Mucins 3A and 3B Are Expressed in the Epithelium of Human Large Airway
Abstract
:1. Introduction
2. Results
2.1. Demographics of the Study Groups
2.2. Immunohistochemical MUC3A and MUC3B Expression in Airway Epithelium
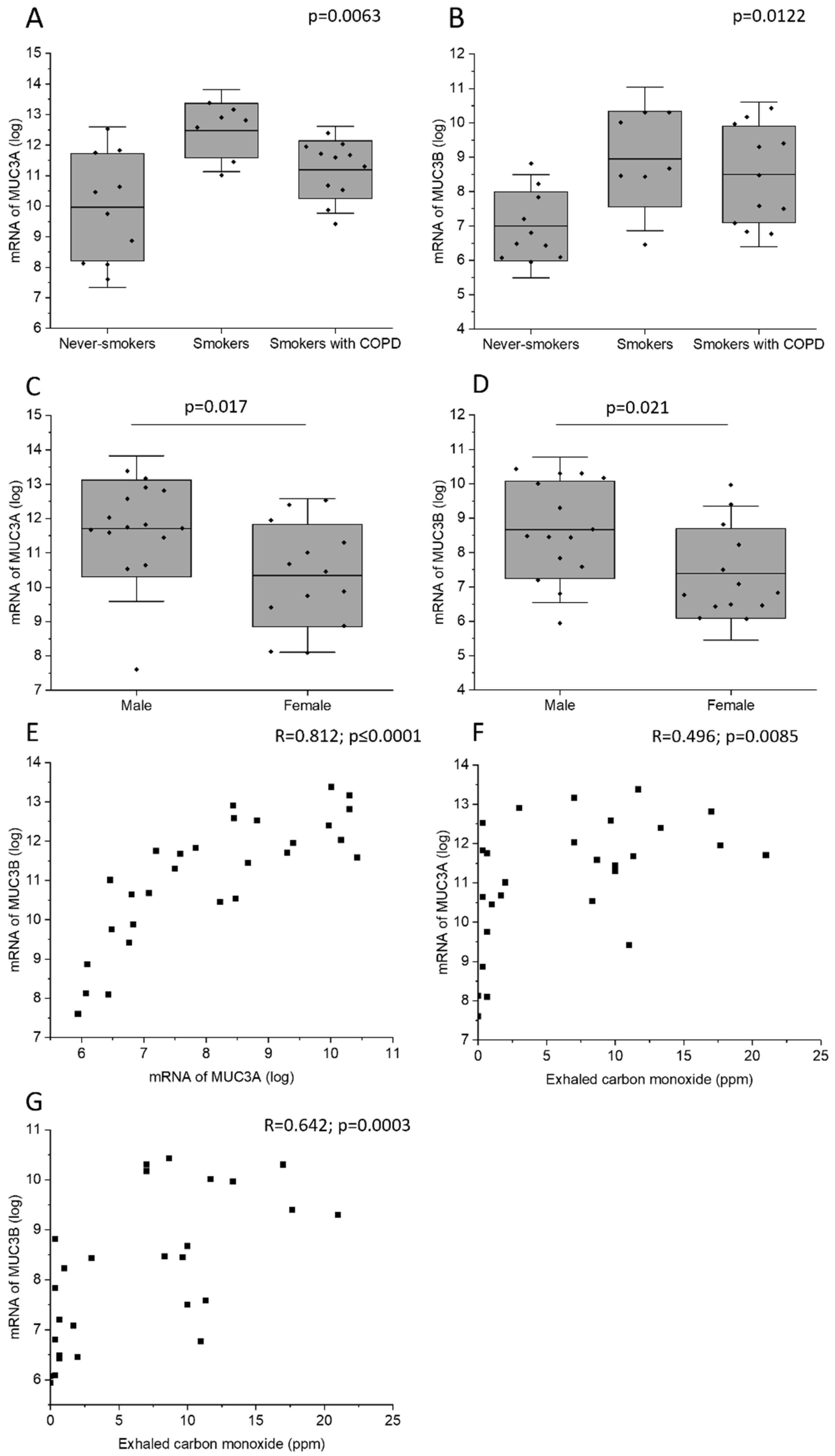
2.3. MUC3A and MUC3B mRNA Levels across the Study Groups
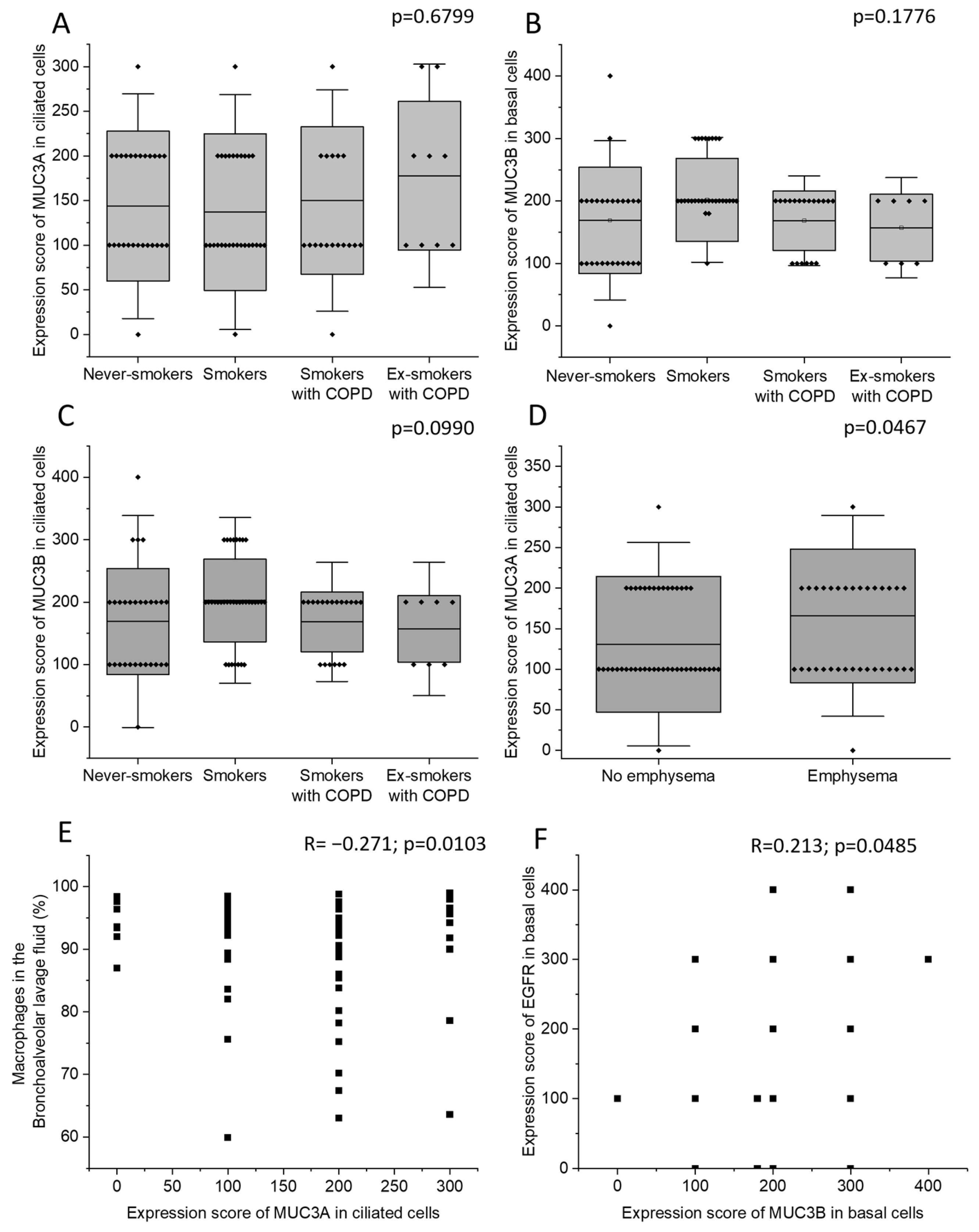
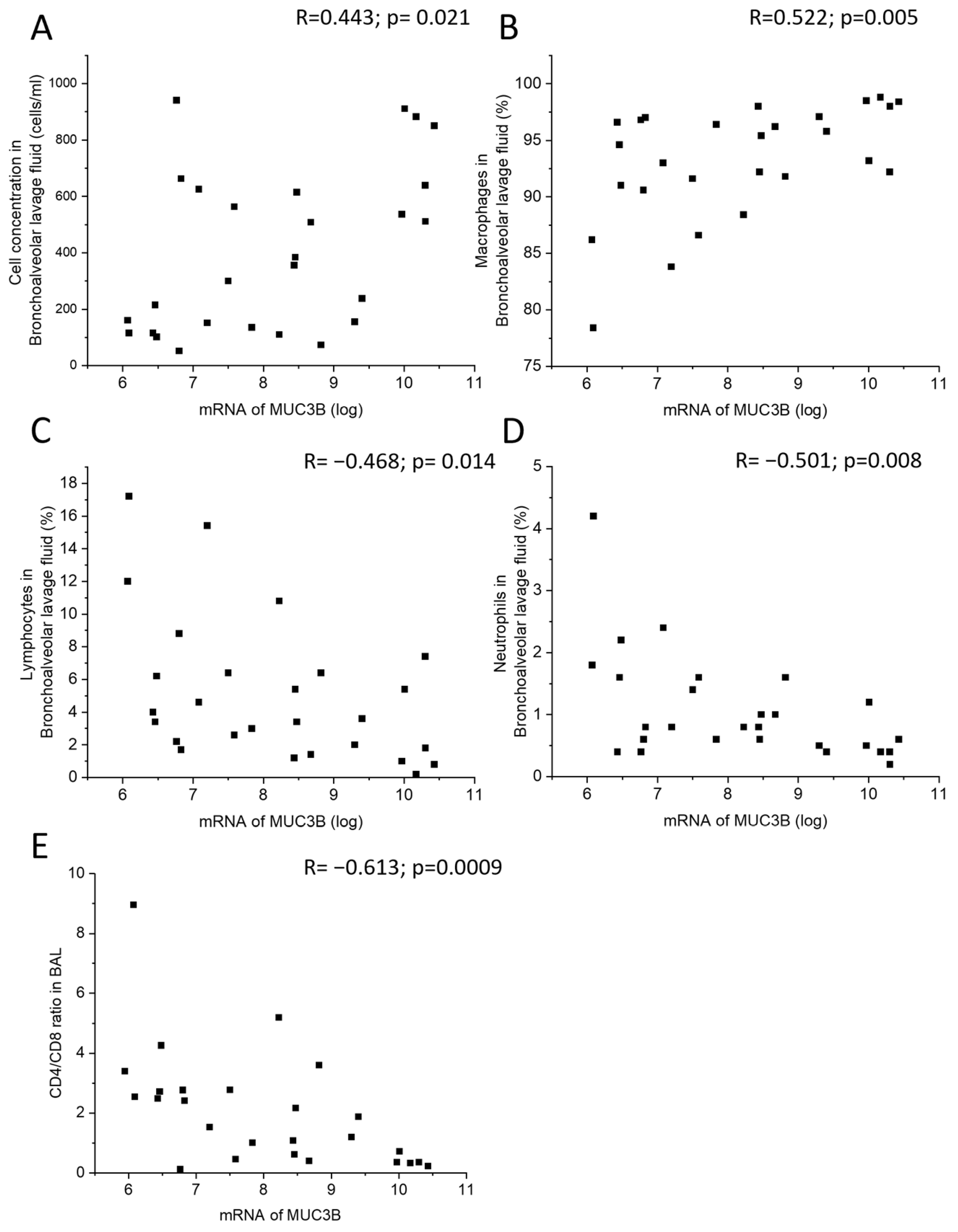
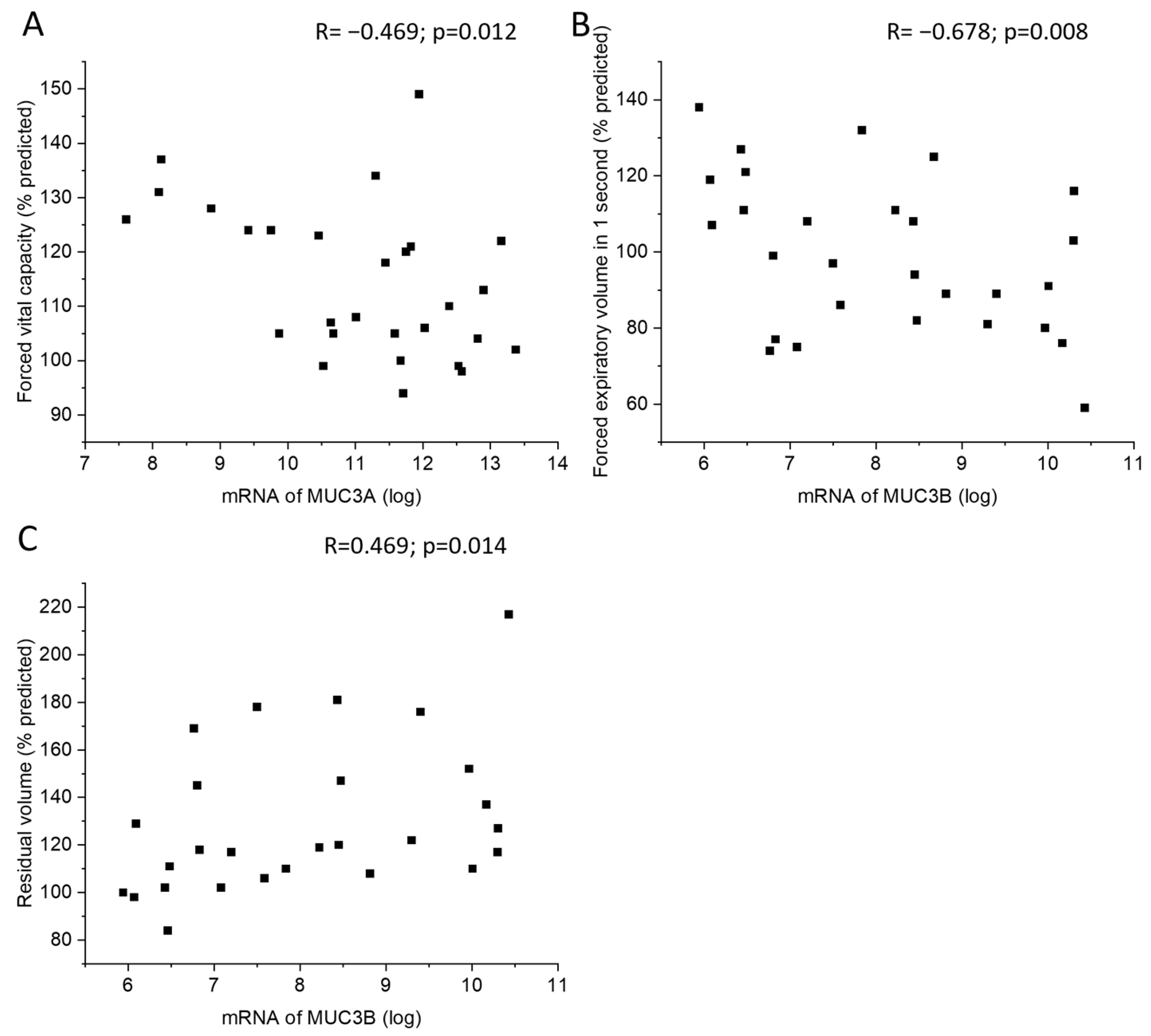
2.4. Correlations of MUC3A and MUC3B mRNA Levels with Clinical Parameters and BALF
3. Discussion
4. Materials and Methods
4.1. Study Subjects
4.2. Bronchoscopy and Inflammatory Markers
4.3. Immunohistochemical Staining and Analysis of Immunohistochemical Protein Expression
4.4. MUC3A and MUC3B mRNA Quantification by Microarray
4.5. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BALF | bronchoalveolar lavage fluid |
| COPD | chronic obstructive pulmonary disease |
| COSMIC | Clinical & Systems Medicine Investigations of Smoking-related Chronic Obstructive Pulmonary Disease |
| CT | computed tomography |
| EGF | epidermal growth factor |
| EGFR | epidermal growth factor receptor |
| FEV1 | forced expiratory volume in one second |
| VC | vital capacity |
| FVC | forced vital capacity |
| mRNA | messenger RNA |
| MUC | mucin |
| RV | residual volume |
References
- Caramori, G.; Casolari, P.; Barczyk, A.; Durham, A.L.; di Stefano, A.; Adcock, I. COPD immunopathology. Semin. Immunopathol. 2016, 38, 497–515. [Google Scholar] [PubMed]
- Shin, S.H.; Kwon, S.O.; Kim, V.; Silverman, E.K.; Kim, T.H.; Kim, D.K.; Hwang, Y.I.; Yoo, K.H.; Kim, W.J.; Park, H.Y. Association of body mass index and COPD exacerbation among patients with chronic bronchitis. Respir. Res. 2022, 23, 52. [Google Scholar]
- Lahousse, L.; Seys, L.J.M.; Joos, G.F.; Franco, O.H.; Stricker, B.H.; Brusselle, G.G. Epidemiology and impact of chronic bronchitis in chronic obstructive pulmonary disease. Eur. Respir. J. 2017, 50, 1602470. [Google Scholar] [PubMed]
- Marshall, D.C.; Al Omari, O.; Goodall, R.; Shalhoub, J.; Adcock, I.M.; Chung, K.F.; Salciccioli, J.D. Trends in prevalence, mortality, and disability-adjusted life-years relating to chronic obstructive pulmonary disease in Europe: An observational study of the global burden of disease database, 2001–2019. BMC Pulm. Med. 2022, 22, 289. [Google Scholar]
- Institute for Health Metrics and Evaluation. Global Burden of Disease Study 2019 (GBD 2019) Results; Institute for Health Metrics and Evaluation: Seattle, WA, USA, 2019. [Google Scholar]
- Terry, P.D.; Dhand, R. The 2023 GOLD Report: Updated Guidelines for Inhaled Pharmacological Therapy in Patients with Stable COPD. Pulm. Ther. 2023, 9, 345–357. [Google Scholar]
- Adeloye, D.; Song, P.; Zhu, Y.; Campbell, H.; Sheikh, A.; Rudan, I.; NIHR RESPIRE Global Respiratory Health Unit. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: A systematic review and modelling analysis. Lancet Respir. Med. 2022, 10, 447–458. [Google Scholar]
- Vij, N.; Chandramani-Shivalingappa, P.; Van Westphal, C.; Hole, R.; Bodas, M. Cigarette smoke-induced autophagy impairment accelerates lung aging, COPD-emphysema exacerbations and pathogenesis. Am. J. Physiol. Cell Physiol. 2018, 314, C73–C87. [Google Scholar] [PubMed]
- Rathnayake, S.N.H.; Ditz, B.; van Nijnatten, J.; Sadaf, T.; Hansbro, P.M.; Brandsma, C.A.; Timens, W.; van Schadewijk, A.; Hiemstra, P.S.; Ten Hacken, N.H.T.; et al. Smoking induces shifts in cellular composition and transcriptome within the bronchial mucus barrier. Respirology 2023, 28, 132–142. [Google Scholar] [PubMed]
- Kim, V.; Jeong, S.; Zhao, H.; Kesimer, M.; Boucher, R.C.; Wells, J.M.; Christenson, S.A.; Han, M.K.; Dransfield, M.; Paine, R., 3rd; et al. Current smoking with or without chronic bronchitis is independently associated with goblet cell hyperplasia in healthy smokers and COPD subjects. Sci. Rep. 2020, 10, 20133. [Google Scholar]
- Alsharairi, N.A. Antioxidant Intake and Biomarkers of Asthma in Relation to Smoking Status—A Review. Curr. Issues Mol. Biol. 2023, 45, 5099–5117. [Google Scholar]
- Bradicich, M.; Schuurmans, M.M. Smoking status and second-hand smoke biomarkers in COPD, asthma and healthy controls. ERJ Open Res. 2020, 6, 00192–02019. [Google Scholar] [PubMed]
- Rabe, K.F.; Watz, H. Chronic obstructive pulmonary disease. Lancet 2017, 389, 1931–1940. [Google Scholar] [PubMed]
- Fermont, J.M.; Masconi, K.L.; Jensen, M.T.; Ferrari, R.; Di Lorenzo, V.A.P.; Marott, J.M.; Schuetz, P.; Watz, H.; Waschki, B.; Müllerova, H.; et al. Biomarkers and clinical outcomes in COPD: A systematic review and meta-analysis. Thorax 2019, 74, 439–446. [Google Scholar] [PubMed]
- Vivodtzev, I.; Tamisier, R.; Baguet, J.P.; Borel, J.C.; Levy, P.; Pépin, J.L. Arterial stiffness in COPD. Chest 2014, 145, 861–875. [Google Scholar]
- Papaioannou, A.I.; Mazioti, A.; Kiropoulos, T.; Tsilioni, I.; Koutsokera, A.; Tanou, K.; Nikoulis, D.J.; Georgoulias, P.; Zakynthinos, E.; Gourgoulianis, K.I.; et al. Systemic and airway inflammation and the presence of emphysema in patients with COPD. Respir. Med. 2010, 104, 275–282. [Google Scholar] [PubMed]
- Zhang, Y.; Tedrow, J.; Nouraie, M.; Li, X.; Chandra, D.; Bon, J.; Kass, D.J.; Fuhrman, C.R.; Leader, J.K.; Duncan, S.R.; et al. Elevated plasma level of Pentraxin 3 is associ-ated with emphysema and mortality in smokers. Thorax 2021, 76, 335–342. [Google Scholar] [PubMed]
- Ma, J.; Rubin, B.K.; Voynow, J.A. Mucins, Mucus, and Goblet Cells. Chest 2018, 154, 169–176. [Google Scholar]
- O’Donnell, R.A.; Richter, A.; Ward, J.; Angco, G.; Mehta, A.; Rousseau, K.; Swallow, D.M.; Holgate, S.T.; Djukanovic, R.; Davies, D.E.; et al. Expression of ErbB receptors and mucins in the airways of long term current smokers. Thorax 2004, 59, 1032–1040. [Google Scholar]
- Merikallio, H.; Kaarteenaho, R.; Lindén, S.; Padra, M.; Karimi, R.; Li, C.X.; Lappi-Blanco, E.; Wheelock, Å.M.; Sköld, C.M. Smoking-associated increase in mucins 1 and 4 in human airways. Respir. Res. 2020, 21, 239. [Google Scholar]
- Kato, K.; Chang, E.H.; Chen, Y.; Lu, W.; Kim, M.M.; Niihori, M.; Hecker, L.; Kim, K.C. MUC1 contributes to goblet cell metaplasia and MUC5AC expression in response to cigarette smoke in vivo. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L82–L90. [Google Scholar]
- Khorasani, A.M.; Mohammadi, B.; Saghafi, M.R.; Mohammadi, S.; Ghaffari, S.; Mirsadraee, M.; Khakzad, M.R. The association between MUC5AC and MUC5B genes expression and remodeling progression in severe neutrophilic asthma: A direct relationship. Respir. Med. 2023, 213, 107260. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhang, X.; Xu, Y.; Liu, L.; Sun, X.; Wang, B.; Yu, K.; Zhang, H.; Zhao, X.; Wang, X. BMAL1 regulates MUC1 overexpression in ovalbumin-induced asthma. Mol. Immunol. 2023, 156, 77–84. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Q.; Lu, H.; Wang, C.; Xia, L. Suppression of lncRNA MALAT1 Reduces LPS- or IL-17A-Induced Inflammatory Response in Human Middle Ear Epithelial Cells via the NF-κB Signaling Pathway. BioMed Res. Int. 2021, 2021, 8844119. [Google Scholar] [CrossRef]
- Jono, H.; Xu, H.; Kai, H.; Lim, D.J.; Kim, Y.S.; Feng, X.H.; Li, J.D. Transforming growth factor-beta-Smad signaling pathway negatively regulates nontypeable Haemophilus influenzae-induced MUC5AC mucin transcription via mitogen-activated protein kinase (MAPK) phosphatase-1-dependent inhibition of p38 MAPK. J. Biol. Chem. 2003, 278, 27811–27819. [Google Scholar] [CrossRef]
- Fischer, B.; Voynow, J. Neutrophil elastase induces MUC5AC messenger RNA expression by an oxidant-dependent mechanism. Chest 2000, 117, 317S–320S. [Google Scholar] [CrossRef]
- Wu, M.; Lai, T.; Jing, D.; Yang, S.; Wu, Y.; Li, Z.; Wu, Y.; Zhao, Y.; Zhou, L.; Chen, H.; et al. Epithelium-derived IL17A promotes cigarette smoke-induced inflammation and mucus hyperproduction. Am. J. Respir. Cell Mol. Biol. 2021, 65, 581–592. [Google Scholar] [PubMed]
- Nishida, Y.; Yagi, H.; Ota, M.; Tanaka, A.; Sato, K.; Inoue, T.; Yamada, S.; Arakawa, N.; Ishige, T.; Kobayashi, Y.; et al. Oxidative stress induces MUC5AC expression through mitochondrial damage-dependent STING signaling in human bronchial epithelial cells. FASEB BioAdvances 2023, 5, 171–181. [Google Scholar] [CrossRef]
- Deng, J.; Tang, H.; Zhang, Y.; Yuan, X.; Ma, N.; Hu, H.; Wang, X.; Liu, C.; Xu, G.; Li, Y.; et al. House dust mite-induced endo-plasmic reticulum stress mediates MUC5AC hypersecretion via TBK1 in airway epithelium. Exp. Lung Res. 2023, 49, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; Huang, L.Z.X.; Mykytyn, A.Z.; Wang, C.; Lamers, M.M.; Westendorp, B.; Wubbolts, R.W.; van Putten, J.P.M.; Bosch, B.J.; Haagmans, B.L.; et al. Glycosylated extracellu-lar mucin domains protect against SARS-CoV-2 infection at the respiratory surface. PLoS Pathog. 2023, 19, e1011571. [Google Scholar] [CrossRef]
- Arruda, B.L.; Kanefsky, R.A.; Hau, S.; Janzen, G.M.; Anderson, T.K.; Vincent Baker, A.L. Mucin 4 is a cellular biomarker of necrotizing bronchiolitis in influenza A virus infection. Microbes Infect. 2023, 105169. [Google Scholar] [CrossRef]
- Pratt, W.S.; Crawley, S.; Hicks, J.; Ho, J.; Nash, M.; Kim, Y.S.; Gum, J.R.; Swallow, D.M. Multiple transcripts of MUC3: Evidence for two genes, MUC3A and MUC3B. Biochem. Biophys. Res. Commun. 2000, 275, 916–923. [Google Scholar] [CrossRef]
- Kyo, K.; Muto, T.; Nagawa, H.; Lathrop, G.M.; Nakamura, Y. Associations of distinct variants of the intestinal mucin gene MUC3A with ulcerative colitis and Crohn’s disease. J. Hum. Genet. 2001, 46, 5–20. [Google Scholar] [CrossRef]
- Dohrman, A.; Tsuda, T.; Escudier, E.; Cardone, M.; Jany, B.; Gum, J.; Kim, Y.; Basbaum, C. Distribution of lysozyme and mucin (MUC2 and MUC3) mRNA in human bronchus. Exp. Lung Res. 1994, 20, 367–380. [Google Scholar] [CrossRef] [PubMed]
- López-Ferrer, A.; Curull, V.; Barranco, C.; Garrido, M.; Lloreta, J.; Real, F.X.; de Bolós, C. Mucins as Differentiation Markers in Bronchial Epithelium. Am. J. Respir. Cell Mol. Biol. 2001, 24, 22–29. [Google Scholar] [CrossRef]
- Copin, M.C.; Devisme, L.; Buisine, M.P.; Marquette, C.H.; Wurtz, A.; Aubert, J.P.; Gosselin, B.; Porchet, N. From normal respiratory mucosa to epidermoid carcinoma: Expression of human mucin genes. Int. J. Cancer 2000, 86, 162–168. [Google Scholar] [CrossRef]
- Copin, M.C.; Buisine, M.P.; Devisme, L.; Leroy, X.; Escande, F.; Gosselin, B.; Aubert, J.P.; Porchet, N. Normal respiratory mucosa, precursor lesions and lung carcinomas: Differential expression of human mucin genes. Front. Biosci. 2001, 6, D1264–D1275. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, S.; Dorofieiev, A. The Content of Mucin MUC-2, -3 and -4 Antigens in the Bronchial Mucosa Membrane of Chronic Obstructive Pulmonary Disease Patients dur-ing Acute Exacerbation—Initial Report. Adv. Respir. Med. 2017, 85, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Gum, J.R.; Hicks, J.W.; Crawley, S.C.; Dahl, C.M.; Yang, S.C.; Roberton, A.M.; Kim, Y.S. Initiation of transcription of the MUC3A human intestinal mucin from a TATA-less promoter and comparison with the MUC3B amino terminus. J. Biol. Chem. 2003, 278, 49600–49609. [Google Scholar] [CrossRef]
- Weidner, J.; Jogdand, P.; Jarenbäck, L.; Åberg, I.; Helihel, D.; Ankerst, J.; Westergren-Thorsson, G.; Bjermer, L.; Erjefält, J.S.; Tufvesson, E. Expression, activity and localization of lysosomal sulfatases in Chronic Obstructive Pulmonary Disease. Sci. Rep. 2019, 9, 1991. [Google Scholar] [CrossRef]
- Perez, T.A.; Castillo, E.G.; Ancochea, J.; Pastor Sanz, M.T.; Almagro, P.; Martínez-Camblor, P.; Miravitlles, M.; Rodríguez-Carballeira, M.; Navarro, A.; Lamprecht, B.; et al. Sex differences between women and men with COPD: A new analysis of the 3CIA study. Respir. Med. 2020, 171, 106105. [Google Scholar] [CrossRef]
- Machado, G.C.; Ferrer, V.P. MUC17 mutations and methylation are associated with poor prognosis in adult-type diffuse glioma patients. J. Neurol. Sci. 2023, 452, 120762. [Google Scholar] [CrossRef]
- Czogalla, B.; Dötzer, K.; Sigrüner, N.; von Koch, F.E.; Brambs, C.E.; Anthuber, S.; Frangini, S.; Burges, A.; Werner, J.; Mahner, S.; et al. Combined Expression of HGFR with Her2/neu, EGFR, IGF1R, Mucin-1 and Integrin α2β1 Is Associated with Aggressive Epithelial Ovarian Cancer. Biomedicines 2022, 10, 2694. [Google Scholar] [CrossRef]
- Kumar, A.R.; Devan, A.R.; Nair, B.; Nair, R.R.; Nath, L.R. Biology, Significance and Immune Signaling of Mucin 1 in Hepatocellular Carcinoma. Curr. Cancer Drug Targets 2022, 22, 725–740. [Google Scholar]
- Lin, S.; Tian, C.; Li, J.; Liu, B.; Ma, T.; Chen, K.; Gong, W.; Wang, J.M.; Huang, J. Differential MUC22 expression by epigenetic alterations in human lung squamous cell carcinoma and adenocarcinoma. Oncol. Rep. 2021, 45, 78. [Google Scholar] [CrossRef] [PubMed]
- Taverna, C.; Maggiore, G.; Cannavicci, A.; Bonomo, P.; Santucci, M.; Franchi, A. Immunohistochemical profiling of mucins in sinonasal adenocarcinomas. Pathol. Res. Pract. 2019, 215, 152439. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, T.; Okudela, K.; Nakashima, Y.; Mitsui, H.; Matsumura, M.; Umeda, S.; Arai, H.; Baba, T.; Suzuki, T.; Koike, C.; et al. Unique expression profiles of mucin proteins in interstitial pneumonia-associated lung adenocarcinomas. Histol. Histopathol. 2019, 34, 1243–1254. [Google Scholar]
- Tu, J.; Tang, M.; Li, G.; Chen, L.; Wang, Y.; Huang, Y. Expression of Mucin Family Proteins in Non-Small-Cell Lung Cancer and its Role in Evaluation of Prognosis. J. Oncol. 2022, 2022, 4181658. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, M.; Matsushima, M.; Carreras, J.; Hirabayashi, K.; Kikuti, Y.; Ueda, T.; Kaneko, M.; Fujimoto, R.; Sano, M.; Teramura, E.; et al. Whole-genome copy number and immunohistochemical analyses on surgically resected intracholecystic papillary neoplasms. Pathol. Int. 2021, 71, 823–830. [Google Scholar] [CrossRef]
- Robinson, L.; van Heerden, M.B.; Ker-Fox, J.G.; Hunter, K.D.; van Heerden, W.F.P. Expression of Mucins in Salivary Gland Mucoepidermoid Carcinoma. Head Neck Pathol. 2021, 15, 491–502. [Google Scholar] [CrossRef]
- Yokoyama, S.; Hamada, T.; Higashi, M.; Matsuo, K.; Maemura, K.; Kurahara, H.; Horinouchi, M.; Hiraki, T.; Sugimoto, T.; Akahane, T.; et al. Predicted Prognosis of Patients with Pancreatic Cancer by Machine Learning. Clin. Cancer Res. 2020, 26, 2411–2421. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zuo, D.; Liu, T.; Yin, L.; Li, C.; Wang, L. Prognostic and Clinicopathological Significance of MUC Family Members in Colorectal Cancer: A Systematic Review and Meta-Analysis. Gastroenterol. Res. Pract. 2019, 2019, 2391670. [Google Scholar] [CrossRef] [PubMed]
- Ratan, C.; Cicily, K.D.D.; Nair, B.; Nath, L.R. MUC Glycoproteins: Potential Biomarkers and Molecular Targets for Cancer Therapy. Curr. Cancer Drug Targets 2021, 21, 132–152. [Google Scholar] [CrossRef] [PubMed]
- Behl, A.; Sarwalia, P.; Kumar, S.; Behera, C.; Mintoo, M.J.; Datta, T.K.; Gupta, P.N.; Chhillar, A.K. Codelivery of Gemcita-bine and MUC1 Inhibitor Using PEG-PCL Nanoparticles for Breast Cancer Therapy. Mol. Pharm. 2022, 19, 2429–2440. [Google Scholar] [CrossRef]
- Lee, D.H.; Choi, S.; Park, Y.; Jin, H.S. Mucin1 and Mu-cin16: Therapeutic Targets for Cancer Therapy. Pharmaceuticals 2021, 14, 1053. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Chaudhary, S.; Lakshmanan, I.; Aithal, A.; Kisling, S.G.; Sorrell, C.; Marimuthu, S.; Gautam, S.K.; Rauth, S.; Kshirsagar, P.; et al. Chi-meric antibody targeting unique epitope on onco-mucin16 reduces tumor burden in pancreatic and lung malignancies. NPJ Precis. Oncol. 2023, 7, 74. [Google Scholar] [CrossRef]
- Schicht, M.; Reichle, A.; Schapher, M.; Garreis, F.; Kleinsasser, B.; Aydin, M.; Sahin, A.; Iro, H.; Paulsen, F. The Translational Role of MUC8 in Salivary Glands: A Potential Biomarker for Salivary Stone Disease? Diagnostics 2021, 11, 2330. [Google Scholar] [CrossRef]
- Lewandowska, K.B.; Szturmowicz, M.; Lechowicz, U.; Franczuk, M.; Błasińska, K.; Falis, M.; Błaszczyk, K.; Sobiecka, M.; Wyrostkiewicz, D.; Siemion-Szcześniak, I.; et al. The Presence of T Allele (rs35705950) of the MUC5B Gene Predicts Lower Baseline Forced Vital Capacity and Its Subsequent Decline in Patients with Hypersensitivity Pneumonitis. Int. J. Mol. Sci. 2023, 24, 10748. [Google Scholar] [CrossRef]
- Packer, R.J.; Shrine, N.; Hall, R.; Melbourne, C.A.; Thompson, R.; Williams, A.T.; Paynton, M.L.; Guyatt, A.L.; Allen, R.J.; Lee, P.H.; et al. Genome-wide association study of chronic sputum production implicates loci in-volved in mucus production and infection. Eur. Respir. J. 2023, 61, 2201667. [Google Scholar] [CrossRef]
- Tajiri, T.; Matsumoto, H.; Jinnai, M.; Kanemitsu, Y.; Nagasaki, T.; Iwata, T.; Inoue, H.; Nakaji, H.; Oguma, T.; Ito, I.; et al. Pathophysiological relevance of sputum MUC5AC and MUC5B levels in patients with mild asthma. Allergol. Int. 2022, 71, 193–199. [Google Scholar] [CrossRef]
- Shah, B.K.; Singh, B.; Wang, Y.; Xie, S.; Wang, C. Mucus Hypersecretion in Chronic Obstruc-tive Pulmonary Disease and Its Treatment. Mediat. Inflamm. 2023, 2023, 8840594. [Google Scholar] [CrossRef]
- Kumar, S.S.; Binu, A.; Devan, A.R.; Nath, L.R. Mucus targeting as a plausible approach to improve lung function in COVID-19 patients. Med. Hypotheses 2021, 156, 110680. [Google Scholar] [CrossRef]
- Kesimer, M.; Ford, A.A.; Ceppe, A.; Radicioni, G.; Cao, R.; Davis, C.W.; Doerschuk, C.M.; Alexis, N.E.; Anderson, W.H.; Henderson, A.G.; et al. Airway Mucin Concentration as a Marker of Chronic Bronchitis. N. Engl. J. Med. 2017, 377, 911–922. [Google Scholar] [CrossRef]
- Radicioni, G.; Ceppe, A.; Ford, A.A.; Alexis, N.E.; Barr, R.G.; Bleecker, E.R.; Christenson, S.A.; Cooper, C.B.; Han, M.K.; Hansel, N.N.; et al. Airway mucin MUC5AC and MUC5B concentrations and the initiation and progression of chronic obstructive pulmonary disease: An analysis of the SPIROMICS cohort. Lancet Respir. Med. 2021, 9, 1241–1254. [Google Scholar] [CrossRef]
- Forsslund, H.; Yang, M.; Mikko, M.; Karimi, R.; Nyrén, S.; Engvall, B.; Grunewald, J.; Merikallio, H.; Kaarteenaho, R.; Wahlström, J.; et al. Gender differences in the T-cell profiles of the airways in COPD patients associated with clinical phenotypes. Int. J. Chronic Obstr. Pulm. Dis. 2016, 12, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Balgoma, D.; Yang, M.; Sjödin, M.; Snowden, S.; Karimi, R.; Levänen, B.; Merikallio, H.; Kaarteenaho, R.; Palmberg, L.; Larsson, K.; et al. Linoleic acid-derived lipid mediators increase in a female-dominated subphenotype of COPD. Eur. Respir. J. 2016, 47, 1645–1656. [Google Scholar] [CrossRef] [PubMed]
- Karimi, R.; Tornling, G.; Forsslund, H.; Mikko, M.; Wheelock, Å.M.; Nyrén, S.; Sköld, C.M. Differences in regional air trapping in current smokers with normal spirometry. Eur. Respir. J. 2017, 49, 1600345. [Google Scholar] [CrossRef]
- Karimi, R.; Tornling, G.; Forsslund, H.; Mikko, M.; Wheelock, Å.M.; Nyrén, S.; Sköld, C.M. Lung density on high resolution computer tomography (HRCT) reflects degree of inflammation in smokers. Respir. Res. 2014, 15, 23. [Google Scholar] [CrossRef]
- Kohler, M.; Sandberg, A.; Kjellqvist, S.; Thomas, A.; Karimi, R.; Nyrén, S.; Eklund, A.; Thevis, M.; Sköld, C.M.; Wheelock, Å.M. Gender differences in the bronchoalveolar lavage cell proteome of patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2013, 131, 743–751. [Google Scholar] [CrossRef]
- Sandberg, A.S.; Sköld, C.M.; Grunewald, J.; Eklund, A.; Wheelock, Å.M. Assessing recent smoking status by measuring exhaled carbon monoxide levels. PLoS ONE 2011, 6, e28864. [Google Scholar] [CrossRef] [PubMed]
- Karimi, R.; Tornling, G.; Grunewald, J.; Eklund, A.; Sköld, C.M. Cell recovery in bronchoalveolar lavage fluid in smokers is dependent on cumulative smoking history. PLoS ONE 2012, 7, e34232. [Google Scholar] [CrossRef]
- Forsslund, H.; Mikko, M.; Karimi, R.; Grunewald, J.; Wheelock, Å.M.; Wahlström, J.; Sköld, C.M. Distribution of T-cell subsets in BAL fluid of patients with mild to moderate COPD depends on current smoking status and not airway obstruction. Chest 2014, 145, 711–722. [Google Scholar] [CrossRef]
- Löfdahl, J.M.; Cederlund, K.; Nathell, L.; Eklund, A.; Sköld, C.M. Bronchoalveolar lavage in COPD: Fluid recovery correlates with the degree of emphysema. Eur. Respir. J. 2005, 25, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Kohler, M.; Heyder, T.; Forsslund, H.; Garberg, H.K.; Karimi, R.; Grunewald, J.; Berven, F.S.; Nyrén, S.; Sköld, C.M. Proteomic profiling of lung immune cells reveals dysregulation of phagocytotic pathways in female-dominated molecular COPD phenotype. Respir. Res. 2018, 19, 39. [Google Scholar] [CrossRef]
- Lee, H.J.; Xu, X.; Choe, G.; Chung, D.H.; Seo, J.W.; Lee, J.H.; Lee, C.T.; Jheon, S.; Sung, S.W.; Chung, J.H. Protein overexpression and gene amplification of epidermal growth factor receptor in nonsmall cell lung carcinomas: Comparison of four commercially available antibodies by immunohistochemistry and fluorescence in situ hybridization study. Lung Cancer 2010, 68, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Lappi-Blanco, E.; Mäkinen, J.M.; Lehtonen, S.; Karvonen, H.; Sormunen, R.; Laitakari, K.; Johnson, S.; Mäkitaro, R.; Bloigu, R.; Kaarteenaho, R. Mucin-1 correlates with survival, smoking status, and growth patterns in lung adenocarcinoma. Tumor Biol. 2016, 37, 13811–13820. [Google Scholar] [CrossRef]
- Li, C.X.; Wheelock, C.E.; Sköld, C.M.; Wheelock, Å.M. Integration of multi-omics datasets enables molecular classification of COPD. Eur. Respir. J. 2018, 51, 1701930. [Google Scholar] [CrossRef]
- Bolstad, B.M.; Irizarry, R.A.; Åstrand, M.; Speed, T.P. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 2003, 19, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004, 5, R80. [Google Scholar] [CrossRef] [PubMed]
- Smyth, G.K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 2004, 3, 3. [Google Scholar] [CrossRef]
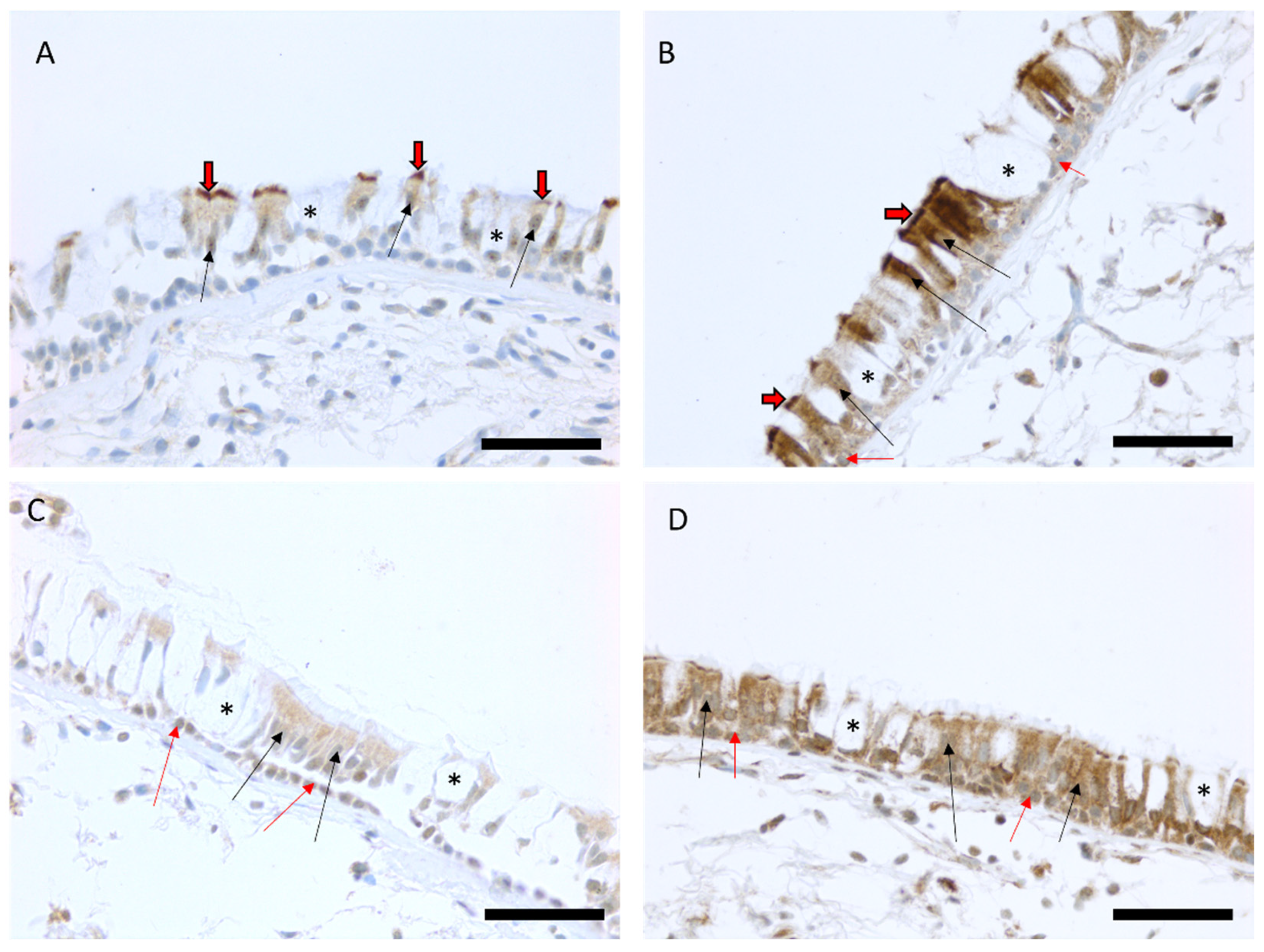
| Healthy Never-Smokers (n = 40) | Smokers (n = 40) | COPD Smokers (n = 27) | COPD Ex-Smokers (n = 11) | |
|---|---|---|---|---|
| Age (years) | 59.5 (51.0–63.8) | 53.0 (49.0–58.6) | 60.4 (56.0–63.0) | 63.0 (54.1–65.0) |
| Female subjects (n (%)) | 20 (50) | 20 (50) | 12 (44) | 6 (55) |
| Number of mRNA samples (n (%)) | 10 (25) | 7 (18) | 11 (41) | 0 (0) |
| Current cigarette consumption (per day) | 0 (0–0) | 17 (12–20) | 20 (11–20) | 0 (0–0) |
| Smoking history (pack-years) | 0 (0–0) | 33.5 (28.5–40.0) | 42 (36–48) | 30 (20–38) |
| Exhaled carbon monoxide (ppm) | 0.33 (0.00–0.84) | 9.84 (6.67–11.50) | 9.67 (2.67–13.33) | 0.33 (0.00–5.33) |
| Postbronchodilator FEV1 (% predicted) | 119.5 (109.5–127.0) | 108.5 (103.0–116.5) | 78.0 (74.0–85.0) | 81.0 (65.0–88.0) |
| Postbronchodilator FVC (% predicted) | 121.0 (110.5–128.0) | 113.0 (106.5–124.0) | 105.0 (94.0–110.0) | 104.0 (95.0–116.0) |
| Postbronchodilator VC (% predicted) | 120.5 (110.5–129.5) | 114.0 (106.5–126.5) | 106.0 (94.0–119.0) | 111.0 (101.0–119.0) |
| Diffusing capacity (% predicted) | 89.5 (84.0–96.5) | 77.5 (72.0–85.0) | 63.0 (60.0–74.0) | 71.0 (49.0–77.0) |
| Residual volume (% predicted) | 100.5 (91.5–111.0) | 115.0 (94.0–127.0) | 132.5 (107.0–165.0) | 143.0 (124.0–160.0) |
| Emphysema (n (%)) | 1 (3) | 22 (55) | 21 (78) | 8 (73) |
| Chronic bronchitis (n (%)) | 0 (0) | 10 (25) | 7 (26) | 2 (18) |
| Provided mRNA (n = 28) | Did Not Provide mRNA (n = 90) | p-Value | |
|---|---|---|---|
| Female subjects (n (%), current smokers (n, %)) | 13 (46), 7 (54) | 45 (50), 25 (56) | 0.830, 0.192 |
| Male subjects (n (%), current smokers (n, %)) | 15 (54), 11 (73) | 45 (50), 24 (53) | 0.830, 0.192 |
| Age (years) | 58.0 (50.0–62.9) | 57.5 (52.0–63.0) | 0.770 |
| Current cigarette consumption (per day) | 15 (0–20) | 10 (0–17) | 0.088 |
| Smoking history (pack-years) | 34 (0–41) | 26 (0–40) | 0.318 |
| Exhaled carbon monoxide (ppm) | 7.0 (0.7–11.0) | 2.7 (0.3–10.0) | 0.475 |
| Postbronchodilator FEV1 (% predicted) | 92.5 (80.0–108.0) | 111.5 (92.0–121.0) | 0.017 |
| Postbronchodilator FVC (% predicted) | 111.5 (104.5–124.0) | 111.5 (103.0–123.0) | 0.823 |
| Postbronchodilator VC (% predicted) | 111.5 (98.5–124.0) | 114.0 (106.0–128.0) | 0.168 |
| Diffusing capacity (% predicted) | 80.0 (63.0–92.0) | 79.0 (70.5–88.0) | 0.761 |
| Residual volume (% predicted) | 119 (108–147) | 109 (94–129) | 0.018 |
| Emphysema (n (%)) | 12 (43) | 40 (44) | 1.000 |
| Chronic bronchitis (n (%)) | 6 (21) | 13 (14) | 0.387 |
| Producer | Kit | Antigen Retrieval | Dilution | |
|---|---|---|---|---|
| MUC3A | Atlas Antibodies | Envision | Citrate pH 6 | 1/150 |
| MUC3B | Abgent (C-term E881) | Flex | Citrate pH 6 | 1/1000 |
| EGFR1 | Novocastra. NCL-L-EGFR_384 | Envision | Citrate pH 6 | 1/100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merikallio, H.; Pincikova, T.; Kotortsi, I.; Karimi, R.; Li, C.-X.; Forsslund, H.; Mikko, M.; Nyrén, S.; Lappi-Blanco, E.; Wheelock, Å.M.; et al. Mucins 3A and 3B Are Expressed in the Epithelium of Human Large Airway. Int. J. Mol. Sci. 2023, 24, 13546. https://doi.org/10.3390/ijms241713546
Merikallio H, Pincikova T, Kotortsi I, Karimi R, Li C-X, Forsslund H, Mikko M, Nyrén S, Lappi-Blanco E, Wheelock ÅM, et al. Mucins 3A and 3B Are Expressed in the Epithelium of Human Large Airway. International Journal of Molecular Sciences. 2023; 24(17):13546. https://doi.org/10.3390/ijms241713546
Chicago/Turabian StyleMerikallio, Heta, Terezia Pincikova, Ioanna Kotortsi, Reza Karimi, Chuan-Xing Li, Helena Forsslund, Mikael Mikko, Sven Nyrén, Elisa Lappi-Blanco, Åsa M. Wheelock, and et al. 2023. "Mucins 3A and 3B Are Expressed in the Epithelium of Human Large Airway" International Journal of Molecular Sciences 24, no. 17: 13546. https://doi.org/10.3390/ijms241713546





