabetes: A review. J. Clin. Biochem. Nutr. 2009, 45, 1–8. [Google Scholar] [CrossRef]
- Jamwal, S.; Sharma, S. Vascular endothelium dysfunction: A conservative target in metabolic disorders. Inflamm. Res. 2018, 67, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Vlassara, H.; Kirstein, M.; Yamada, Y.; Striker, G.E.; Striker, L.J. Receptor-specific increase in extracellular matrix production in mouse mesangial cells by advanced glycosylation end products is mediated via platelet-derived growth factor. Proc. Natl. Acad. Sci. USA 1992, 89, 2873–2877. [Google Scholar] [CrossRef] [PubMed]
- Sahakyan, G.; Vejux, A.; Sahakyan, N. The Role of Oxidative Stress-Mediated Inflammation in the Development of T2DM-Induced Diabetic Nephropathy: Possible Preventive Action of Tannins and Other Oligomeric Polyphenols. Molecules 2022, 27, 9035. [Google Scholar] [CrossRef]
- Elmarakby, A.A.; Sullivan, J.C. Relationship between Oxidative Stress and Inflammatory Cytokines in Diabetic Nephropathy. Cardiovasc. Ther. 2012, 30, 49–59. [Google Scholar] [CrossRef]
- El-Azab, M.F.; Al-Karmalawy, A.A.; Antar, S.A.; Hanna, P.A.; Tawfik, K.M.; Hazem, R.M. A novel role of Nano selenium and sildenafil on streptozotocin-induced diabetic nephropathy in rats by modulation of inflammatory, oxidative, and apoptotic pathways. Life Sci. 2022, 303, 120691. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Kulkarni, Y.A. NF-κβ: A Potential Target in the Management of Vascular Complications of Diabetes. Front. Pharmacol. 2017, 8, 798. [Google Scholar] [CrossRef]
- Li, H.; Meng, Y.; He, S.; Tan, X.; Zhang, Y.; Zhang, X.; Wang, L.; Zheng, W. Macrophages, Chronic Inflammation, and Insulin Resistance. Cells 2022, 11, 3001. [Google Scholar] [CrossRef]
- Charlton, A.; Garzarella, J.; Jandeleit-Dahm, K.A.; Jha, J.C. Oxidative stress and inflammation in renal and cardiovascular complications of diabetes. Biology 2020, 10, 18. [Google Scholar] [CrossRef]
- Hayden, M.R. The Mighty Mitochondria Are Unifying Organelles and Metabolic Hubs in Multiple Organs of Obesity, Insulin Resistance, Metabolic Syndrome, and Type 2 Diabetes: An Observational Ultrastructure Study. Int. J. Mol. Sci. 2022, 23, 4820. [Google Scholar] [CrossRef]
- Tan, Y.; Cheong, M.S.; Cheang, W.S. Roles of Reactive Oxygen Species in Vascular Complications of Diabetes: Therapeutic Properties of Medicinal Plants and Food. Oxygen 2022, 2, 246–268. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Wronka, M.; Krzemińska, J.; Młynarska, E.; Rysz, J.; Franczyk, B. The influence of lifestyle and treatment on oxidative stress and inflammation in diabetes. Int. J. Mol. Sci. 2022, 23, 15743. [Google Scholar] [CrossRef] [PubMed]
- Martinez de Toda, I.; Ceprián, N.; Díaz-Del Cerro, E.; De la Fuente, M. The role of immune cells in oxi-inflamm-aging. Cells 2021, 10, 2974. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45. [Google Scholar]
- Shi, H.; Sui, Y.; Wang, X.; Luo, Y.; Ji, L. Hydroxyl radical production and oxidative damage induced by cadmium and naphthalene in liver of Carassius auratus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2005, 140, 115–121. [Google Scholar] [CrossRef]
- Yokoyama, K.; Hashiba, K.; Wakabayashi, H.; Hashimoto, K.; Satoh, K.; Kurihara, T.; Motohashi, N.; Sakagami, H. Inhibition of LPS-stimulated NO production in mouse macrophage-like cells by tropolones. Anticancer Res. 2004, 24, 3917–3922. [Google Scholar]
- Yoshioka, T.; Iwamoto, N.; Lto, K. An applicationof Electron Paramagnetic Resonance to evaluate nitric oxide and its quenchers. J. Am. Soc. Nephrol. 1996, 7, 961–965. [Google Scholar] [CrossRef]
- Plaser, Z.A.; Cushman, L.L.; Jonson, B.C. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal. Biochem. 1966, 16, 359–364. [Google Scholar] [CrossRef]

| Variables | Controls (n = 36) | DMT2N0 with Normal Renal Function (n = 21), Stage 1 | DMT2N1 with Mild-to-Moderate Renal Failure (n = 43), Stage 3a | DMT2N2 with Moderate-to-Severe Renal Failure (n = 47), Stage 3b |
|---|---|---|---|---|
| Age | 41.25 ± 7.78 | 51.27 ± 6.07 | 54.41 ± 5.74 | 56.68 ± 8.91 |
| Sex (M/F) | 14 M/24 F | 10 M/11 F | 10 M/13 F | 14 M/10 F |
| Disease duration | - | 10.7 ± 7.3 | 12.7 ± 8.6 | 14.8 ± 6.1 |
| BMI (kg/m2), mean ± SD | 26.07 ± 3.52 | 32.11 ± 5.44 | 33.56 ± 6.21 | 34.76 ± 6.14 |
| Blood sugar (mmol/L), mean ± SD | 4.98 ± 0.32 | 8.05 ± 5.11 | 9.55 ± 4.89 | 9.82 ± 5.41 |
| HbA1C% | 5.69 ± 0.44 | 6.37 ± 0.85 | 9.87 ± 1.27 | 9.99 ± 1.98 |
| GFR (mL/min/1.73 m2) | 110.08 ± 9.32 | 95.66 ± 5.60 | 49.83 ± 2.57 | 36.95 ±4.50 |
| UAE (mg/day) | 1.20 ± 035 | 21.80 ± 1.7 | 213.55 ± 6.85 | 651.08 ± 8.92 |
| FBC (mmol/L) | 5.31 ± 0.28 | 6.43 ± 0.89 | 12.72 ± 5.15 | 5.47± 0.6 |
| Cholesterol (mmol/L) | 4.17 ± 0.3 | 5.01 ± 0.7 | 5.47 ± 0.6 | 5.47 ± 0.6 |
| Triglycerides (mmol/L) | 1.31 ± 0.15 | 2.13 ± 0.2 | 2.65 ± 0.2 | 3.01 |
| HDL (mmol/L) | 0.91 ± 0.019 | 1.19 ± 0.04 | 1.32 ± 0.05 | 1.33 ± 0.01 |
| LDL (mmol/L) | 2.03 ± 0.11 | 2.72 ± 0.24 | 2.96 ± 0.2 | 3.2 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goycheva, P.; Petkova-Parlapanska, K.; Georgieva, E.; Karamalakova, Y.; Nikolova, G. Biomarkers of Oxidative Stress in Diabetes Mellitus with Diabetic Nephropathy Complications. Int. J. Mol. Sci. 2023, 24, 13541. https://doi.org/10.3390/ijms241713541
Goycheva P, Petkova-Parlapanska K, Georgieva E, Karamalakova Y, Nikolova G. Biomarkers of Oxidative Stress in Diabetes Mellitus with Diabetic Nephropathy Complications. International Journal of Molecular Sciences. 2023; 24(17):13541. https://doi.org/10.3390/ijms241713541
Chicago/Turabian StyleGoycheva, Petya, Kamelia Petkova-Parlapanska, Ekaterina Georgieva, Yanka Karamalakova, and Galina Nikolova. 2023. "Biomarkers of Oxidative Stress in Diabetes Mellitus with Diabetic Nephropathy Complications" International Journal of Molecular Sciences 24, no. 17: 13541. https://doi.org/10.3390/ijms241713541
APA StyleGoycheva, P., Petkova-Parlapanska, K., Georgieva, E., Karamalakova, Y., & Nikolova, G. (2023). Biomarkers of Oxidative Stress in Diabetes Mellitus with Diabetic Nephropathy Complications. International Journal of Molecular Sciences, 24(17), 13541. https://doi.org/10.3390/ijms241713541









