The Impact of 5-Aminolevulinic Acid Supplementation on Redox Balance and Aerobic Capacity
Abstract
1. Introduction
2. Results
2.1. Primary Analysis
2.2. Secondary Analysis
2.2.1. Heart Rate

2.2.2. Blood Lactate Concentrations (La)

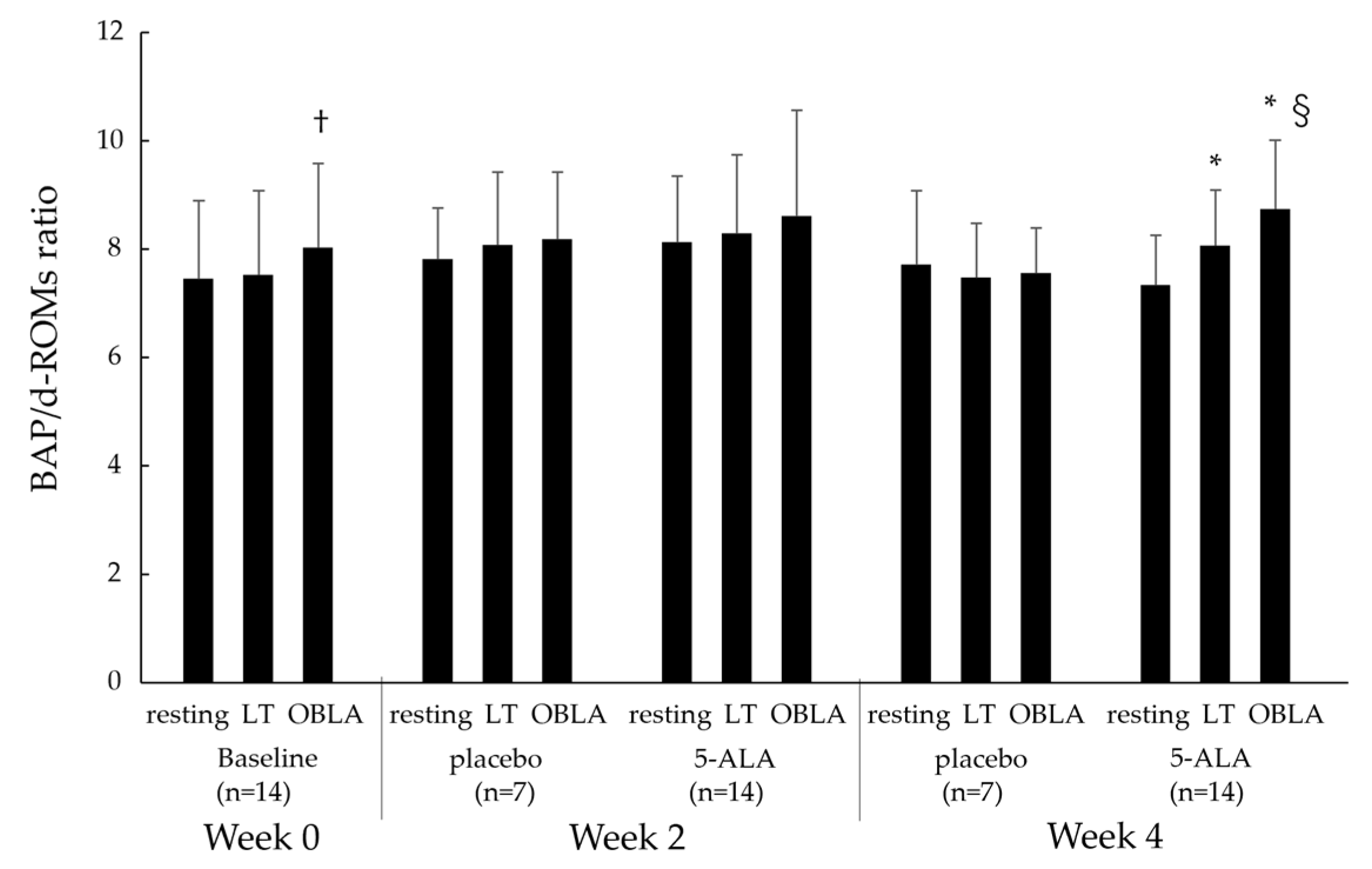
2.2.3. Diacron Reactive Oxygen Metabolites (d-ROMs) and Biological Antioxidant Potential (BAP)


2.2.4. Salivary Cortisol and Secretory Immunoglobulin A Levels (s-IgA)
2.2.5. Rating of Perceived Exertion (RPE)
2.2.6. Profile of Mood States
2.2.7. Fatigue
3. Discussion
3.1. Oxidative Stress and Antioxidant Potential
3.2. Aerobic Capacity
3.3. Profile of Mood States and Fatigue
4. Materials and Methods
4.1. Participants

4.2. Clinical Trial Design

4.3. Submaximal Incremental Cycling Test
4.4. Measurements
4.4.1. HR
4.4.2. La
4.4.3. d-ROMs and BAP
4.4.4. Salivary Cortisol and s-IgA
4.4.5. RPE
4.4.6. Profile of Mood States
4.4.7. Fatigue
4.5. Statistical Analysis
4.5.1. Primary Analysis
4.5.2. Secondary Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodriguez, B.L.; Curb, J.D.; Davis, J.; Shintani, T.; Perez, M.H.; Apau-Ludlum, N.; Johnson, C.; Harrigan, R.C. Use of the dietary supplement 5-aminiolevulinic acid (5-ALA) and its relationship with glucose levels and hemoglobin A1C among individuals with prediabetes. Clin. Transl. Sci. 2012, 5, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Ogura, S.I.; Maruyama, K.; Hagiya, Y.; Sugiyama, Y.; Tsuchiya, K.; Takahashi, K.; Abe, F.; Tabata, K.; Okura, I.; Nakajima, M.; et al. The effect of 5-aminolevulinic acid on cytochrome c oxidase activity in mouse liver. BMC Res. Notes 2011, 4, 66. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Yasuzawa, T.; Uesaka, A.; Izumi, Y.; Kamiya, A.; Tsuchiya, K.; Kobayashi, Y.; Kuwahata, M.; Kido, Y. Type 2 diabetic conditions in Otsuka Long-Evans Tokushima Fatty rats are ameliorated by 5-aminolevulinic acid. Nutr. Res. 2014, 34, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Masuki, S.; Morita, A.; Kamijo, Y.I.; Ikegawa, S.; Kataoka, Y.; Ogawa, Y.; Sumiyoshi, E.; Takahashi, K.; Tanaka, T.; Nakajima, M.; et al. Impact of 5-aminolevulinic acid with iron supplementation on exercise efficiency and home-based walking training achievement in older women. J. Appl. Physiol. 2016, 120, 87–96. [Google Scholar] [CrossRef]
- Radák, Z.; Pucsok, J.; Mecseki, S.; Csont, T.; Ferdinandy, P. Muscle soreness-induced reduction in force generation is accompanied by increased nitric oxide content and DNA damage in human skeletal muscle. Free Radic. Biol. Med. 1999, 26, 1059–1063. [Google Scholar] [CrossRef]
- Varamenti, E.; Tod, D.; Pullinger, S.A. Redox Homeostasis and Inflammation Responses to Training in Adolescent Athletes: A Systematic Review and meta-analysis. Sports Med. Open 2020, 6, 34. [Google Scholar] [CrossRef]
- Levada-Pires, A.C.; Cury-Boaventura, M.F.; Gorjao, R.; Hirabara, S.M.; Puggina, E.F.; Pellegrinotti, I.L.; Domingues Filho, L.A.; Curi, R.; Pithon-Curi, T.C. Induction of lymphocyte death by short- and long-duration triathlon competitions. Med. Sci. Sports Exerc. 2009, 41, 1896–1901. [Google Scholar] [CrossRef]
- Tanskanen, M.M.; Uusitalo, A.L.; Kinnunen, H.; Häkkinen, K.; Kyröläinen, H.; Atalay, M. Association of military training with oxidative stress and overreaching. Med. Sci. Sports Exerc. 2011, 43, 1552–1560. [Google Scholar] [CrossRef]
- Margonis, K.; Fatouros, I.G.; Jamurtas, A.Z.; Nikolaidis, M.G.; Douroudos, I.; Chatzinikolaou, A.; Mitrakou, A.; Mastorakos, G.; Papassotiriou, I.; Taxildaris, K.; et al. Oxidative stress biomarkers responses to physical overtraining: Implications for diagnosis. Free Radic. Biol. Med. 2007, 43, 901–910. [Google Scholar] [CrossRef]
- Tanskanen, M.; Atalay, M.; Uusitalo, A. Altered oxidative stress in overtrained athletes. J. Sports Sci. 2010, 28, 309–317. [Google Scholar] [CrossRef]
- Castro, C.E. Mechanisms of reaction of hemeproteins with oxygen and hydrogen peroxide in the oxidation of organic substrates. Pharmacol. Ther. 1980, 10, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Cai, S.; Kitajima, Y.; Fujino, M.; Ito, H.; Takahashi, K.; Abe, F.; Tanaka, T.; Ding, Q.; Li, X.K. 5-Aminolevulinic acid combined with ferrous iron induces carbon monoxide generation in mouse kidneys and protects from renal ischemia-reperfusion injury. Am. J. Physiol. Renal. Physiol. 2013, 305, F1149–F1157. [Google Scholar] [CrossRef]
- Fujino, M.; Nishio, Y.; Ito, H.; Tanaka, T.; Li, X.K. 5-aminolevulinic acid regulates the inflammatory response and alloimmune reaction. Int. Immunopharmacol. 2016, 37, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.; Choi, A.M.K. Heme oxygenase-1 the “emerging molecule” has arrived. Am. J. Respir. Cell Mol. Biol. 2002, 27, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fujino, M.; Zhu, S.; Isaka, Y.; Ito, H.; Takahashi, K.; Nakajima, M.; Tanaka, T.; Zhu, P.; Li, X.K. 5-ALA/SFC en-hances HO-1 expression through the MAPK/Nrf2 antioxidant pathway and attenuates murine tubular epithelial cell apoptosis. FEBS Open Bio 2019, 9, 1928–1938. [Google Scholar] [CrossRef]
- Higashikawa, F.; Noda, M.; Awaya, T.; Tanaka, T.; Sugiyama, M. 5-aminolevulinic acid, a precursor of heme, reduces both fasting and postprandial glucose levels in mildly hyperglycemic subjects. Nutrition 2013, 29, 1030–1036. [Google Scholar] [CrossRef]
- Powers, S.K.; Ji, L.L.; Kavazis, A.N.; Jackson, M.J. Reactive oxygen species: Impact on skeletal muscle. Compr. Physiol. 2011, 1, 941–969. [Google Scholar]
- Tryfidou, D.V.; McClean, C.; Nikolaidis, M.G.; Davison, G.W. DNA Damage Following Acute Aerobic Exercise: A Systematic Review and Meta-analysis. Sports Med. 2020, 50, 103–127. [Google Scholar] [CrossRef]
- Lewis, N.A.; Howatson, G.; Morton, K.; Hill, J.; Pedlar, C.R. Alterations in redox homeostasis in the elite endurance athlete. Sports Med. 2015, 45, 379–409. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Tsukioka, K.; Naito, H. Changes in the blood redox balance during a simulated duathlon race and its relationship with athletic performance. Physiol. Rep. 2019, 7, e14277. [Google Scholar] [CrossRef]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Calvani, R.; Cesari, M.; Buford, T.W.; Lorenzi, M.; Behnke, B.J.; Leeuwenburgh, C. Mitochondrial dysfunction and sarcopenia of aging: From signaling pathways to clinical trials. Int. J. Biochem. Cell Biol. 2013, 45, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Mcmillin, J.B.; Taffet, G.E.; Taegtmeyer, H.; Hudson, E.K.; Tate, C.A. Mitochondrial metabolism and substrate competition in the aging Fischer rat heart. Cardiovasc. Res. 1993, 27, 2222–2228. [Google Scholar] [CrossRef] [PubMed]
- Nojima, J.; Motoki, Y.; Tsuneoka, H.; Kuratsune, H.; Matsui, T.; Yamamoto, M.; Yanagihara, M.; Hinoda, Y.; Ichihara, K. ‘Oxidation stress index’ as a possible clinical marker for the evaluation of non-Hodgkin lymphoma. Br. J. Haematol. 2011, 155, 528–530. [Google Scholar] [CrossRef]
- Maurya, P.K.; Kumar, P.P.; Chandra, P. Biomarkers of oxidative stress in erythrocytes as a function of human age. World J. Methodol. 2015, 5, 216–222. [Google Scholar] [CrossRef]
- Kendall, B.; Eston, R. Exercise-induced muscle damage and the potential protective role of estrogen. Sports Med. 2002, 32, 103–123. [Google Scholar] [CrossRef]
- Aquino, R.; Perez, M.; Sil, P.; Shintani, T.; Harrigan, R.; Rodriguez, B. The relationship of 5-aminolevulinic acid on mood and coping ability in prediabetic middle aged and older adults. Geriatrics 2018, 3, 17. [Google Scholar] [CrossRef]
- Higashikawa, F.; Kanno, K.; Ogata, A.; Sugiyama, M. Reduction of fatigue and anger-hostility by the oral administration of 5-aminolevulinic acid phosphate: A randomized, double-blind, placebo-controlled, parallel study. Sci. Rep. 2020, 10, 16004. [Google Scholar] [CrossRef]
- Ota, U.; Sugihara, H.; Abe, F.; Nakajima, M.; Ogura, S.; Tanaka, T. 5-Aminolevulinic Acid (5-ALA): A precursor of heme: Fermentation, metabolism and usage. ALA-Porphyr. Sci. 2013, 2, 3–17. [Google Scholar]
- Bonaventura, J.M.; Sharpe, K.; Knight, E.; Fuller, K.L.; Tanner, R.K.; Gore, C.J. Reliability and Accuracy of Six Hand-Held Blood Lactate Analysers. J. Sports Sci. Med. 2015, 14, 203–214. [Google Scholar]
- Trotti, R.; Carratelli, M.; Barbieri, M. Performance and clinical application of a new, fast method for the detection of hydroperoxides in serum. Panminerva Med. 2002, 44, 37–40. [Google Scholar] [PubMed]
- Pasquini, A.; Luchetti, E.; Marchetti, V.; Cardini, G.; Iorio, E.L. Analytical performances of d-ROMs test and BAP test in canine plasma. Definition of the normal range in healthy Labrador dogs. Vet. Res. Commun. 2008, 32, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Izawa, S.; Shirotsuki, K.; Sugaya, N.; Ogawa, N.; Suzuki, K.; Nomura, S. The application of saliva to an assessment of stress: Procedures for collecting and analyzing saliva and characteristics of salivary substances. Jpn. J. Complement. Altern. Med. 2007, 4, 91–101. [Google Scholar] [CrossRef][Green Version]
- Borg, G. Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar] [CrossRef]
- Heuchert, J.P.; McNair, D.M.; Yokoyama, K.; Watanabe, K. POMS Japanese Manual; Yokoyama, K., Trans, K.W., Eds.; Kanekoshobo: Tokyo, Japan, 2015; p. 2. (In Japanese) [Google Scholar]
- Heuchert, J.P.; McNair, D.M. Test Review: The Profile of Mood States 2nd Edition. J. Psychoeduc. Assess. 2014, 32, 273–277. [Google Scholar]
- Lee, K.A.; Hicks, G.; Nino-Murcia, G. Validity and reliability of a scale to assess fatigue. Psychiatry Res. 1991, 36, 291–298. [Google Scholar] [CrossRef]
- Corsetti, R.; Villa, M.; Pasturenzi, M.; Finco, A.; Cornelli, U. Redox State in Professional Cyclists Following Competitive Sports Activity. Open Sports Med. J. 2012, 6, 34–41. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
| Week 0 | Week 2 | Week 4 | ||||
|---|---|---|---|---|---|---|
| POMS 2 Scales | Baseline | Pla | ALA | Pla | ALA | |
| Profile of Mood States | Anger/Hostility | 3.1 ± 2.9 | 3.0 ± 2.4 | 4.9 ± 4.6 | 3.4 ± 2.3 | 4.2 ± 4.9 |
| Confusion/Bewilderment | 7.3 ± 3.2 | 7.0 ± 3.8 | 6.1 ± 2.7 | 9.1 ± 4.4 | 6.6 ± 4.2 | |
| Depression/Dejection | 4.1 ± 3.1 | 4.3 ± 4.9 | 3.5 ± 2.8 | 4.9 ± 3.3 | 3.2 ± 3.6 | |
| Fatigue/Inertia | 6.4 ± 3.3 | 6.9 ± 2.8 | 6.9 ± 3.9 | 9.6 ± 3.8 | 7.1 ± 4.4 | |
| Tension/Anxiety | 6.9 ± 3.5 | 7.4 ± 4.3 | 6.8 ± 2.8 | 8.1 ± 4.9 | 5.9 ± 3.8 | |
| Vigor/Activity | 11.3 ± 4.5 | 12.0 ± 3.1 | 10.0 ± 4.0 | 9.0 ± 2.8 | 10.0 ± 2.8 | |
| Friendliness | 10.3 ± 3.2 | 9.6 ± 2.8 | 9.1 ± 3.3 | 9.7 ± 3.0 | 9.6 ± 3.2 | |
| Total Mood Disturbance | 26.7 ± 13.8 | 26.1 ± 17.4 | 27.7 ± 15.8 | 27.0 ± 13.4 | 26.6 ± 17.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saga, N.; Hu, A.; Yamaguchi, T.; Naraoka, Y.; Kobayashi, H. The Impact of 5-Aminolevulinic Acid Supplementation on Redox Balance and Aerobic Capacity. Int. J. Mol. Sci. 2024, 25, 988. https://doi.org/10.3390/ijms25020988
Saga N, Hu A, Yamaguchi T, Naraoka Y, Kobayashi H. The Impact of 5-Aminolevulinic Acid Supplementation on Redox Balance and Aerobic Capacity. International Journal of Molecular Sciences. 2024; 25(2):988. https://doi.org/10.3390/ijms25020988
Chicago/Turabian StyleSaga, Norio, Ailing Hu, Takuji Yamaguchi, Yuna Naraoka, and Hiroyuki Kobayashi. 2024. "The Impact of 5-Aminolevulinic Acid Supplementation on Redox Balance and Aerobic Capacity" International Journal of Molecular Sciences 25, no. 2: 988. https://doi.org/10.3390/ijms25020988
APA StyleSaga, N., Hu, A., Yamaguchi, T., Naraoka, Y., & Kobayashi, H. (2024). The Impact of 5-Aminolevulinic Acid Supplementation on Redox Balance and Aerobic Capacity. International Journal of Molecular Sciences, 25(2), 988. https://doi.org/10.3390/ijms25020988






